Significance
Patients with compromised T-cell function are at risk for opportunistic fungal infections. We have developed a novel approach to restore immunity by using a fungal pattern-recognition receptor Dectin-1 to redirect T-cell specificity to carbohydrate antigen in the fungal cell wall. We did so by genetically modifying T cells using the nonviral Sleeping Beauty gene-transfer system to enforce expression of a chimeric antigen receptor (CAR) that recapitulates the specificity of Dectin-1 (D-CAR). The D-CAR+ T cells can be electroporated and propagated on artificial activating and propagating cells in a manner suitable for human application, enabling this immunology to be translated into immunotherapy. This approach has implications for genetically modifying T cells to express CARs with specificity for carbohydrate and thus broadening their application in the investigational treatment of pathogens and malignancies.
Keywords: T-cell therapy; β-1,3-glucan; fungus; adoptive immunotherapy
Abstract
Clinical-grade T cells are genetically modified ex vivo to express chimeric antigen receptors (CARs) to redirect their specificity to target tumor-associated antigens in vivo. We now have developed this molecular strategy to render cytotoxic T cells specific for fungi. We adapted the pattern-recognition receptor Dectin-1 to activate T cells via chimeric CD28 and CD3-ζ (designated “D-CAR”) upon binding with carbohydrate in the cell wall of Aspergillus germlings. T cells genetically modified with the Sleeping Beauty system to express D-CAR stably were propagated selectively on artificial activating and propagating cells using an approach similar to that approved by the Food and Drug Administration for manufacturing CD19-specific CAR+ T cells for clinical trials. The D-CAR+ T cells exhibited specificity for β-glucan which led to damage and inhibition of hyphal growth of Aspergillus in vitro and in vivo. Treatment of D-CAR+ T cells with steroids did not compromise antifungal activity significantly. These data support the targeting of carbohydrate antigens by CAR+ T cells and provide a clinically appealing strategy to enhance immunity for opportunistic fungal infections using T-cell gene therapy.
Opportunistic invasive fungal infections (IFI) by Aspergillus spp. cause morbidity and mortality in immunocompromised patients. Mortality rates associated with invasive Aspergillus (IA) are 22% in patients receiving solid-organ transplants and 60–85% in patients receiving hematopoietic stem cell transplants (HSCT) (1, 2). Antifungal agents such as polyenes, triazoles, and echinocandins can be rendered ineffective by suboptimal host immunity, emergence of drug-resistant strains, and attendant toxicities in the recipients (3, 4). Thus, new approaches for treating IAs are needed. Because the adoptive transfer of genetically modified T cells expressing CD19-specific chimeric antigen receptors (CARs) has resulted in successful treatment of patients with B-cell malignancies (5–9), we sought to determine if a CAR could be developed to redirect T-cell specificity to Aspergillus.
Among immunocompetent individuals, intact innate immunity can prevent and eradicate IFI. Endogenous alveolar macrophages recognize, phagocytize, and kill fungal spores (10), and neutrophils can recognize and eliminate germinating hyphae (11). Immunotherapeutic strategies such as adoptive transfer of preselected CD4+ T-cell clones can help control Aspergillus infection indirectly by producing interferon-gamma (IFN-γ) (12). An immunotherapeutic strategy deploying cytotoxic T cells appears to be clinically advantageous, because such cells have an endogenous ability to kill, be propagated to large numbers ex vivo, be genetically modified to recognize desired target antigens, and contribute to immunologic memory (8).
We previously have redirected the specificity of genetically modified T cells via the stable introduction of a CD19-specific second-generation CAR (8, 9). Recognition of CD19 was achieved by a CAR exodomain composed of a CD19-specific single-chain variable fragment attached to the T-cell surface via a modified IgG4 hinge and fragment-crystallized (Fc) region (13). CAR-dependent and MHC-independent T-cell activation can be achieved through the CAR endodomain composed of CD3-ζ and CD28 (14, 15). We modified this prototypical CAR design to achieve recognition of carbohydrate by accommodating the pattern-recognition properties of Dectin-1 (16, 17). This dectin is a type II transmembrane protein expressed on macrophages, neutrophils, and dendritic cells (18) and is specific for β-glucans, which are glucose polymers consisting of β-1,3-glucan and β-1,6-glucan expressed on the cell wall of fungi (19). Because Dectin-1 mediates recognition of Aspergillus fumigatus (20), we hypothesized that the extracellular portion of Dectin-1 could be adapted as the specificity domain for a CAR (designated “D-CAR”) on T cells to redirect their specificity for this fungus.
We report that T cells can be genetically modified by using the Sleeping Beauty (SB) transposon/transposase system to express D-CAR stably and can be propagated selectively on artificial activating and propagating cells (aAPCs). We demonstrate that the D-CAR+ T cells (i) bind specifically to laminarin (which is rich in β-1,3-glucan), (ii) exhibit a central memory phenotype, (iii) inhibit growth of Aspergillus hyphae even in the presence of dexamethasone, and (iv) target Aspergillus infection in the skin and lung of immunocompromised mice. Thus, using a gene transfer and propagation approach adapted for human application (21, 22), we have demonstrated that an innate immune response to fungi can be harnessed by bioengineering cytotoxic T cells. This demonstration provides the foundation for testing whether D-CAR+ T cells can be infused to improve the survival of immunocompromised patients who develop opportunistic IA infection.
Results
Generating D-CAR+ Human T Cells.
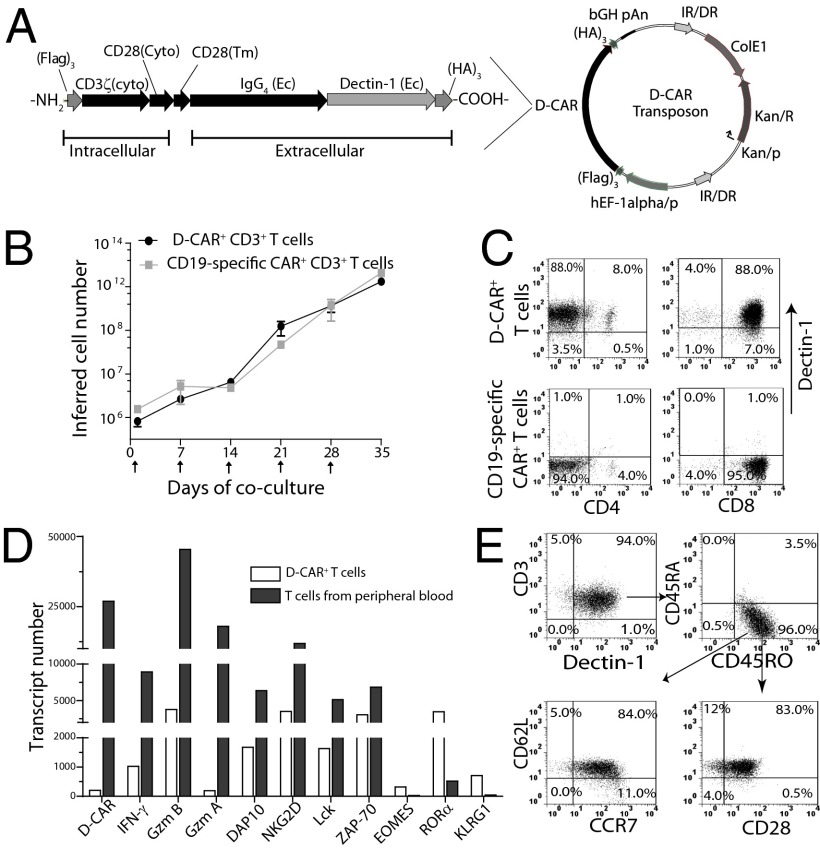
As expected, neither circulating nor propagated human αβ T cells express Dectin-1 or Dectin-2 and thus do not directly recognize Aspergillus using these pattern-recognition receptors. To generate a CAR with the specificity of Dectin-1 capable of activating T cells from peripheral blood, we fused the extracellular domain of human Dectin-1 (23) to our standard CAR cassette (15) encoding a modified IgG4 hinge/Fc (13), human CD28 transmembrane and cytoplasmic domains, and the CD3-ζ signaling motif. The resulting D-CAR construct was subcloned as an SB transposon (Fig. 1A) and electro-transferred with SB11 transposase into peripheral blood-derived human primary T cells (Fig. S1). These data demonstrate that a type II transmembrane receptor can be fashioned into a CAR (containing HA and FLAG epitope tags to validate assembly) and stably expressed on T cells following transposition (Fig. S1B). The propagation of D-CAR+ T cells was not significantly different from CD19-specific CAR+ T cells cocultured with aAPC (clone #4) (24) that activates/propagates T cells via transgenic human CD19. The addition of γ-irradiated aAPC (Fig. S2) every 7 d was calculated to support a 6-log numeric expansion (from 106 to 1012) for both D-CAR+ and CD19-specific CAR+ T cells (Fig. 1B). There was no outgrowth of CD3− cells [e.g., natural killer (NK) cells]. Thirty-five days after electroporation, the propagated T cells were stained with mAb specific for Dectin-1 and analyzed by flow cytometry. Almost all (average 97.6% ± 1.6; n = 3) of the T cells coexpressed CD3 and D-CAR; 90–95% of the propagated D-CAR+ T cells coexpressed CD8 (Fig. 1C); and, as anticipated, there was no expression of D-CAR on CD19-specific CAR+CD8+ or CAR+CD4+ T cells. As such, the CD19-specific CAR+ T cells serve as a negative control (hereafter termed “control T cells”). These data demonstrate that D-CAR+ T cells can be expanded numerically on aAPCs using an approach similar to that used for the manufacture of clinical-grade CD19-specific T cells in our ongoing clinical trials.
Fig. 1.
Bioengineering of D-CAR+ T cells. (A) Components of D-CAR. The extracellular (Ec) sugar-binding domain of human Dectin-1 was fused in frame to a modified human IgG4 hinge and Fc region and the transmembrane (Tm) and cytoplasmic (Cyto) domain of human CD28, and Cyto CD3-ζ. HA3 and FLAG3 epitope tags were fused on the C and N termini, respectively. (B) Numeric expansion of T cells cocultured with aAPC clone #4 preloaded with Dectin-1–specific mAb in the presence of soluble IL-2 and IL-21. Data are plotted as mean ± SD from three different donors. Upward arrows indicate the addition of γ-irradiated aAPC. (C) Expression of D-CAR on CD4+ and CD8+ T cells after electroporation and propagation for 35 d. CD19-specific CAR+ T cells from the same donor were used as a gating control. (D) Comparative gene-expression profiles of the expanded D-CAR+ T cells and circulating T cells from healthy donors. Abundance of mRNA transcripts was measured by digital gene expression using the nCounter analysis system. (E) D-CAR+ T cells propagated for 35 d were costained with anti-CD3 and anti–Dectin-1 mAbs, in addition to staining with anti-CD45RA, CD45RO, CD28, CD62L, and CCR7, and were analyzed by flow cytometry. D-CAR+CD3+CD45RA−RO+ T cells were gated for expression of CD62L, CD28, and CCR7 to define central memory T cells.
Genetic Signature and Immunophenotype of D-CAR+ T Cells.
We used nCounter analysis of direct digital readouts to quantify mRNA abundance (25), coding for IFN-γ (a Th1 proinflammatory marker), granzymes A/B (markers of cytotoxicity), DNAX-activating protein (DAP-10) for PI 3-kinase signaling, NK-cell--activating receptor (NKG2D) for costimulation, and zeta-chain–associated protein kinase 70 (ZAP70) and lymphocyte-specific protein tyrosine kinase (Lck) for T-cell activation. Compared with unmodified T cells in peripheral blood, the D-CAR+ T cells exhibited an increase in levels of IFN-γ (eightfold), granzyme A (94-fold), granzyme B (12-fold), DAP10 (threefold), NKG2D (threefold), Lck (threefold), and ZAP-70 (twofold) (Fig. 1D). In addition, there was a 15-fold decrease in killer cell lectin-like receptor subfamily G, member 1 (KLRG1), which is consistent with the preservation of T cells that avoid replication senescence (26, 27), and a six- to sevenfold decrease in the nuclear receptor retinoic acid receptor–related orphan receptor α (RORα), which contributes to the development of CD4+ Th17 cells (28). The therapeutic activity of CD19-specific CAR+ T cells in vivo has been shown to correlate positively with T-cell persistence and is associated with a central memory (TCM) phenotype (29). Gating on the CD3+ D-CAR+ subset showed that 96% of the cells coexpress CD45RO, as is consistent with an outgrowth of memory T cells. Further analysis of the CD45RO+ T cells revealed that 84% exhibited a TCM immunophenotype on the basis of coexpression of CD28, CD62L, and CCR7 (Fig. 1E). These data suggest that D-CAR+ T cells numerically expanded on aAPCs may sustain persistence after adoptive transfer with the potential to provide long-term protection from IA. Moreover, using our direct T-cell receptor (TCR) expression analysis (DTEA) method, we found no significant difference in the abundance and diversity of TCR Vα and Vβ family members expressed by unmodified and genetically modified D-CAR+ T cells (Fig. S3). These data indicate that D-CAR+ T cells emerge from and maintain a polyclonal population.
Redirected Specificity by D-CAR+ T Cells.
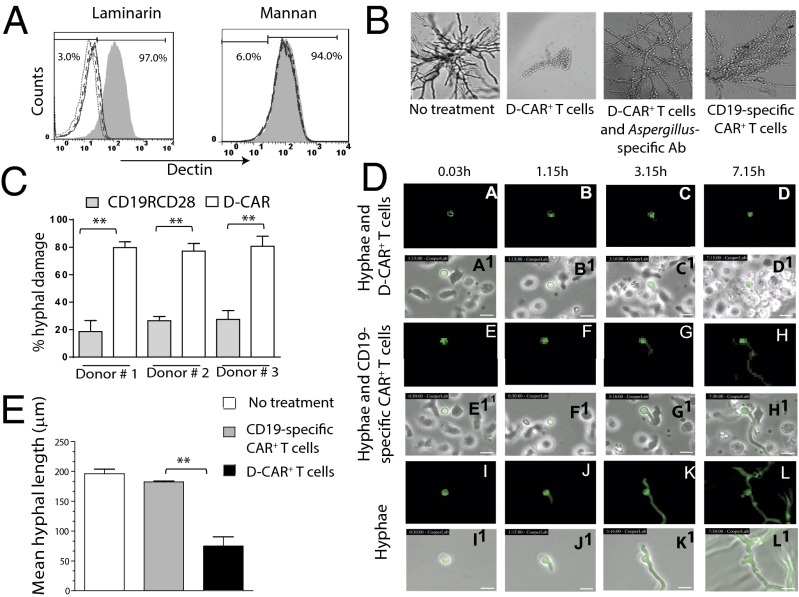
Dectin-1 binds to the glucose polymer laminarin that is similar to the sugar moiety found in fungal walls but does not bind to mannan, the mannose polymer derived from Saccharomyces cerevisiae (30). D-CAR+ T cells exhibited the same binding preference, because laminarin, but not mannan, abrogated binding of mAb specific for Dectin-1 to D-CAR on genetically modified T cells in a dose-dependent manner (Fig. 2A). The specificity was evaluated further by coculturing genetically modified T cells with germinating Aspergillus spores using three in vitro assays. We used microscopy to demonstrate that fungal growth was inhibited significantly by D-CAR+ T cells as compared with CD19-specific CAR+ control T cells (Fig. 2B). Preincubation of a rabbit polyclonal antibody raised against the soluble extract of A. fumigatus did prevent the ability of D-CAR+ T cells to damage fungal growth, suggesting that the redirected specificity is achieved by a specific interaction with the fungal cell wall. Next, we investigated the capacity of genetically modified T cells to kill germinating conidia using a colorimetric assay to reveal the viability of Aspergillus based on the ability to reduce a tetrazolium dye (31). Coculture of D-CAR+ T cells led to an ∼75–80% loss of viability in germinating spores compared with a 15–30% loss of viability when cocultured with CD19-specific CAR+ T cells (P < 0.01; Fig. 2C). In the third in vitro assay, we undertook video time-lapsed microscopy (VTLM) to visualize serially the targeting of germinating Aspergillus by D-CAR+ T cells (Movie S1) and CD19-specific CAR+ T cells (Movie S2) compared with untreated controls (Movie S3). D-CAR+ T cells and background low levels of CD19-specific T cells bound to germlings (Fig. 2 D, c and g) by 3 h. However, by 7 h there was a rapid influx of D-CAR+ T cells (Fig. 2 D, d) but not of control T cells (Fig. 2 D, h). The consequence of this binding of D-CAR+ T cells was destruction of hyphal growth (Fig. 2 D, d) compared with the control T cells (Fig. 2 D, h) and in the absence of T cells (Fig. 2 D, k and l). The mean hyphal length at 24 h was calculated from VTLM to quantify the ability of T cells to destroy hyphae. Compared with control T cells, D-CAR+ T cells reduced hyphal length from 180 μm to 70 μm (62% inhibition). In the absence of T cells, hyphae grew on average to 200 μm over same time period (Fig. 2E). These imaging data support the ability of D-CAR+ T cells to target the germinating hyphae specifically and as desired, and not the dormant Aspergillus conidia.
Fig. 2.
Specificity and damage to Aspergillus hyphae by D-CAR+ T cells. (A) Binding of D-CAR+ T cells by Dectin-1–specific mAb in the absence or presence (200 μg/mL, solid line; 400 μg/mL, dashed line; and 600 μg/mL, dotted line) laminarin or mannan, as evaluated by flow cytometry. Shaded and open regions in the histograms represent mAb binding in the absence or presence of sugar, respectively. (B) Preincubation with Aspergillus-specific polyclonal antibody blocked binding of D-CAR+ T cells to hyphae. Fungal growth was inhibited more by D-CAR+ T cells than by CD19-specific CAR+ T cells. (C) Comparison by XTT assay of the ability of D-CAR+ and CD19-specific CAR+ T cells to damage germinating A. fumigatus hyphae specifically; **P < 0.01. (D) D-CAR+ effector (E) T cells were coincubated with targets (T) GFP+AF293 swollen conidia (E:T ratio of 2:1) and imaged continuously by VTLM. Conidia germlings cocultured with CD19-specific CAR+ T cells and without T cells served as negative control. (See Movies S1–S3.) Pictures of conidia taken at various time points under fluorescent light (a–l) and with white light (a1–l1) show inhibition of growth by germinating conidia (green fluorescence) by D-CAR+ T cells (a–d), CD19-specific CAR+ T cells (e–h), and in the absence of T cells (i–l). (Scale bars: 10 μm.) (E) At 24 h, the ability of T cells to inhibit Aspergillus germination as measured by hyphae length obtained from VTLM. Data are shown as mean ± SD. **P < 0.01; n = 3.
Effect of Dexamethasone on D-CAR+ T Cells.
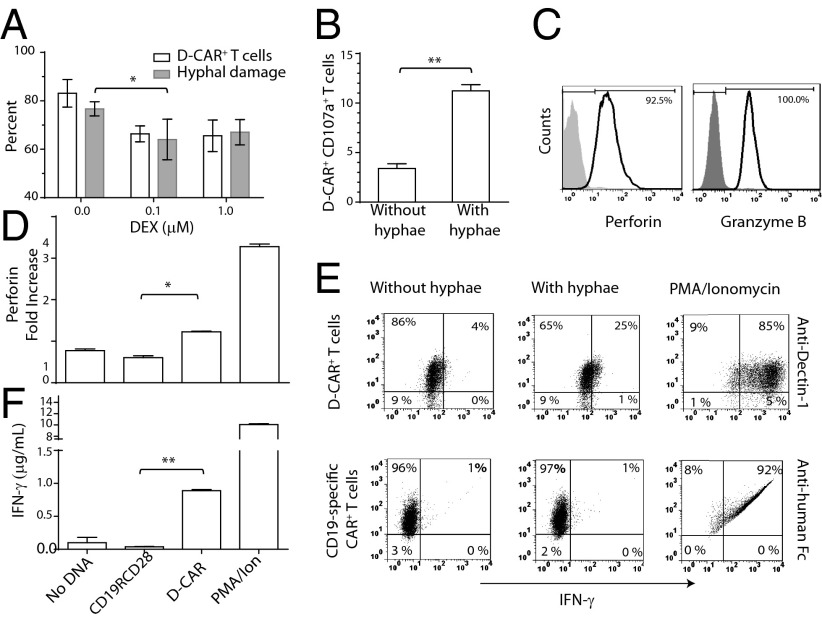
A likely translational application for infusing D-CAR+ T cells will be in recipients of solid-organ transplants or allogeneic HSCT. Dexamethasone (DEX), a corticosteroid systemically administered for immunosuppression, has been demonstrated to down-regulate endogenous Dectin-1 expression (32) and predisposes patients to fungal infections (33). We evaluated the effect of DEX on the expression of D-CAR on T cells and found that levels of the introduced immunoreceptor diminished partially in a dose-dependent manner, so that 64% (n = 2) of genetically modified T cells continued to express D-CAR in 1 μM of DEX. Despite this down-regulation of the introduced immunoreceptor, these T cells retained their ability to damage hyphae (Fig. 3A).
Fig. 3.
D-CAR+ T cells are activated by germinating Aspergillus. (A) D-CAR+ T cells cultured for 1 wk on aAPC preloaded with Dectin-1–specific mAb in the presence of DEX and soluble IL-2 and IL-21 were analyzed for expression of D-CAR by flow cytometry (open bars; D-CAR+ T cells cultured in the absence of DEX served as a control) and for their ability to kill A. fumigatus hyphae by XTT assay (gray bars; A. fumigatus cultured without T cells served as control). Data are shown as mean ± SD; n = 2. (B) CD107a expression on D-CAR+ T cells in the absence or presence of A. fumigatus hyphae, analyzed by flow cytometry. Data are shown as mean ± SD; n = 3. (C) Expression of perforin and granzyme B analyzed by flow cytometry; shaded regions are isotype controls. (D) Perforin secretion in conditioned T-cell culture supernatants after stimulation by aAPCs loaded with Dectin-1–specific mAb. Fold changes in perforin level were calculated by normalizing against unstimulated T cells. (E) D-CAR+ T cells and CD19-specific CAR+ T cells (expressing CD19RCD28) were incubated with germinating A. fumigatus hyphae for 4–6 h. The percentages of D-CAR+IFN-γ+ T cells and CD19-specific CAR+IFN-γ+ T cells were measured by flow cytometry. T cells stimulated with phorbol12-myristate13-acetate/ionomycin served as positive controls. Shown are representative dot plots from two independent experiments. (F) IFN-γ cytokine levels were measured in T-cell culture conditioned supernatants after stimulation with 50 µg/mL Aspergillus cell lysate.*P < 0.05; **P < 0.01.
Activation of D-CAR+ T Cells by Aspergillus Germlings.
To corroborate the hyphal killing, we evaluated up-regulation of CD107a (LAMP1) that is expressed on the T-cell surface following cytotoxic degranulation (34). We observed a threefold up-regulation of CD107a on D-CAR+ T cells after coculture with Aspergillus germlings (Fig. 3B). Damage to hyphae likely was mediated by perforin and granzymes, because D-CAR+ T cells expressed perforin (92%) and granzyme B (100%) (Fig. 3C). Furthermore, perforin secretion was increased (P < 0.05) in conditioned supernatant after stimulation with aAPCs (clone #4) preloaded with Dectin-1–specific mAb (Fig. 3D). Results obtained from our customized bar-coded probe set used to measure abundance of mRNA levels suggested that D-CAR+ T cells produced higher IFN-γ levels than T cells from peripheral blood (Fig. 1D). Therefore, IFN-γ expression was measured in D-CAR+ T cells exposed to germinating Aspergillus, and we also observed a six- to sevenfold increase in IFN-γ protein levels (Fig. 3E). In contrast, no change in IFN-γ levels was observed in control T cells cocultured with germinating Aspergillus. We also validated the presence of soluble IFN-γ in conditioned supernatants of D-CAR+ T cells (P < 0.01) cultured with fungal hyphae extract (Fig. 3F). We detected no secreted IL-17, as is consistent with decreased levels of mRNA coding for RORα (28). In aggregate, these data demonstrate that electroporated and propagated D-CAR+ T cells are cytotoxic in response to hyphae from Aspergillus and can secrete proinflammatory cytokines.
Targeting Aspergillus Infection in Mice by D-CAR+ T Cells.
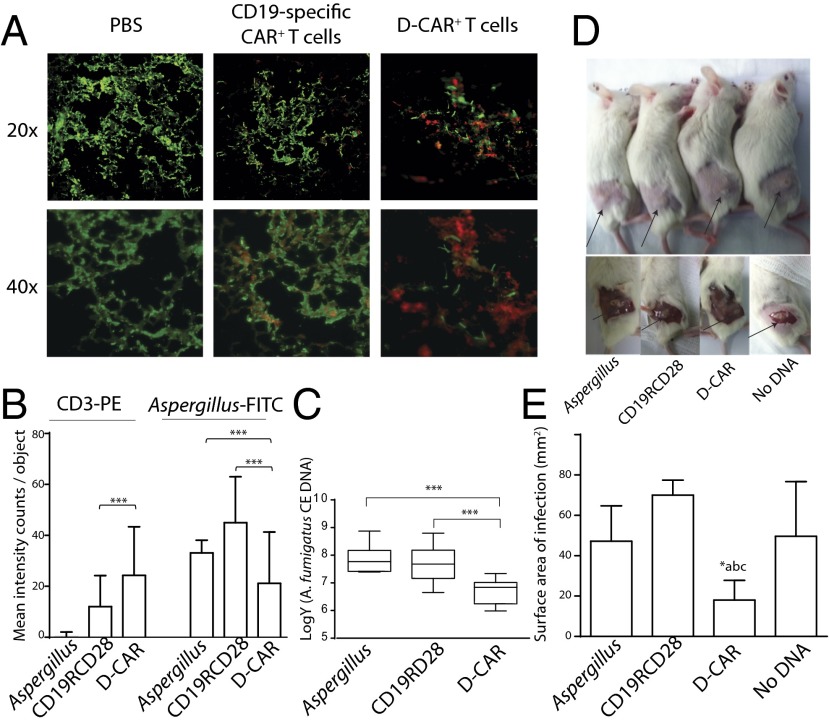
To assess the in vivo therapeutic efficacy of the D-CAR+ T cells, we treated immunosuppressed NOD SCID-γ (NSG) mice bearing IA with D-CAR+ T cells. Mice that received no T cells (PBS control) or CD19-specific T cells demonstrated Aspergillus infection as shown by staining of lung tissue sections with FITC-conjugated Aspergillus-specific antibody. In contrast, treatment with D-CAR+ T cells resulted in diminished fungal infection and retention of D-CAR+ T cells (Fig. 4A). Indeed, infused T cells identified by phycoerythrin (PE)-conjugated CD3-specific mAb revealed that fungal hyphae were preferentially covered with D-CAR+ T cells rather than CD19-specific CAR+ T cells. An antifungal effect mediated by D-CAR+ T cells was supported by the observation that the number of visualized genetically modified T cells, as assessed by red fluorescence, was greater in the mice that received D-CAR+ T cells than in the two controls (P < 0.001; Fig. 4B). In aggregate, quantification of fluorescence showed that mice receiving D-CAR+ T cells had a significantly lower pulmonary Aspergillus load than mice treated with control CAR+ T cells or no T cells (P < 0.001; Fig. 4B). This result was supported by measurements of fungal burden in lungs by quantitative PCR (qPCR), which revealed that the introduced germlings underwent greater damage in the mice receiving D-CAR+ T cells than in controls (P < 0.001; Fig. 4C). We extended our observation to another clinically relevant model of IA. Previously, it has been shown that skin lesions and fungal burden are directly proportional, and thus the infected surface area is an objective measure of the degree of fungal infection (35). We observed that surface area of cutaneous fungal lesions (Fig. 4D) was smaller in mice that received D-CAR+ T cells than in control mice that received CD19-specific CAR+ T cells or no T cells (P < 0.01; Fig. 4E). These in vivo data support our in vitro observations that D-CAR+ T cells can target IA.
Fig. 4.
Targeting Aspergillus infection in mice by D-CAR+ T cells: (A) Pulmonary Aspergillus infection in NSG mice. Fluorescent microscopic pictures of lung sections of Aspergillus (AF293) in mice that received PBS, CD19-specific CAR+ T cells, and D-CAR+ T cells. (B) Mean fluorescence intensity for staining with anti-human CD3-PE and anti-Aspergillus-FITC was calculated using Inform imaging software. Values are shown as mean fluorescent intensity (counts per image objects) from three lung slides per group. ***P < 0.001. (C) Quantification of A. fumigatus burden in lungs of mice 4 d after infection, as determined by qPCR. Data are expressed as the number of conidial equivalent (CE) copies of DNA in aliquots of lung homogenates. Data are shown as mean ± SD; ***P < 0.001 compared with control mice receiving PBS (labeled “Aspergillus”) or CD19-specific CAR+ T cells; n = 5 mice. (D) Cutaneous Aspergillus infection in NSG mice. Mice received no therapy, CD19-specific (CD19RCD28) CAR+ T cells, D-CAR+ T cells, and T cells expressing no CAR (No DNA). Site of fungal lesions are indicated by arrows. After 96 h, mice were anesthetized, and skin lesions were measured. (E) Cutaneous fungal burden of mice treated with D-CAR+ T cells compared with Aspergillus without treatment, CD19-specific CAR+ T-cells, or (c) no DNA electroporated T-cells. *P < 0.05, treatment with D-CAR+ cells vs. other three conditions; n = 5.
Bispecific T Cells Coexpressing D-CAR and CD19-Specific CAR.
Patients at risk for progression of B-cell malignancies, such as patients with advanced disease undergoing HSCT, are also vulnerable for IA. Therefore, we investigated if the SB system could be adopted to coexpress CD19-specific CAR and D-CAR in T cells. T cells that underwent double transposition were propagated on aAPC clone #4 (36) and were demonstrated by flow cytometry to coexpress the two CARs (Fig. S4A). To establish the ability of D-CAR to activate T cells expressing two CAR species, we again demonstrated that hyphae growth could be targeted on Aspergillus germlings (Fig. S4B) with damage proportional to the ratio of T cells to fungal burden (Fig. S4C). Specificity for CD19 was validated by chromium release assay for CD19+ vs. CD19− tumor cells (Fig. S4D). These data demonstrate that T cells can be engineered to have specificity for both carbohydrate and protein antigens.
Discussion
We demonstrate a previously unidentified approach for immunotherapy of Aspergillus based on redirecting T-cell specificity through a CAR that recognizes carbohydrate antigen: D-CAR with specificity of Dectin-1 fused to CD28 and CD3-ζ cytoplasmic signaling domain that delivers a fully competent T-cell–activation signal as defined by killing, cytokine production, and proliferation. We genetically modified primary circulating T cells using our SB transposon/transposase system that we adapted for human gene transfer. Indeed, we have achieved institutional and federal regulatory approvals for four clinical trials (NCT00968760, NCT01497184, NCT01362452, NCT01653717) infusing autologous and allogeneic T cells genetically modified with an SB encoding a CD19-specific CAR and propagation on aAPC (clone #4) (21, 22, 37). To generate clinically relevant numbers of D-CAR+ T cells for adoptive transfer, T cells were expanded numerically on “designer” aAPC. These genetically modified T cells coexpress perforin and granzyme and exhibit an ability to recognize and lyse Aspergillus germlings. The therapeutic potential of these T cells is highlighted further by the majority of D-CAR+ T cells (84%) exhibiting a TCM phenotype that likely can self-renew as well as differentiate into effector T cells in vivo (38). nCounter Digital profiling was used to examine the abundance of mRNA species; both the transcription factors Eomesodermin and KLRG1 were down-regulated in propagated D-CAR+ T cells, supporting the observation that infused D-CAR+ T cells can sustain proliferation without terminal differentiation and avoid replicative senescence (39, 40).
Because this is the first time, to our knowledge, that a pattern-recognition receptor has been adapted to redirect T-cell specificity, we used multiple experiments (fungal cell-killing assay, cytokine production, up-regulation of CD107a, VTLM, and two mouse models) to assess the ability of D-CAR+ T cells to target germinating Aspergillus. Based on the results obtained from in vitro and in vivo studies, D-CAR+ T cells can directly target and control Aspergillus infection. These assays also demonstrated that D-CAR is able to activate proinflammatory and cytolytic machinery such as the perforin/granzyme pathway of genetically modified T cells. The exocytosis of cytolytic granules apparently can disrupt fungal germination directly, as evident from the reports that granzyme-deficient mice are susceptible to IFI (41, 42). The production of IFN-γ from the D-CAR+ T cells may further augment immunity to IFIs, because pharmacological dosing of recombinant IFN-γ (43) or of IFN-γ derived from CD4+ helper T cells or NK cells has been shown to augment anti-Aspergillus activity (44, 45). However, it remains to be determined whether the D-CAR–dependent production of IFN-γ can contribute directly to the clearance of fungal infections or indirectly through the activation of granulocytes. Other cytokines, such as IL-17, produced by subsets of T cells also may participate in antifungal immunity by activating neutrophils (46). However, we did not observe IL-17 secretion by D-CAR+ T cells, as is is consistent with decreased expression of RORα, which plays a critical role in augmenting IL-17 production from T cells (28, 47).
Targeting by D-CAR+ T cells may be augmented by combination therapies. For example, Aspergillus preexposed to caspofungin can unmask β-glucan residues in the cell wall of fungi and enhance antifungal activity mediated by neutrophils (31). Engineering a T-cell response to Aspergillus hyphae also may overcome congenital impairment of the innate immune system, as with the Dectin-1 Y238X polymorphism (48). This mutation is associated with diminished Dectin-1 receptor activity, increased susceptibility to IFI among recipients of HSCT (48), and loss of TLR4-mediated signals during hyphal germination, thereby contributing to the evasion of immune recognition by Aspergillus (49).
To help translate our findings to clinical practice, D-CAR+ T cells must maintain effector function in the presence of systemically administered immunosuppressive medications. The corticosteroid DEX can down-regulate expression of Dectin-1 on granulocytes and thereby increase recipients’ susceptibility to IFI. Although we did observe a decrease in D-CAR expression in electroporated/propagated T cells exposed to DEX, the level of D-CAR expression still was sufficient to damage germinating Aspergillus hyphae. This result indicates that adoptive transfer of D-CAR+ T cells may remain effective in patients at risk for IFI because of the systemic administration of DEX. In addition, we generated bispecific T cells using the SB system that coexpress CD19-specific CAR (CD19RCD28) and D-CAR. These T cells exhibited specificity for both CD19 and Aspergillus, raising the possibility that one population of T cells can be customized to target relapse and infection, which are two common causes of morbidity and mortality after allogeneic HSCT.
In summary, we report a bioengineering approach to redirect the specificity of T cells by the expression of a novel CAR that incorporates the pattern-recognition ability of Dectin-1 to recognize Aspergillus germlings. The D-CAR design enabled us to adapt an immunoreceptor that operates within the landscape of innate immunity for expression in T cells to co-opt their endogenous cytolytic machinery to target Aspergillus hyphae. This approach is clinically appealing, because long-lived T cells can be genetically modified (e.g., using the SB system) and propagated to large numbers (e.g., using γ-irradiated aAPC) ex vivo in compliance with cGMP for Phase I/II trials. One proposed clinical application for the administration of donor-derived D-CAR+ T cells is administration following allogeneic HSCT, because the introduced D-CAR is capable of recognizing β-glucan moieties present on opportunistic fungal infections. Furthermore, the recognition of carbohydrate antigens by CAR broadens the translational appeal of genetically modified T cells to target pathogens as well as tumor-associated carbohydrate antigens.
Materials and Methods
Plasmids.
D-CAR design was based on our second-generation CD19-specific CAR, CD19RCD28 (15). In brief, the sequence encoding the extracellular (Ec) sugar-binding domain of human Dectin-1 (GenBank accession no. AY026769) was fused to the modified human IgG4 hinge and Fc regions (13), which in turn were combined with the transmembrane and cytoplasmic residues of human CD28 molecule and then the human cytoplasmic CD3-ζ chain. The human codon optimized (CoOp) D-CAR (CoOp D-CAR), synthesized by GeneArt, was fused at the (Ec) C terminus to a triplicate HA epitope (amino acid sequence YPYDVPDYA) and to the FLAG3 epitope (amino acid sequence DYKDDDDC) on the N terminus by PCR to obtain FLAG(CoOp)–D-CAR–HA3 (Fig. 1A) with flanking 5′ SpeI and 3′ NotI restriction enzyme sites. After sequence validation, the FLAG3(CoOp)–D-CAR–HA3 fragment was subcloned into the SB transposon DNA plasmid CoOpCD19RCD28/pSBSO (used to express CD19RCD28 for human application), by restriction digestion at SpeI-NruI and NheI-SmaI sites, thus replacing the CD19RCD28 CAR fragment with the FLAG3(CoOp)–D-CAR–HA3 fragment to create the final plasmid CoOpD D-CAR/pSBSO (designated “pSBD-CAR”). The DNA plasmid pCMV-SB11 expresses the SB11 transposase (21).
Cells.
Primary human T cells were isolated by density gradient centrifugation over Ficoll-Paque-Plus (GE Healthcare Bio-Sciences AB) from peripheral blood obtained from the Gulf Coast Regional Blood Center (Houston, TX) after informed consent. All primary cells and cell lines were cultured in RPMI medium 1640 (HyClone Laboratories) supplemented with 2 mM Glutamax-1 (catalog no. 35050-061; Life Technologies) and 10% heat-inactivated FBS (HyClone). D-CAR+ T cells were generated as previously described for CD19-specific CAR+ T cells (37). In brief, T cells were electroporated with DNA plasmids from the SB system coding for D-CAR and SB11 and were propagated on aAPCs (clone #4) preloaded with Dectin-1–specific mAb in the presence of IL-2 and IL-21. Bispecific CD19-specific CAR+ T cells coexpressing D-CAR were generated using double transposition whereby the SB system was used to coexpress two CARs. Mock-electroporated (“no DNA”) CAR− T cells were propagated on aAPCs (clone #4) preloaded with CD3-specific mAb OKT3 (catalog no. 16-0037-85; eBioscience) in the presence of IL-2 and IL-21 (50). All cell lines used in this study were authenticated by finger printing at the MD Anderson Cancer Center sequencing core facility. Additional details are provided in SI Materials and Methods.
Fungal Isolates, Growth, and Germination.
Two A. fumigatus isolates were used: a reference wild-type strain, A. fumigatus 293 (AF293), and a strain of A. fumigatus expressing GFP (GFP-AF293, GFP+AF), a kind gift from Kieren Marr (The Johns Hopkins University School of Medicine, Baltimore, MD) (51). Both isolates were grown on potato dextrose agar for 7 d at 37 °C before conidia were harvested by flooding the plates with PBS containing 0.1% Tween 20 (35). For killing assays, conidia were counted using a hemocytometer and resuspended at a concentration of 105 spores/mL in RPMI containing 10% FBS. One milliliter containing 105 spores in Eppendorf tubes was shaken (220 rpm, digital incubator shaker, Innova 4300, New Brunswick Scientific, Inc.) at 37 °C for 12–16 h for germination. Similarly, for VTLM, harvested GFP+AF conidia were incubated and shaken for 4–5 h before analysis.
XTT Assay.
The targeting of Aspergillus was assessed using a 2,3-bis (2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino) carbonyl]-sH-tetrazolium hydroxide (XTT)-based colorimetric assay (31). Germinating conidia were incubated for 2–3 h with 106 T cells (effector:target ratio, 10:1) in RPMI 1640 with 10% FBS. CD19-specific CAR+ T cells were used as a negative control. After incubation, T cells were lysed with cold, sterile water (hypotonic lysis). Damage to germlings was calculated following the manufacturer’s protocol (XTT assay, catalog no. X4251; Sigma).
Animal Studies.
All animal experiments were approved by the University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee. Eight-week-old female NSG mice (Jackson Laboratory) weighing 18–20 g were used and housed in a sterile facility. Cyclophosphamide (100 or 150 mg/kg of body weight) was injected i.p. to achieve immunosuppression 4 d and 1 d before inoculation with Aspergillus (AF293) spores (defined as day 0). Pulmonary fungal infection was induced by infecting with AF293 conidia (1.5 × 106 per mouse) via the sino-pulmonary route (52). The mice were grouped (n = 5) into four groups. The first group received no T cells; the second group received CD19-specific CAR+ T cells; the third group received D-CAR+ T cells; and the fourth group received T cells that did not express CAR (“no DNA”). T cells were dosed at 2 × 107 by i.v. injection every day for 3 days beginning on day 0. All surviving mice were killed on day 4 to determine fungal burden. The same experimental design was used to establish a cutaneous fungal infection, except that T cells were administered s.c. into the same site at a 1:10 ratio of AF293 conidia (1.5 × 106) per mouse) to T cells (15 × 106 per mouse). Skin lesions were measured daily using digital calipers (Fisher Scientific), and the longest and shortest diameters were recorded (35).
Statistics.
Graphs were plotted using Prism v. 4.0 software (Graph Pad Software). Statistical analysis was carried out using SAS 9.3 (SAS Inc.). A two-tailed P value of <0.05 was considered statistically significant. Skin lesion areas and fungal burden were compared among groups of mice using an unpaired Student t test.
Supplementary Material
Acknowledgments
We thank the flow cytometry and cellular imaging core facility at the MD Anderson Cancer Center (MDACC) [supported by MDACC Cancer Center Support Grant (CCSG) CA016672]; Dr. Perry Hackett (University of Minnesota) for assistance with the SB system; Dr. Kieren Marr (The John Hopkins University) for helping develop the concept; Dr. Judy Moyes (MDACC) for editing; Dr. George McNamara (MDACC) for help with image processing; and Dr. Jianlang Dai (MDACC) for statistical analysis. This work was supported by Cancer Center Core Grant CA16672; National Institutes of Health (NIH) Grants R01 CA124782, CA120956, CA141303, and CA141303; NIH Grant R33 CA116127; NIH Grant P01 CA148600; the Burroughs Wellcome Fund; the Cancer Prevention and Research Institute of Texas; the Chronic Lymphocytic Leukemia Global Research Foundation; the Defense Advanced Research Projects Agency (Defense Sciences Office); the Department of Defense; the estate of Noelan L. Bibler; the Gillson Longenbaugh Foundation; the Harry T. Mangurian, Jr. Fund for Leukemia Immunotherapy; the Institute of Personalized Cancer Therapy; the Leukemia and Lymphoma Society; the Lymphoma Research Foundation; MDACC’s Sister Institution Network Fund; the Miller Foundation; Mr. Herb Simons; Mr. and Mrs. Joe H. Scales; Mr. Thomas Scott; the National Foundation for Cancer Research; the Pediatric Cancer Research Foundation; and the William Lawrence and Blanche Hughes Children's Foundation.
Footnotes
Conflict of interest statement: L.J.N.C. founded and owns InCellerate, Inc. He has patents with Sangamo BioSciences with artificial nucleases. He consults with Targazyme, Inc. (formerly American Stem Cells, Inc.), GE Healthcare, Ferring Pharmaceuticals, Inc., and Bristol-Myers Squibb. He receives honoraria from Miltenyi Biotec.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312789111/-/DCSupplemental.
References
- 1.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: Changes in epidemiology and risk factors. Blood. 2002;100(13):4358–4366. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 2.Steinbach WJ, et al. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect. 2012;65(5):453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Kontoyiannis DP. Antifungal prophylaxis in hematopoietic stem cell transplant recipients: The unfinished tale of imperfect success. Bone Marrow Transplant. 2011;46(2):165–173. doi: 10.1038/bmt.2010.256. [DOI] [PubMed] [Google Scholar]
- 4.Georgiadou SP, Kontoyiannis DP. The impact of azole resistance on aspergillosis guidelines. Ann N Y Acad Sci. 2012;1272:15–22. doi: 10.1111/j.1749-6632.2012.06795.x. [DOI] [PubMed] [Google Scholar]
- 5.Brentjens RJ, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5(177):177ra138. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grupp SA, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kochenderfer JN, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012;119(12):2709–2720. doi: 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116(7):1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kebriaei P, et al. Infusing CD19-directed T cells to augment disease control in patients undergoing autologous hematopoietic stem-cell transplantation for advanced B-lymphoid malignancies. Hum Gene Ther. 2012;23(5):444–450. doi: 10.1089/hum.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldorf AR, Levitz SM, Diamond RD. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- 11.Schaffner A, Douglas H, Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perruccio K, et al. Transferring functional immune responses to pathogens after haploidentical hematopoietic transplantation. Blood. 2005;106(13):4397–4406. doi: 10.1182/blood-2005-05-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper LJ, et al. T-cell clones can be rendered specific for CD19: Toward the selective augmentation of the graft-versus-B-lineage leukemia effect. Blood. 2003;101(4):1637–1644. doi: 10.1182/blood-2002-07-1989. [DOI] [PubMed] [Google Scholar]
- 14.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 15.Kowolik CM, et al. CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells. Cancer Res. 2006;66(22):10995–11004. doi: 10.1158/0008-5472.CAN-06-0160. [DOI] [PubMed] [Google Scholar]
- 16.Adachi Y, et al. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72(7):4159–4171. doi: 10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown GD. Dectin-1: A signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 18.Taylor PR, et al. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol. 2002;169(7):3876–3882. doi: 10.4049/jimmunol.169.7.3876. [DOI] [PubMed] [Google Scholar]
- 19.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. BioEssays. 2006;28(8):799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 20.Werner JL, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182(8):4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huls MH, et al. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J Vis Exp. 2013;(72):e50070. doi: 10.3791/50070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maiti SN, et al. Sleeping beauty system to redirect T-cell specificity for human applications. J Immunother. 2013;36(2):112–123. doi: 10.1097/CJI.0b013e3182811ce9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willment JA, Gordon S, Brown GD. Characterization of the human beta -glucan receptor and its alternatively spliced isoforms. J Biol Chem. 2001;276(47):43818–43823. doi: 10.1074/jbc.M107715200. [DOI] [PubMed] [Google Scholar]
- 24.Suhoski MM, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15(5):981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiss GK, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26(3):317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 26.Henson SM, et al. KLRG1 signaling induces defective Akt (ser473) phosphorylation and proliferative dysfunction of highly differentiated CD8+ T cells. Blood. 2009;113(26):6619–6628. doi: 10.1182/blood-2009-01-199588. [DOI] [PubMed] [Google Scholar]
- 27.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectinlike receptor G1 (KLRG1) Blood. 2002;100(10):3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 28.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28(1):29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalos M, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams EL, et al. Differential high-affinity interaction of dectin-1 with natural or synthetic glucans is dependent upon primary structure and is influenced by polymer chain length and side-chain branching. J Pharmacol Exp Ther. 2008;325(1):115–123. doi: 10.1124/jpet.107.133124. [DOI] [PubMed] [Google Scholar]
- 31.Lamaris GA, et al. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J Infect Dis. 2008;198(2):186–192. doi: 10.1086/589305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willment JA, et al. Dectin-1 expression and function are enhanced on alternatively activated and GM-CSF-treated macrophages and are negatively regulated by IL-10, dexamethasone, and lipopolysaccharide. J Immunol. 2003;171(9):4569–4573. doi: 10.4049/jimmunol.171.9.4569. [DOI] [PubMed] [Google Scholar]
- 33.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 34.Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- 35.Ben-Ami R, Lewis RE, Leventakos K, Latgé JP, Kontoyiannis DP. Cutaneous model of invasive aspergillosis. Antimicrob Agents Chemother. 2010;54(5):1848–1854. doi: 10.1128/AAC.01504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hurton LV, et al. Tethered IL-15 mutein on CD19-specific T cells sustains persistence when tumor antigen is low and can treat minimal residual disease. Mol Ther. 2013;21:S237–S237. [Google Scholar]
- 37.Singh H, et al. Manufacture of clinical-grade CD19-specific T cells stably expressing chimeric antigen receptor using Sleeping Beauty system and artificial antigen presenting cells. PLoS ONE. 2013;8(5):e64138. doi: 10.1371/journal.pone.0064138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearce EL, et al. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302(5647):1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 40.Intlekofer AM, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6(12):1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 41.Voskoboinik I, Dunstone MA, Baran K, Whisstock JC, Trapani JA. Perforin: Structure, function, and role in human immunopathology. Immunol Rev. 2010;235(1):35–54. doi: 10.1111/j.0105-2896.2010.00896.x. [DOI] [PubMed] [Google Scholar]
- 42.Schmidt S, et al. Human natural killer cells exhibit direct activity against Aspergillus fumigatus hyphae, but not against resting conidia. J Infect Dis. 2011;203(3):430–435. doi: 10.1093/infdis/jiq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelleher P, et al. Interferon-gamma therapy in two patients with progressive chronic pulmonary aspergillosis. Eur Respir J. 2006;27(6):1307–1310. doi: 10.1183/09031936.06.00021705. [DOI] [PubMed] [Google Scholar]
- 44.Beck O, et al. Generation of highly purified and functionally active human TH1 cells against Aspergillus fumigatus. Blood. 2006;107(6):2562–2569. doi: 10.1182/blood-2005-04-1660. [DOI] [PubMed] [Google Scholar]
- 45.Bouzani M, et al. Human NK cells display important antifungal activity against Aspergillus fumigatus, which is directly mediated by IFN-γ release. J Immunol. 2011;187(3):1369–1376. doi: 10.4049/jimmunol.1003593. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19(6):409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunha C, et al. Dectin-1 Y238X polymorphism associates with susceptibility to invasive aspergillosis in hematopoietic transplantation through impairment of both recipient- and donor-dependent mechanisms of antifungal immunity. Blood. 2010;116(24):5394–5402. doi: 10.1182/blood-2010-04-279307. [DOI] [PubMed] [Google Scholar]
- 49.Netea MG, et al. Aspergillus fumigatus evades immune recognition during germination through loss of toll-like receptor-4-mediated signal transduction. J Infect Dis. 2003;188(2):320–326. doi: 10.1086/376456. [DOI] [PubMed] [Google Scholar]
- 50.O’Connor CM, et al. Adoptive T-cell therapy improves treatment of canine non-Hodgkin lymphoma post chemotherapy. Sci Rep. 2012;2:249. doi: 10.1038/srep00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bretz C, et al. MyD88 signaling contributes to early pulmonary responses to Aspergillus fumigatus. Infect Immun. 2008;76(3):952–958. doi: 10.1128/IAI.00927-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lewis RE, Albert ND, Kontoyiannis DP. Efficacy of single-dose liposomal amphotericin B or micafungin prophylaxis in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2008;52(11):4178–4180. doi: 10.1128/AAC.00715-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.