Significance
Secondary organic aerosol (SOA) formed from the atmospheric oxidation of gaseous combustion emissions is an important component of global fine-particle pollution, which influences the Earth’s energy budget and affects human health. However, existing models underpredict the amount of SOA measured in laboratory experiments and in the atmosphere. We analyze smog chamber and emissions data to demonstrate that unspeciated organics in combustion emissions are a major class of SOA precursors. We develop source-specific parameterizations for these emissions using surrogate chemical compounds. We find that unspeciated organics dominate SOA mass formed from combustion emissions in the United States; therefore, unspeciated organics must be included in models to simulate ambient fine particulate matter concentrations.
Keywords: particulate matter, air quality, photochemical oxidation, volatile organic compounds, emissions inventory
Abstract
Secondary organic aerosol (SOA) formed from the atmospheric oxidation of nonmethane organic gases (NMOG) is a major contributor to atmospheric aerosol mass. Emissions and smog chamber experiments were performed to investigate SOA formation from gasoline vehicles, diesel vehicles, and biomass burning. About 10–20% of NMOG emissions from these major combustion sources are not routinely speciated and therefore are currently misclassified in emission inventories and chemical transport models. The smog chamber data demonstrate that this misclassification biases model predictions of SOA production low because the unspeciated NMOG produce more SOA per unit mass than the speciated NMOG. We present new source-specific SOA yield parameterizations for these unspeciated emissions. These parameterizations and associated source profiles are designed for implementation in chemical transport models. Box model calculations using these new parameterizations predict that NMOG emissions from the top six combustion sources form 0.7 Tg y−1 of first-generation SOA in the United States, almost 90% of which is from biomass burning and gasoline vehicles. About 85% of this SOA comes from unspeciated NMOG, demonstrating that chemical transport models need improved treatment of combustion emissions to accurately predict ambient SOA concentrations.
Combustion sources such as motor vehicles, fireplaces, and wildfires emit a complex mixture of gaseous and particulate pollutants that influence Earth’s climate, ecology, and human health (1). Although many aspects of particulate matter are well understood, large uncertainties still exist concerning the formation and evolution of secondary organic aerosol (SOA) from the atmospheric oxidation of nonmethane organic gases (NMOG) (2). (In this work, NMOG and volatile organic compound are used synonymously.) Globally, SOA concentrations exceed primary organic aerosol (POA) levels and account for one third or more of the dry fine-aerosol mass in the atmosphere (3). However, chemical transport models often significantly underpredict SOA concentrations (4), especially during photochemical episodes (5).
Recent smog chamber experiments have demonstrated substantial SOA mass formation from diluted exhaust of major combustion sources, including light-duty gasoline vehicles, medium- and heavy-duty diesel vehicles, and biomass burning (6–11). However, speciated SOA precursors such as single-ring aromatics, isoprene, terpenes, and large alkanes that are commonly included in atmospheric models only explained a fraction of the measured SOA mass (12).
Robinson et al. (12) proposed that much of the unexplained SOA arises from the oxidation of high-molecular-weight organic vapors (C12 and higher) that are difficult to speciate with standard gas chromatography (GC)-based techniques used to develop emission profiles. One major challenge for GC analysis is the exponential increase in the number of constitutional isomers with increasing carbon number (13); another is the polarity of partially oxidized emissions (which elute very slowly if at all). This leads to two classes of unspeciated organics: unresolved and uneluted (14). Unresolved organics coelute from a GC column, making it hard to identify individual compounds (e.g., isoalkanes) and causing them to appear as an unresolved complex mixture (UCM). Uneluted organics often do not pass through a GC column at all (e.g., substituted polar compounds while using a nonpolar column). Numerous studies have reported significant UCM mass while analyzing particle and gas-phase organic emissions from combustion sources (14–17). For fossil fuel sources, the UCM emissions typically greatly exceed the uneluted component (18). There have been recent advances in understanding the composition of UCM emissions (19), but substantial work remains before they are comprehensively speciated, independently studied, and appropriately included as SOA precursors in models.
Chemical transport models and reaction mechanisms were developed largely to address photochemical ozone formation, driven by the national ambient air quality standard for ozone. The unspeciated NMOG is a small enough fraction of total NMOG emissions that models can map the unspeciated organics onto species in a reaction mechanism without degrading model performance for ozone predictions. However, this may be a much riskier omission for simulating SOA formation. The few models that include SOA formation from unspeciated organics report improved model performance (20–22). However, their inventories [based on gas-particle partitioning data (12) or surrogate scaling (22)] and SOA pathways [assumed to be source independent and based on limited experimental data (12)] are poorly constrained; thus, the organic aerosol (OA) budgets predicted by these models remain uncertain.
In this work, we analyze published smog chamber and emissions data to develop source-specific parameterizations for SOA mass formation from unspeciated organics emitted by major combustion sources. We determine, based on a review of emission profiles and inventories, that contemporary chemical transport models misclassify emissions of unspeciated organics. We develop source profiles and a US inventory for unspeciated organics from combustion sources and use the new source-specific parameterizations to show that unspeciated emissions contribute substantially to SOA production.
Results and Discussion
SOA Production from Dilute Exhaust.
We analyzed published data compiled from 138 emissions and 58 smog chamber experiments conducted by Carnegie Mellon University’s Center for Atmospheric Particle Studies using 15 on-road gasoline vehicles, 3 on-road diesel vehicles, and biomass burning of 12 different fuels collected from different regions of North America. The vehicles spanned a wide range of engine and emissions control technologies; details are in SI Appendix. According to the US National Emissions Inventory (NEI), these source categories—on-road gasoline, on-road diesel, and biomass burning—account for 72% of all combustion-related NMOG emissions in the United States and therefore are likely important contributors to SOA mass formation (23); combustion emissions account for 50% of nonbiogenic NMOG emissions in the United States. Evaporative, refueling, and other nontailpipe NMOG emissions from these sources are not thought to contribute substantially to SOA mass formation (19, 24).
POA and NMOG emissions were characterized at low levels of dilution (e.g., in a constant volume sampler) using standard procedures. SOA formation was characterized by injecting dilute emissions from each source into a smog chamber and photochemically aging them under urban-like conditions (relatively high NOx and moderate organic aerosol concentrations). The chamber experiments were performed at low relative humidity (<20%) and modest OH exposures (<2.3 × 107 molecule-hr cm−3, comparable to a few hours of atmospheric oxidation); therefore, SOA formation was dominated by gas-phase oxidation (rather than aqueous processing) with limited multigenerational chemistry. The POA, SOA, and NMOG data compared well with previous studies (SI Appendix, Table S1). Additional experimental details are in SI Appendix.
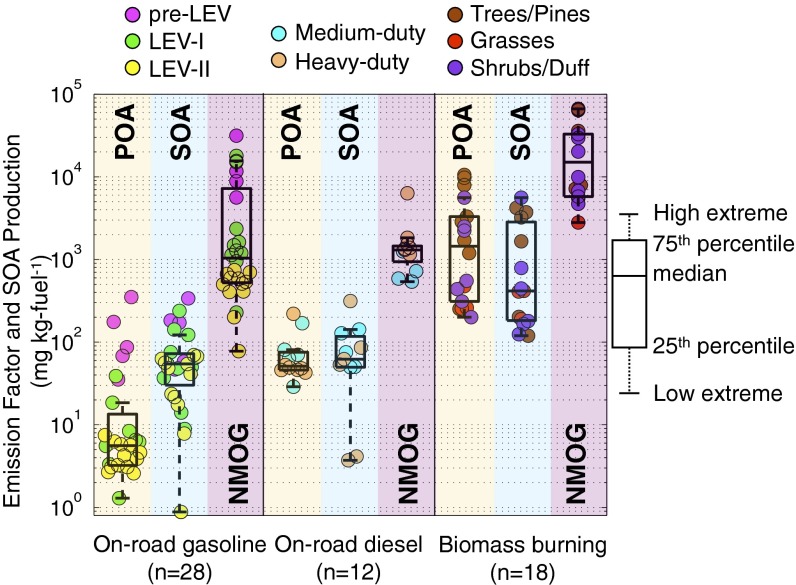
In Fig. 1, we compare emission/production factors for POA, SOA, and NMOG. There were clear differences in emissions between the three source categories. Across the three categories, the median POA emission factor varied by almost three orders of magnitude; it was highest for biomass burning and lowest for on-road gasoline vehicles. Biomass burning also had the highest NMOG emissions, which were an order of magnitude higher than those for the median gasoline and diesel vehicle. NMOG emissions from some older gasoline vehicles (pre-1995) were comparable to the biomass burning emissions. The significant variation within each category was caused by source-to-source variability in emissions, not experimental uncertainty (SI Appendix).
Fig. 1.
Smog chamber emission/production factors for POA, SOA, and NMOG for on-road gasoline, on-road diesel, and biomass burning. Solid symbols are data from individual experiments. Boxes show 25th and 75th percentiles, and whiskers show the low and high extremes across the data. The low extreme extends to the data point closest to, but larger than Q1 − 1.5 × IQR, and the high extreme extends to the data point closest to, but smaller than Q3 + 1.5 × IQR, where Q1 is the 25th percentile, Q3 is the 75th percentile, and IQR is the interquartile range. The scatter in the data are driven by source-to-source variability in the emissions. NMOG is calculated by subtracting the methane mass from the total organic gas mass measured with a flame ionization detector. The on-road gasoline source category includes data from 28 experiments conducted on emissions from three pre-Low Emission Vehicle (LEV), six LEV-I, and six LEV-II light-duty gasoline vehicles. The on-road diesel source category includes data from 10 experiments conducted on one heavy-duty diesel vehicle and 5 experiments conducted on two medium-duty diesel vehicles. The on-road diesel data are from vehicles without exhaust aftertreatment (e.g., diesel particulate filters), the most common type of diesel vehicle currently on the road. The biomass burning source category includes data from 18 experiments conducted on 12 different biomass types (trees/pines, grass, and shrubs/duff).
For all three source categories, the NMOG emissions exceeded the POA emissions by an order of magnitude or more. This underscores the large potential for SOA mass formation. In general, more SOA formation was measured in experiments with higher NMOG emissions. The scatter in the SOA data plotted in Fig. 1 is largely driven by source-to-source variability in the magnitude and composition of the NMOG emissions (SI Appendix). Biomass burning produced, on average, about an order of magnitude more SOA than gasoline and diesel vehicles on a mass of fuel burned basis, similar to the average difference in NMOG emissions. For the gasoline and diesel categories, the SOA production was comparable to or higher than the POA emissions, consistent with the many field studies reporting that summertime ambient SOA concentrations greatly exceed those of POA, even in urban areas (3). For biomass burning, the median SOA mass formation was somewhat lower than the median POA emissions. The smog chamber experiments represented only a few hours of photochemical aging; therefore the SOA production factors plotted in Fig. 1 likely underestimate the ultimate SOA production from these sources. We expect SOA production to be substantially larger than POA emissions with continued oxidation.
Fig. 1 indicates that gasoline emissions have much higher SOA-to-POA ratios than diesel emissions, yet the total OA from those sources was similar; therefore gasoline vehicles effectively “catch up” to diesel vehicles when one accounts for SOA production. For on-road gasoline vehicles, there was an order of magnitude reduction in the NMOG emissions from newer, lower-mileage vehicles that met stricter emissions standards (pre-LEV to LEV-I to LEV-II) (25). However, those reductions did not translate into a similar reduction in SOA (10). The emissions controls appear to have selectively removed a greater fraction of the lower-molecular-weight organics, while allowing the more efficient SOA precursors to remain in the emissions (10). There were no discernible trends in POA, SOA, and NMOG values within the on-road diesel and biomass burning subcategories.
Fig. 1 focuses on existing sources. Recent regulations require the installation of aftertreatment devices (diesel particulate filter, diesel oxidation catalyst, and selective catalytic reduction) on diesel vehicles in the United States and Europe. Catalyzed diesel particulate filters effectively eliminate primary particulate matter emissions and SOA production (6, 11).
SOA from Speciated Organics.
Under the conditions of these experiments, SOA was formed from the gas-phase oxidation of high-molecular-weight NMOG, but most chemical transport models only account for the oxidation of speciated NMOG. We predicted the SOA mass from the oxidation of speciated NMOG (SOAsp) using the SOA model in the Community Multiscale Air Quality model (CMAQ)—a chemical transport model widely used for regulatory and policy analyses in the United States (4). To do this, we quantified the emissions of speciated SOA precursors (e.g., aromatics and larger alkanes) using standard one-dimensional GC-based techniques. The speciation data for on-road vehicles are in May et al. (25); the biomass burning data are in SI Appendix, Table S2. The ratio of speciated SOA precursors to the sum of the speciated NMOG emissions compared well with literature data (SI Appendix, Fig. S1).
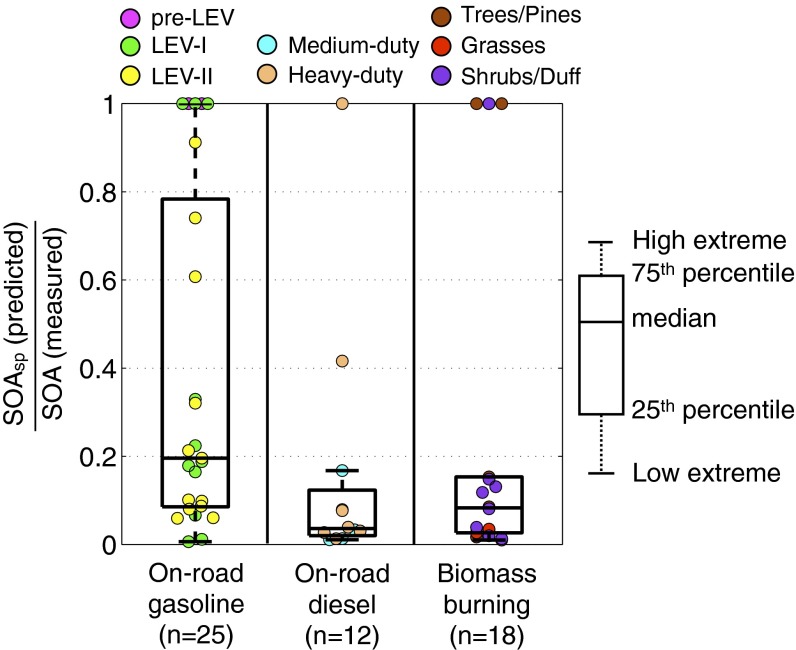
In Fig. 2, we plot the ratio of SOAsp to the total measured SOA mass for all of our smog chamber experiments. For all three source categories, the speciated SOA precursors explained only a small fraction of the measured SOA mass with median SOAsp-to-measured-SOA ratios ranging between 0.04 and 0.20. About 80% of the experiments had ratios less than 0.5. In only 16% of the experiments, the SOAsp matched or exceeded the measured SOA mass. The SOA yields in CMAQ may be uncertain by a factor of two, but this does not substantially alter the conclusion that speciated SOA precursors explain only a small fraction of the SOA (SI Appendix). This implies that a large set of SOA precursors remain unaccounted for.
Fig. 2.
Box plots of ratios of predicted SOA mass (SOAsp) to the SOA mass measured in the smog chamber experiments conducted with dilute exhaust. The solid circles are ratios from individual experiments. SOAsp is predicted using the parameterization in CMAQ v4.7 and the speciated NMOG data (6). For some experiments, SOAsp exceeded measured SOA formation; there we assumed that the model fully explained the SOA formation, and the ratios are restricted to unity.
SOA Yields of Unspeciated Organics.
Although the GC-based speciation techniques used by this study provide comprehensive data (e.g., 202 individual organics were quantified in gasoline exhaust) and are commonly used to develop source profiles, a portion of the NMOG emissions was not speciated. It seems plausible that the difference between the measured SOA mass and the SOA mass predicted from speciated precursors (SOAsp) could be largely due to these unspeciated organics.
The mass of unspeciated organics was defined as the difference between the total measured NMOG mass minus the sum of the speciated organics, plus any evaporated POA. The total NMOG mass was measured using a flame ionization detector, calibrated with propane, at low dilution (high OA concentrations), following standard protocols (25). This measurement did not include any compounds evaporated from the POA between measurements made at low dilution (defined by standard sampling protocols) and the more atmospheric-like conditions inside the smog chamber. The evaporated POA was defined as the difference between POA mass measured at low dilution and that measured inside the smog chamber at higher levels of dilution (SI Appendix) (26–28). On average, semivolatile POA vapors contributed ∼20% to unspeciated organics from on-road diesel, ∼65% to unspeciated organics from biomass burning, and a negligible fraction of unspeciated organics from on-road gasoline inside the smog chamber.
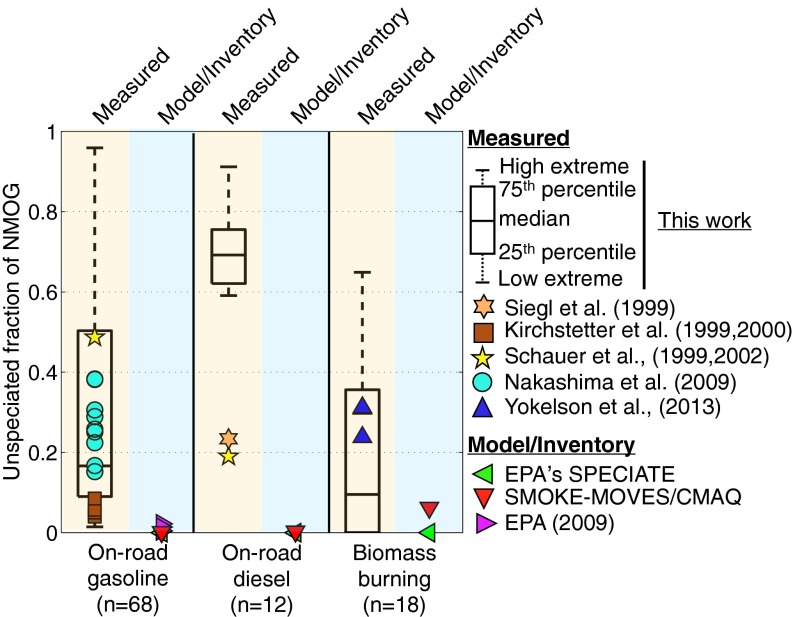
In general, 10–20% of total NMOG emissions from these source categories are not speciated by commonly applied GC-based techniques. Our data, shown in Fig. 3, confirm this. For on-road gasoline sources, we quantified the emissions of 202 individual species (mainly hydrocarbons); together these species accounted for 84% of the total NMOG emissions, leaving 16% unspeciated. For biomass burning, 66 individual species were quantified, which accounted for 90% of the total NMOG emissions, leaving 10% unspeciated. For diesel emissions, we only speciated midweight SOA precursors (C6-C12 organics) but not lower-molecular-weight species. Schauer et al. (16) and Siegl et al. (29) performed more comprehensive speciation analysis on diesel exhaust; they report unspeciated fractions of 19% and 23%, respectively, for diesel exhaust. Our measurements of speciated SOA precursors agreed well with the data of Schauer et al. (16) and Siegl et al. (29) (SI Appendix, Fig. S1); thus, we assumed that 20% of the NMOG from diesel vehicles is unspeciated.
Fig. 3.
Box plots of the unspeciated fraction of NMOG. Solid symbols represent literature values.
We estimated the SOA mass yield for the unspeciated organics by fitting the unexplained SOA (the difference between the measured SOA mass and model-predicted SOA mass from speciated organics). Because little is known about the unspeciated NMOG, we treated it as a lumped species—one such surrogate species for each combustion source category (gasoline, diesel, and biomass). We represented the reaction rate and SOA mass yield of these surrogate species using n-alkane data from Presto et al. (30) to facilitate efficient implementation in chemical transport models. The n-alkane surrogate for the unspeciated NMOG emissions for each source category was chosen by minimizing the error between the predicted and measured SOA mass. Each surrogate species represents the average yield of the complex mixture of thousands of compounds comprising the unspeciated NMOG for a given source category.
The unspeciated organic emissions for different source categories had different SOA yields, but in all cases these yields were significant, ranging between 10% and 40% depending on conditions. The unspeciated organic emissions from on-road diesel vehicles had yields comparable to n-pentadecane (C15 n-alkane), which is not surprising because diesel fuel is dominated by high-molecular-weight species (>C10) (19). The unspeciated organic emissions from on-road gasoline vehicles had lower SOA yields than diesel, similar to n-tridecane (C13 n-alkane). Presumably, this is due to differences in composition of gasoline and diesel fuel. Finally, the SOA yield of unspeciated emissions from biomass burning was similar to n-pentadecane (C15 n-alkane). The fact that the SOA yields for unspeciated organics are different for different source categories indicates that the composition of these emissions varies by source category.
The skill of the parameterization is shown in SI Appendix, Fig. S2. It changed the model measurement bias from a severe underestimation (substantial unexplained SOA) when only speciated precursors are included in the model to almost unbiased when both speciated precursors and unspeciated NMOG are included. The change in the median bias was from −80% to −12% for on-road gasoline, −95% to +3% for on-road diesel, and −94% to −2% for biomass burning.
Although the new parameterization removed model measurement bias, significant scatter remained (although less scatter than for the speciated-only model). The scatter was lower for the gasoline and diesel data than for biomass burning. This reflects real world source variability, which is greatest for biomass burning. A key issue is source-to-source variability in SOA precursor composition. For example, the composition of the speciated SOA precursors varied by more than ±50% within a given source category (SI Appendix, Fig. S1). The model captured the effects of this variability on the SOA production. However, the composition of the unspeciated organics likely varied by a similar amount, but this variability was not captured because the SOA production from the unspeciated NMOG emissions from each source category (not an individual source) was represented using a single n-alkane surrogate. This is appropriate because chemical transport models simulate the average behavior of a source category, not the variation among individual sources within a given source category. Additional variability is likely due to known uncertainty in the inputs (SOA yields for speciated organics and reaction rates of unspeciated organics) and to a combination of differences in gas-particle partitioning, oxidant exposure, and NMOG-to-NOx ratio. For more discussion, refer to SI Appendix.
Estimating US Unspeciated Organic Emissions.
Unspeciated organics are not appropriately included in current emission inventories and, in turn, chemical transport models. The problems with existing inventories are illustrated in Fig. 3, which compares actual emissions data to the emission profiles contained in three commonly used databases/tools (31, 32). Inventories are created by multiplying source-based NMOG emission rates by normalized emission profiles (33), which map the NMOG emissions onto surrogate species used by chemical mechanisms. These profiles are typically based on the speciated emissions from one-dimensional GC analysis similar to that performed here. However, existing emission databases, and therefore emission inventories and chemical transport models, do not explicitly account for emissions of unspeciated organics. For example, Fig. 3 indicates that current inventories have essentially no emissions of unspeciated organics for on-road gasoline sources, which stands in contrast to the data presented here and in the literature (16, 17, 29, 34–37).
A schematic of the inventory development process is presented in SI Appendix, Fig. S3, to illustrate how unspeciated organics are dropped. The emission profiles are normalized to the sum of the speciated organics rather than the entire NMOG emissions (38) to include all of the NMOG mass in the model. Therefore, even if the total NMOG emissions are correct, speciated organics are overrepresented, and unspeciated organics are absent (or, at best, underrepresented) in the inventory. The misallocation likely does not have a large effect on ozone modeling; however, as shown by the chamber data, unspeciated organics have a greater SOA formation potential than speciated organics—even speciated SOA precursors. This bias implies that current models probably underpredict SOA mass formation from combustion emissions.
Table 1 lists an estimate of the unspeciated organic emissions from six major sources (on-road gasoline, off-road gasoline, biomass burning, wood burning, off-road diesel, and on-road diesel) based on the 2008 NEI in the United States (23). Together these sources contribute 97% of all combustion-related NMOG emissions in the United States.
Table 1.
NMOG, POA, and estimated unspeciated organic emissions in the United States from the top six combustion sources considered in this work
| Source | NMOG emissions (Tg y−1) | Unspeciated fraction of NMOG (%) | POA emissions (Tg y−1) | Evaporated fraction of POA (%) | Unspeciated emissions (Tg y−1) | SOA surrogate for unspeciated emissions |
| Biomass burning (wild, prescribed, and agricultural fires) | 4.17 | 20 | 1.03 | 65 | 1.51 | n-pentadecane (C15) |
| Wood burning (residential wood combustion) | 0.34 | 7 | 0.24 | 65 | 0.18 | n-pentadecane (C15) |
| On-road gasoline (tailpipe) | 1.67 | 25 | 0.046 | 50 | 0.44 | n-tridecane (C13) |
| Off-road gasoline (tailpipe) | 1.54 | 25 | 0.035 | 50 | 0.40 | n-tridecane (C13) |
| On-road diesel (tailpipe) | 0.19 | 20 | 0.053 | 60 | 0.070 | n-pentadecane (C15) |
| Off-road diesel (tailpipe) | 0.15 | 20 | 0.027 | 60 | 0.046 | n-pentadecane (C15) |
| Other combustion | 0.28 | |||||
| Noncombustion | 7.77 | |||||
| Total | 16.1 | 2.65 |
We estimated the mass fraction of unspeciated organics for on-road gasoline and open burning as an average of all of the speciation data in Fig. 3. For on-road diesel, we averaged the Schauer et al. (16) and Siegl et al. (29) data, omitting our data because we only characterized SOA precursor emissions. For residential wood burning, we estimated the unspeciated fraction by averaging the Schauer et al. (39) and McDonald et al. (40) data. We assumed that the unspeciated fraction for off-road gasoline/diesel was the same as that for on-road gasoline/diesel.
The 2008 NEI estimates for NMOG emissions do not include the POA mass that evaporates with dilution to atmospheric concentrations (20). The issue is that POA emission factors used to construct the NEI were measured at low levels of dilution (<100) that create high particulate matter concentrations (>100 µg m−3) (20). This biases the gas-particle partitioning toward the particle phase. The NEI POA emissions data are corrected for dilution using the gas-particle partitioning data from May et al. (26–28). May et al. (26–28) showed that a maximum of 50%, 60%, and 65% of the POA emissions from on-road gasoline vehicles, on-road diesel vehicles, and biomass burning could evaporate when they were diluted from dilution sampler conditions to a typical ambient OA concentration of 5 µg m−3. Total unspeciated emissions are calculated as the sum of unspeciated NMOG emissions plus the mass of POA emissions that evaporate.
We estimated that the total unspeciated organic emissions from the six combustion sources are 2.65 Tg y−1 in the United States, which was slightly less than one third of all combustion-related NMOG emissions. Our estimate is a factor of six higher than previous estimates (33). The smog chamber data demonstrate that unspeciated emissions are potent SOA precursors, and their omission may bias SOA model predictions.
Atmospheric Implications.
The contribution of first-generation oxidation of unspeciated organics to the ambient SOA budget can be estimated by combining the previously described source-specific parameterizations and the new emissions data in Table 1 in a box model. A box model allowed us to isolate the effect of our new parameterization in a controlled context where we could comprehensively track the chemistry and partitioning for different sources. We ran two box model calculations: traditional and updated. The traditional model used the POA and SOA treatment from CMAQ (4). Briefly, POA was treated as nonvolatile and nonreactive, and SOAsp was calculated as described in the section SOA from Speciated Organics using emission profiles from SMOKE-MOVES/CMAQ (emission profile numbers are listed in SI Appendix, Table S7, and model speciation is listed in SI Appendix, Tables S8 and S9).
The updated model renormalized the SMOKE-MOVES/CMAQ emission profiles to account for unspeciated organics using the data in Table 1. Accounting for unspeciated organics reduced emissions of speciated organics; therefore, SOAsp was recalculated. Furthermore, POA was considered to be semivolatile, and the evaporated POA vapors contributed to the unspeciated organic mass. The n-alkane surrogates listed in Table 1 were used to predict SOA from unspeciated organics (SOAunsp; see also SI Appendix, Table S3). We assumed a constant background OA concentration of 5 µg m−3 and a constant temperature of 298 K to determine gas-particle partitioning of the semivolatile organics.
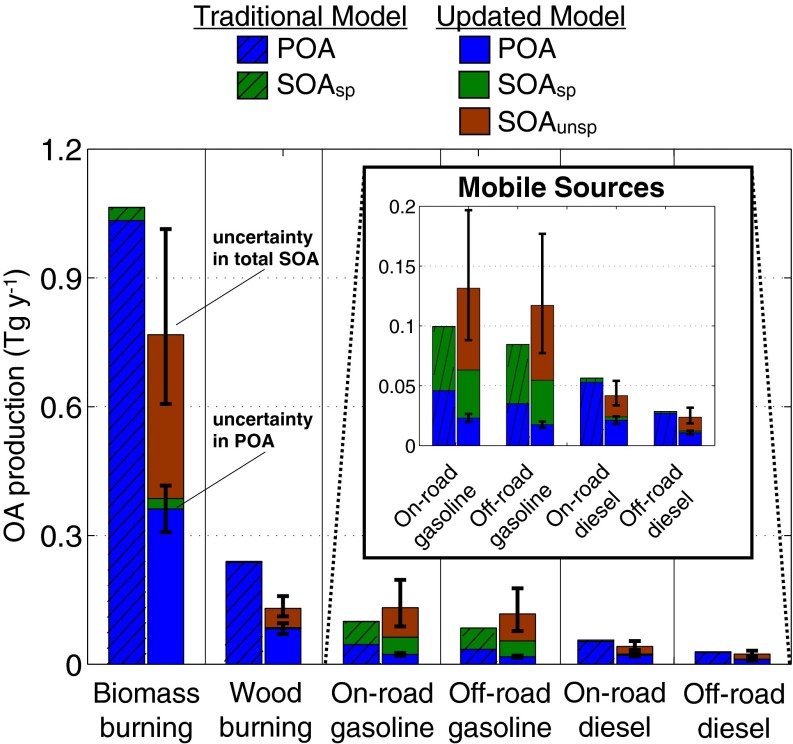
The results from the box model calculations are summarized in Fig. 4. The updated model modestly reduced the total OA (1.2 versus 1.6 Tg y−1) but significantly altered the POA–SOA split (2:3 versus 10:1) bringing it in closer agreement with ambient measurements made using the aerosol mass spectrometer (3). The OA reduction stemmed from the evaporation of POA; only about half of which reacted to form SOA after a single generation of oxidation. The updated model predicted five times more SOA than the traditional model (0.7 versus 0.14 Tg y−1). The difference in SOA production between the traditional and updated models can be attributed to consideration of unspeciated organics (sum of direct unspeciated emissions and volatilized POA), which have higher yields on average compared with speciated compounds previously included in inventories. The updated model predicted that more than 80% of the SOA from combustion emissions was from unspeciated organics.
Fig. 4.
Predictions of POA emissions and SOA production from combustion emissions of the top six combustion sources in the United States using the traditional and updated models (Inset reproduces predictions for mobile sources on a different scale). The traditional model assumes a nonvolatile and nonreactive POA and simulates formation of SOA from speciated organics (SOAsp). The updated model assumes a semivolatile POA and simulates formation of SOAsp and SOA from unspeciated organics (SOAunsp). One set of error bars represent the 5th to 95th percentile confidence intervals on the model predictions for the total SOA (see SI Appendix for more details). The other set of error bars (±15%) represents the uncertainty in the gas-particle partitioning of the POA emissions (26–28).
The updated model predicted that biomass burning dominates both POA emissions and SOA formation from combustion sources at a national scale. Gasoline-powered mobile sources were the second largest source of SOA from combustion sources (and likely the dominant one during summertime in urban environments). Accounting for unspeciated organics more than doubled the predicted contribution of gasoline vehicles to ambient SOA. Both models predicted that gasoline contributes much more SOA than diesel (e.g., six times more in the updated model), which is qualitatively similar to Bahreini et al. (41) but in contrast to Gentner et al. (19). The updated model also showed (not included in Fig. 4) that evaporative, refueling, and other nontailpipe emissions contributed little POA (0.007 Tg y−1) and SOA (0.03 Tg y−1) and are, therefore, only minor sources of OA (19, 24).
Analysis of ambient data suggests that total SOA mass formed in the United States may be significantly larger than the box model predictions (42). The box model only considered first-generation SOA chemistry of combustion emissions; extrapolation to the continental scale requires full consideration of multigenerational aging (43) and aqueous processing (44), both of which will likely produce more SOA. Biogenic emissions are also a major source of SOA not considered by the box model. However, we do not expect that the precursors and processes not included in the box model will alter the relative importance of SOA formed from speciated and unspeciated combustion emissions. Therefore, future modeling studies will likely need to consider unspeciated NMOG emissions from combustion sources to accurately simulate ambient SOA.
Materials and Methods
Speciated-SOA Model.
Each organic species is lumped into a SAPRC07 model species and reacted with the OH radical to form a set of semivolatile surrogate products with mass yields defined using smog chamber data (4). The gas-particle partitioning of these products is treated using absorptive partitioning theory, assuming a quasi-ideal solution (45). The equations of the SOA model are provided in SI Appendix, and the mass yield data are provided in SI Appendix, Table S4.
US Budget Analysis.
Total NMOG and particulate matter (PM)2.5 emissions for the United States used in this analysis were derived from the 2008 Environmental Protection Agency NEI version 3 (23). NMOG and primary PM2.5 emissions were aggregated for source categories presented in Table 1. Details are provided in SI Appendix.
Supplementary Material
Acknowledgments
This research was funded by the US Environmental Protection Agency National Center for Environmental Research through the Science to Achieve Results program (Project RD834554) and by the Coordinating Research Council through projects A74/E96. The US Environmental Protection Agency through its Office of Research and Development collaborated in the research described here. This paper has been subjected to the Agency’s administrative review and approved for publication.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323740111/-/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change . Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC; 2007. Summary for policy makers. [Google Scholar]
- 2.Hallquist M, et al. The formation, properties and impact of secondary organic aerosol: Current and emerging issues. Atmos Chem Phys. 2009;9(14):5155–5236. [Google Scholar]
- 3.Zhang Q, et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys Res Lett. 2007;34(13):L13801. doi: 10.1029/2007GL029979. [DOI] [Google Scholar]
- 4.Carlton AG, et al. Model representation of secondary organic aerosol in CMAQv4.7. Environ Sci Technol. 2010;44(22):8553–8560. doi: 10.1021/es100636q. [DOI] [PubMed] [Google Scholar]
- 5.Volkamer R, et al. Secondary organic aerosol formation from anthropogenic air pollution: Rapid and higher than expected. Geophys Res Lett. 2006;33(17):L17811. doi: 10.1029/2006GL026899. [DOI] [Google Scholar]
- 6.Chirico R, et al. Impact of aftertreatment devices on primary emissions and secondary organic aerosol formation potential from in-use diesel vehicles: Results from smog chamber experiments. Atmos Chem Phys. 2010;10(23):11545–11563. [Google Scholar]
- 7.Hennigan CJ, et al. Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber. Atmos Chem Phys. 2011;11(15):7669–7686. [Google Scholar]
- 8.Platt S, et al. Secondary organic aerosol formation from gasoline vehicle emissions in a new mobile environmental reaction chamber. Atmos Chem Phys. 2013;13(18):9141–9158. [Google Scholar]
- 9.Nordin E, et al. Secondary organic aerosol formation from idling gasoline passenger vehicle emissions investigated in a smog chamber. Atmos Chem Phys. 2013;13(12):6101–6116. [Google Scholar]
- 10.Gordon TD, et al. Secondary organic aerosol formed from light duty gasoline vehicle exhaust dominates primary particulate matter emissions. Atmos Chem Phys. 2014;14(9):4661–4678. [Google Scholar]
- 11.Gordon TD, et al. Secondary organic aerosol production from diesel vehicle exhaust: Impact of aftertreatment, fuel chemistry and driving cycle. Atmos Chem Phys. 2014;14(9):4643–4659. [Google Scholar]
- 12.Robinson AL, et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science. 2007;315(5816):1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein AH, Galbally IE. Known and unknown organic constituents in the Earth’ s atmosphere. Environ Sci Technol. 2007;41(5):1514–1521. doi: 10.1021/es072476p. [DOI] [PubMed] [Google Scholar]
- 14.Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT. Sources of fine organic aerosol. 2. Noncatalyst and catalyst-equipped automobiles and heavy-duty diesel trucks. Environ Sci Technol. 1993;27(4):636–651. [Google Scholar]
- 15.Fraser MP, Cass GR, Simoneit BRT, Rasmussen R. Air quality model evaluation data for organics. 4. C2-C36 non-aromatic hydrocarbons. Environ Sci Technol. 1997;31(8):2356–2367. [Google Scholar]
- 16.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Environ Sci Technol. 1999;33(10):1578–1587. [Google Scholar]
- 17.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 5. C1-C32 organic compounds from gasoline-powered motor vehicles. Environ Sci Technol. 2002;36(6):1169–1180. doi: 10.1021/es0108077. [DOI] [PubMed] [Google Scholar]
- 18.Hildemann LM, Mazurek MA, Cass GR, Simoneit BR. Quantitative characterization of urban sources of organic aerosol by high-resolution gas chromatography. Environ Sci Technol. 1991;25(7):1311–1325. [Google Scholar]
- 19.Gentner DR, et al. Elucidating secondary organic aerosol from diesel and gasoline vehicles through detailed characterization of organic carbon emissions. Proc Natl Acad Sci USA. 2012;109(45):18318–18323. doi: 10.1073/pnas.1212272109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shrivastava MK, Lane TE, Donahue NM, Pandis SN, Robinson AL. Effects of gas particle partitioning and aging of primary emissions on urban and regional organic aerosol concentrations. J Geophys Res. 2008;113(D18):D18301. doi: 10.1029/2007JD009735. [DOI] [Google Scholar]
- 21.Dzepina K, et al. Evaluation of recently-proposed secondary organic aerosol models for a case study in Mexico City. Atmos Chem Phys. 2009;9(15):5681–5709. [Google Scholar]
- 22.Pye H, Seinfeld J. A global perspective on aerosol from low-volatility organic compounds. Atmos Chem Phys. 2010;10(9):4377–4401. [Google Scholar]
- 23.Environmental Protection Agency 2008 National Emissions Inventory Data and Documentation. 2012. (EPA). Available at www.epa.gov/ttnchie1/net/2008inventory.html. Accessed September 23, 2013.
- 24.Jathar SH, et al. Secondary organic aerosol formation from photo-oxidation of unburned fuel: Experimental results and implications for aerosol formation from combustion emissions. Environ Sci Technol. 2013;47(22):12886–12893. doi: 10.1021/es403445q. [DOI] [PubMed] [Google Scholar]
- 25.May AA, et al. Gas- and particle-phase primary emissions from in-use, on-road gasoline and diesel vehicles. Atmos Environ. 2014;88:247–260. [Google Scholar]
- 26.May AA, et al. Gas-particle partitioning of primary organic aerosol emissions: 3. Biomass burning. J Geophys Res. 2013;118(19):11327–11338. doi: 10.1002/jgrd.50828. [DOI] [Google Scholar]
- 27.May AA, et al. Gas-particle partitioning of primary organic aerosol emissions: (1) Gasoline vehicle exhaust. Atmos Environ. 2013;77:128–139. doi: 10.1021/es400782j. [DOI] [PubMed] [Google Scholar]
- 28.May AA, et al. Gas-particle partitioning of primary organic aerosol emissions: (2) Diesel vehicles. Environ Sci Technol. 2013;47(15):8288–8296. doi: 10.1021/es400782j. [DOI] [PubMed] [Google Scholar]
- 29.Siegl WO, Hammerle RH, Herrmann HM, Wenclawiak BW, Luers-Jongen B. Organic emissions profile for a light-duty diesel vehicle. Atmos Environ. 1999;33(5):797–805. [Google Scholar]
- 30.Presto AA, Miracolo MA, Donahue NM, Robinson AL. Secondary organic aerosol formation from high-NO(x) photo-oxidation of low volatility precursors: n-alkanes. Environ Sci Technol. 2010;44(6):2029–2034. doi: 10.1021/es903712r. [DOI] [PubMed] [Google Scholar]
- 31.Environmental Protection Agency . Exhaust Emission Profiles for EPA SPECIATE Database: Energy Policy Act (EPAct) Low-Level Ethanol Fuel Blends and Tier 2 Light-Duty Vehicles. 2009. (EPA) EPA-420-R-09-002. [Google Scholar]
- 32.Environmental Protection Agency 2013. SPECIATE Version 4.3 (EPA). Available at www.epa.gov/ttn/chief/software/speciate/. Accessed September 23, 2013.
- 33.Simon H, et al. The development and uses of EPA’s SPECIATE database. Atmos Pollut Res. 2010;1:196–206. [Google Scholar]
- 34.Kirchstetter TW, Singer BC, Harley RA, Kendall GR, Hesson JM. Impact of California reformulated gasoline on motor vehicle emissions. 2. Volatile organic compound speciation and reactivity. Environ Sci Technol. 1999;33(2):329–336. [Google Scholar]
- 35.Kirchstetter TW, Harley RA. Impact of Reformulated Fuels on Particle and Gas-Phase Emissions from Motor Vehicles. Berkeley, CA: Calif Air Resour Board and Univ of Calif; 2000. [Google Scholar]
- 36.Nakashima Y, Kamei N, Kobayashi S, Kajii Y. Total OH reactivity and VOC analyses for gasoline vehicular exhaust with a chassis dynamometer. Atmos Environ. 2010;44(4):468–475. [Google Scholar]
- 37.Yokelson RJ, et al. Coupling field and laboratory measurements to estimate the emission factors of identified and unidentified trace gases for prescribed fires. Atmos Chem Phys. 2013;13(1):89–116. [Google Scholar]
- 38.Lindhjem C, Yarwood G, Koo B, Fujita E, Turner J. 2009. Development of modeling inventory factors for mobile source particulate organic carbon and semi-volatile organic compound emissions (ENVIRON International Corporation, Novato, California). Available at www.ladco.org/reports/rpo/emissions/NREL_LADCO_FinalReport09.pdf. Accessed November 2013. [Google Scholar]
- 39.Schauer JJ, Kleeman MJ, Cass GR, Simoneit BRT. Measurement of emissions from air pollution sources. 3. C1-C29 organic compounds from fireplace combustion of wood. Environ Sci Technol. 2001;35(9):1716–1728. doi: 10.1021/es001331e. [DOI] [PubMed] [Google Scholar]
- 40.McDonald JD, et al. Fine particle and gaseous emission rates from residential wood combustion. Environ Sci Technol. 2000;34(11):2080–2091. [Google Scholar]
- 41.Bahreini R, et al. Gasoline emissions dominate over diesel in formation of secondary organic aerosol mass. Geophys Res Lett. 2012;39(6):L06805. doi: 10.1029/2011GL050718. [DOI] [Google Scholar]
- 42.De Gouw J, et al. Budget of organic carbon in a polluted atmosphere: Results from the New England Air Quality Study in 2002. J Geophys Res. 2005;110(D16):D16305. doi: 10.1029/2004JD005623. [DOI] [Google Scholar]
- 43.Cappa C, Wilson K. Multi-generation gas-phase oxidation, equilibrium partitioning, and the formation and evolution of secondary organic aerosol. Atmos Chem Phys. 2012;12(20):9505–9528. [Google Scholar]
- 44.Ervens B, Turpin B, Weber R. Secondary organic aerosol formation in cloud droplets and aqueous particles (aqSOA): A review of laboratory, field and model studies. Atmos Chem Phys. 2011;11(21):11069–11102. [Google Scholar]
- 45.Pankow JF. An absorption model of gas/particle partitioning of organic compounds in the atmosphere. Atmos Environ. 1994;28(2):185–188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.