Significance
Protein crystallography has greatly contributed to our understanding of the structure and function of G protein-coupled receptors (GPCRs). Recent success in the structural investigation of active GPCR conformations was guided by the application of high-affinity agonists and G proteins or G protein mimetic nanobodies. However, poor affinities of agonists prevent the formation of diffraction-quality crystals and hamper the generation of state-specific nanobodies. To overcome this limitation, we present a general approach to covalently binding molecular tools for the construction of stable ligand-receptor complexes capable of G protein activation. Besides the promotion of structural studies, tethered agonist-GPCR complexes may find application in biochemical and biophysical experiments that require reliable labeling of distinct receptor populations, underlining the versatility of covalent agonists for studying GPCR activation.
Keywords: chemical probes, structural biology, chemical biology
Abstract
Structural studies on G protein-coupled receptors (GPCRs) provide important insights into the architecture and function of these important drug targets. However, the crystallization of GPCRs in active states is particularly challenging, requiring the formation of stable and conformationally homogeneous ligand-receptor complexes. Native hormones, neurotransmitters, and synthetic agonists that bind with low affinity are ineffective at stabilizing an active state for crystallogenesis. To promote structural studies on the pharmacologically highly relevant class of aminergic GPCRs, we here present the development of covalently binding molecular tools activating Gs-, Gi-, and Gq-coupled receptors. The covalent agonists are derived from the monoamine neurotransmitters noradrenaline, dopamine, serotonin, and histamine, and they were accessed using a general and versatile synthetic strategy. We demonstrate that the tool compounds presented herein display an efficient covalent binding mode and that the respective covalent ligand-receptor complexes activate G proteins comparable to the natural neurotransmitters. A crystal structure of the β2-adrenoreceptor in complex with a covalent noradrenaline analog and a conformationally selective antibody (nanobody) verified that these agonists can be used to facilitate crystallogenesis.
One of the major obstacles to the investigation of the structural basis of G protein-coupled receptor (GPCR) activation is the flexibility of their seven-transmembrane core, particularly in the active state (1), and the resulting biochemical instability of the solubilized protein (2, 3). Protein crystallography, the most powerful tool for the study of GPCR structure, requires the formation of stable and conformationally homogeneous ligand-receptor complexes (4). High-affinity agonists with dissociation constants in the low to subnanomolar range and low off-rates facilitate stabilization of the protein throughout the process of expression, purification, and crystallogenesis (2); however, endogenous neurotransmitters usually show poor binding affinity. Low binding affinity with rapid association and dissociation rates leads to conformational heterogeneity that prevents the formation of diffraction-quality crystals. The rapid dissociation rate of agonists also makes it difficult to generate active-state stabilizing proteins, such as the camelid antibodies (nanobodies) that have been used to obtain active-state structures of the β2-adrenergic receptor (β2AR) (5) and M2 muscarinic receptor (6).
To prevent ligand dissociation, irreversible ligation of electrophilic moieties like halomethylketones, isothiocyanates, Michael acceptors, or aziridinium groups of small-molecule ligands with a suitably positioned nucleophilic residue in the receptor has been used (7–16). However, irreversible ligands often suffer from incomplete cross-linking (15) and reduced receptor activation when covalent binding leads to loss of agonist efficacy (10, 16). Furthermore, their highly electrophilic nature and the abundance of nucleophilic groups in biological systems may lead to a low coupling selectivity (7, 8).
Disulfide-based cross-linking approaches (17, 18) offer the advantage that the covalent binding of disulfide-containing compounds is chemoselective for cysteine and enforced by the affinity of the ligand-pharmacophore rather than by the electrophilicity of the cross-linking function (19). We refer to the described ligands as covalent rather than irreversible agonists because cleavage may be promoted by reducing agents and the disulfide transfer process is a reversible chemical reaction in general.
Structural information on the target protein facilitates the development of covalent ligand-receptor pairs. Mutation of H932.64 in the β2AR to cysteine introduced an anchor for the disulfide-based covalent agonist FAUC50, which does not perturb ligand binding or the activation of the receptor, and thus enabled, to our knowledge, the first agonist-bound GPCR structure (20). Taking advantage of the high structural homology among aminergic GPCRs, we reasoned that the introduction of cysteine into position X2.64 should also result in a covalently binding receptor mutant for other aminergic GPCRs.
We here report a methodology to generate disulfide-based covalent ligand-receptor pairs to promote structural and functional studies on GPCRs. We demonstrate that even the low-affinity endogenous agonists noradrenaline, dopamine, and serotonin can be converted into efficient covalently binding molecular tools for the β2AR, the dopamine D2 receptor (D2R), and the 5-hydroxytryptamine 2A (5-HT2A) serotonergic subtype representing Gs-, Gi-, and Gq-coupled GPCRs, respectively. Analogous studies were conducted starting from histamine and the receptor subtype H1. We applied this strategy to obtain an active-state crystal structure of the β2ARH93C and a covalent (nor)adrenaline analog.
Results
Design and Synthesis of the Covalent Agonists.
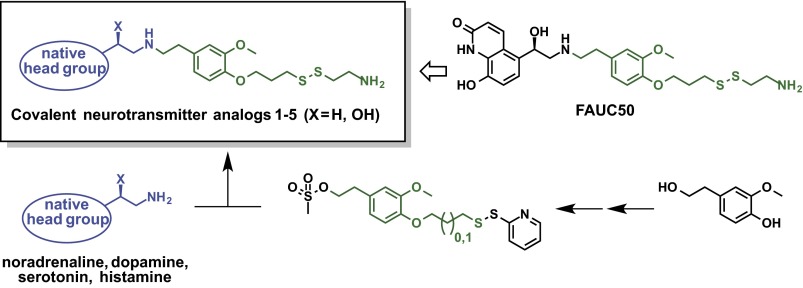
The designed covalent ligands feature the recognition element of neurotransmitters connected to a disulfide moiety via a flexible linker, based on the parent compound FAUC50 (Fig. 1). To develop a general approach to covalent ligands, we planned to exploit the naturally installed nucleophilicity of the neurotransmitters in a chemoselective N-alkylation reaction. Starting from a substituted hydroxyphenylethanol, the required electrophilic building blocks were synthesized (Fig. 1 and SI Appendix, Fig. S3). The chemoselective N-alkylation reaction of the biogenic amines was initially hampered by the instability of the catechol and the 5-hydroxyindole moiety of the endogenous ligands and the disulfide-functionalized derivatives. Catechol groups, for instance, are susceptible to oxidizing conditions, irradiation, and heat, leading to a complex mixture of oxidization products (21). Thus, adjustment of solvent, base, temperature, and handling under an inert atmosphere was necessary to guarantee a good balance between consumption of the starting material and low degradation of the products. Subsequent disulfide exchange and immediate purification by preparative HPLC efficiently yielded the corresponding covalent (nor)adrenaline (1 and 2), dopamine (3), and histamine (5) derivatives (SI Appendix, Fig. S1). Because the serotonergic analog (4) showed substantial impurities, intermediate silyl-protection of the phenolic moiety was necessary to access the pure product (SI Appendix, Fig. S2). Despite their immanent sensitivity to oxygen, irradiation, and heat, all derivatives could be obtained in high quality, as determined by NMR.
Fig. 1.
Covalent β2AR agonist FAUC50 served as a template for the covalent neurotransmitter-derived agonists. Starting from the corresponding hydroxyphenylethanol, regioselective O-alkylation, nucleophilic substitution with potassium thioacetate, methanolysis, treatment with 2,2′-dithiodipyridine, and O-activation gave the required electrophilic building blocks that were used in chemoselective N-alkylation of the biogenic amines. Reagents and conditions are shown in SI Appendix, Figs. S1–S3.
Characterization of the Covalent Ligand-Receptor Pairs.
To construct covalent GPCR-neurotransmitter complexes, our initial investigations were directed to the linkage of the β2ARH93C and the disulfide-functionalized (nor)adrenaline analogs 1 and 2, slightly differing in their spacer length. The efficiency of these compounds in the formation of the covalent bond was evaluated in a time-dependent radioligand depletion assay. Therefore, we used WT β2AR with a single H93C mutation that was purified and reconstituted into HDL particles (22). Both ligands showed considerable covalent binding after incubation with the mutated receptor that could not be reversed by a large excess of radioligand (Fig. 2A and SI Appendix, Fig. S5). Compound 2 was equally effective as FAUC50 in blocking radioligand binding, whereas analog 1 displayed a lower propensity for covalent complex formation, indicating that a shorter linker results in a higher degree of covalent linkage (SI Appendix, Figs. S4 and S5). To determine whether the tethered ligand-receptor complex is capable of activating heterotrimeric Gs protein, reconstituted WT and β2ARH93C mutant receptors were incubated with ligand 2 alone or with ligand 2 followed by an excess of the high-affinity inverse agonist ICI-118,551. After addition of G protein, activation was observed by measuring [35S]GTPγS binding to the Gαs subunit. Fig. 2D shows that an excess of the inverse agonist ICI-118,551 is unable to reverse compound 2-induced G protein activation in the case of β2ARH93C but prevented agonist-induced nucleotide exchange by the WT receptor. Thus, the disulfide-functionalized (nor)adrenaline 2 forms a stable complex with the β2ARH93C that promotes G protein activation.
Fig. 2.
(A) Radioligand depletion assay of the covalent ligands 2 (blue) and FAUC50 (green) incubated with purified β2ARH93C reconstituted into HDL particles yields equally high fractions of covalent ligand-receptor complex compared with the reversible reference alprenolol (black) after dilution with [3H]dihydroalprenolol. (B) Membrane-bound D2RL94C was preincubated with dopamine analog 3 (blue) and the reversible agonist quinpirole (black). After washing the membranes, radioligand displacement with [3H]spiperone indicates nearly complete covalent binding of compound 3 to D2RL94C compared with quinpirole. (C) Preincubation and a radioligand binding assay using membrane-bound 5-HT2AS131C-T134C and compound 4 (blue) show a considerable fraction of nonreversible ligand-receptor complex, whereas membranes treated with the reversible ligand serotonin (black) are fully bound by [3H]ketanserin. (D) Receptor activation of WT β2AR and β2ARH93C was determined by a [35S]GTPγS binding assay using purified and reconstituted receptors and purified heterotrimeric Gs protein. Although compound 2 activates both receptors similar to isoproterenol (iso), the activation at β2ARwt is reduced to basal activity by adding an excess of inverse agonist ICI-118,551 (ICI), whereas the compound 2-β2ARH93C complex displays significant nucleotide binding even in presence of ICI-118,551. An IP accumulation assay was conducted to measure activation of compound 3 at D2RL94C in comparison to the reference quinpirole (quin). Although the effect of quinpirole is fully blocked by an excess of the antagonist haloperidol (halo), the compound 3-D2RL94C complex induces stable and nonreversible IP formation. Similarly, IP accumulation of compound 4 reveals substantial activation of 5-HT2AS131C-T134C, which is equal to the effect of serotonin (serot), but cannot be inhibited by the antagonist ketanserin (ketan). Data represent the mean of three experiments ± SEM, each performed in triplicate.
To investigate covalent receptor binding and activation of the disulfide-functionalized dopamine analog 3, the D2R was chosen as a representative model for a Gi-coupled GPCR and the mutation L94C2.64 was introduced into WT D2R. A radioligand depletion assay, using membranes containing the D2RL94C mutant, revealed extensive blockade of [3H]spiperone binding at the mutated receptor, suggesting a highly efficient covalent binding mode for the dopamine analog 3 (Fig. 2B). The ability of dopamine analog 3 to activate WT D2R, as well as the ability of the covalent 3-D2RL94C complex to induce G protein-promoted signaling, was assessed in an inositol phosphate (IP) formation assay using HEK cells transiently transfected with a chimeric Gαqi5 protein and the WT and mutant receptor, respectively (6, 23) (Fig. 2D and SI Appendix, Fig. S6). In contrast to the reversibility of the control noncovalent agonist quinpirole, agonist-induced IP accumulation by the dopamine analog 3 could not be diminished by an excess of antagonist (Fig. 2D), demonstrating that the dopamine analog 3 is an efficacious covalent agonist for the D2R.
To extend our strategy to a Gq protein-coupling subtype, the serotonin receptor mutant 5-HT2AT134C was generated from WT receptor to evaluate the disulfide-functionalized serotonin surrogate 4. Interestingly, we observed only minor blockade of this receptor mutant in the radioligand depletion assay (SI Appendix, Fig. S7). This result prompted us to evaluate the role of serine residue 131 in transmembrane helix 2 (TM2), positioned below the cysteine anchor, in sterically hindering efficient covalent binding. Consequently, S1312.61 was mutated to glycine. Investigation of the double-mutant 5-HT2AS131G-T134C revealed that the amount of covalently modified receptor increased in the radioligand depletion assay (Fig. 2C), and analogous IP accumulation assays indicated substantial nonreversible Gq protein activation by the covalent neurotransmitter-receptor complex (Fig. 2D and SI Appendix, Fig. S8).
Investigation of the covalent histamine analog 5 directed us to the construction of the histamine H1 receptor mutant H1RY87C as an additional example of a Gq-coupled GPCR. In a corresponding IP accumulation assay, histamine and histamine analog 5 show comparable activation of WT and mutant receptors (SI Appendix, Figs. S9 and S10). Although the response of histamine can be substantially reduced by an excess of the antagonist diphenhydramine, histamine analog 5 still displays significant Gαq activation, thus demonstrating considerable formation of a covalent ligand-receptor complex (SI Appendix, Fig. S10).
Structure of β2ARH93C in Complex with a Covalent (Nor)adrenaline Analog.
To test the applicability of our methodology in structural studies, we conducted crystallography trials for β2ARH93C using compound 2 and the engineered nanobody 6B9 (Nb6B9), which is a high-affinity version of a single-domain antibody fragment obtained from llamas that was further evolved to display optimized properties for crystallography (24). Nb6B9 mimics the G protein by binding at the intracellular surface of the β2AR, and thus stabilizes the active state of the receptor (Fig. 3A). Crystals of the compound 2-bound β2ARH93C-Nb6B9 complex, grown in lipidic mesophase (25), displayed excellent size and stability at 20 °C, in contrast to the crystals obtained for the adrenaline-bound complex of β2AR, stabilized by Nb6B9 (24), which were inherently unstable and had to be harvested shortly after their appearance.
Fig. 3.
(A) Overall structure of the β2ARH93C (blue) forming a covalent complex with compound 2 (magenta) and Nb6B9 (red) that binds at the intracellular G protein interface and stabilizes the active state. (B) Superimposition of the bound compound 2-β2ARH93C complex (blue, ligand in magenta) and the adrenaline-bound active-state structure of the β2AR (orange, ligand in gray). The water molecule and the extended hydrogen-bond network are resolved in the adrenaline-bound structure. Compound 2 forms highly similar polar interactions. It is likely that minor differences may result from low resolution in this part of the compound 2-bound structure in which the water molecule is not clearly resolved and the side chain of H2966.58 and the ECL3 adopt different conformations. (C) Surface view on the binding pocket of both complexes reveals overall identical contraction and shape. The covalent ligand is positioned in an extended binding pocket and does not interfere with ligand binding or activation of the receptor.
The structure of the compound 2-bound β2AR-Nb6B9 complex was solved at a resolution of 3.3 Å with clear electron density for the covalent ligand (SI Appendix, Fig. S11). It reveals a remarkable similarity to the 3.2 Å adrenaline-bound structure. Fig. 3B shows a superimposition of the binding pocket of both complexes. The adrenaline-pharmacophores form identical ionic and hydrophilic interactions. A unique feature of catechol-bound structures is the tilt of TM6 toward the catechol moiety to form a hydrogen bond between the meta-hydroxyl group and Asn2936.55 (24) (SI Appendix, Fig. S12). In the adrenaline-bound structure, a water molecule further mediates an extended hydrogen bond network from Asn2936.55 to His2966.58 and up to Asn301 in extracellular loop 3 (ECL3) (Fig. 3B). The electron density of the covalent complex structure is suggestive of the existence of a corresponding water molecule but is insufficient to confirm its presence definitively. His2966.58 and the Asn301 in ECL3 appear to adopt different positions in the covalent complex structure; however, the electron density maps for this region are relatively weak, which may reflect the inherent flexibility of ECL3.
The linker of the covalent ligand is positioned in an extended pocket formed by TM3, TM7, and ECL2 (SI Appendix, Fig. S13). A comparison of the orthosteric and extended pocket of the adrenaline- and compound 2-bound structures illustrates that the covalent ligand perfectly fills additional space without altering the overall shape of the adrenaline pocket (Fig. 3C). Despite minor differences in ECL3 of the β2AR, our approach yielded a structure that is virtually identical to the adrenaline-β2AR complex.
Discussion
Endogenous neurotransmitters typically bind with a low affinity to their respective receptors. Thus, obtaining active structures often requires synthetic high-affinity agonists. However, appropriate agonists may not be available or may not stabilize physiological conformations. This study presents a general and practical approach for conversion of the native neurotransmitters noradrenaline, dopamine, serotonin, and histamine into covalent GPCR agonists. The convergent synthetic strategy facilitates fast and facile access to the respective disulfide-functionalized molecular tools, allowing one to optimize the linker length between the pharmacophore and the cross-linking functionality (SI Appendix, Figs. S1, S4, and S5). By using different head groups endowed with a primary or secondary amine, this general synthetic approach provides access to a variety of tailored covalent ligands for aminergic GPCRs.
The formation of covalent ligand-receptor complexes was evaluated in radioligand depletion assays for compounds 2, 3, and 4. In addition to synthetic modification of the compounds, the mutation of amino acid residues in proximity to the disulfide-forming cysteine, perturbing covalent bond formation provides an additional tool with which to optimize the target complex. Functional assays confirmed the covalent binding mode for all described agonists and, most importantly, the ability of the covalent ligand–receptor pairs to activate G proteins.
The applicability of the covalent agonists in structural studies is demonstrated by the active-state crystal structure of β2ARH93C in complex with a covalent (nor)adrenaline-derived agonist, verifying that the designed ligands can be used to facilitate crystallogenesis. A comparison of the presented structure and the adrenaline-bound structure revealed that compound 2 induces a virtually identical receptor conformation, providing evidence that the covalent agonists can yield structural insights into the binding mode of endogenous agonists.
In addition to their benefit in structural studies, covalent agonists can find application in biophysical and biochemical assays that require stable ligand-receptor complexes. Although the crystallization of GPCR-G protein complexes is still notoriously difficult (4, 26, 27), a recent key strategy to obtain active-state structures comprised the use of conformationally selective nanobodies that serve as G protein mimetics and stabilize active conformations (5, 6, 24). Covalent ligand-receptor complexes can be useful for the generation of G protein-mimicking nanobodies because they prevent ligand dissociation during the immunization of llamas (28).
A mustard-based covalent agonist FAUC123 facilitated the identification of a rare G protein mimetic nanobody for the M2 muscarinic receptor from a yeast display library using fluorescence-activated cell sorting. The covalent agonist enabled simultaneous staining of yeast with both agonist- and inverse agonist-occupied M2 receptor populations, which were distinguishably labeled with separate fluorophores (6). The disulfide-based approach will further extend the scope and complement the conformational selection of nanobodies.
This strategy may also be useful for studying oligomerization of aminergic GPCRs and allosteric interactions between protomers. In a system that coexpresses WT receptor and a corresponding X2.64C mutant, a covalent complex of a disulfide-based agonist and the mutant receptor could be formed selectively. One could then examine the functional properties of the WT receptor in the presence and absence of receptor bound to covalent agonist.
In conclusion, we demonstrated the generation of efficacious disulfide-based covalent agonists for aminergic GPCRs representing examples for Gs-, Gi-, and Gq-coupled receptors. We believe that this general and adaptable strategy provides a framework for the development of novel covalent agonists for this class of receptors. Applications of covalent ligands extend well beyond structural biology, highlighting their versatility and utility in promoting structural and functional studies on GPCRs.
Materials and Methods
Synthesis and Characterization of the Covalent Agonists.
Detailed schemes and conditions for the synthesis of covalent agonists 1–5 are provided in SI Appendix, Figs. S1 and S2. The synthesis of additional intermediates is summarized in SI Appendix, Fig. S3. Detailed methods and characterization for all compounds are provided in SI Appendix, SI Note S1. 1H and 13C NMR spectra for covalent agonists 1–5 are provided in SI Appendix, SI Note S2.
β2AR Expression and Purification.
For functional experiments, human β2AR bearing an amino-terminal FLAG epitope tag truncated after residue 365 and with or without an H93C mutation was expressed in Sf9 cells using the FastBac baculovirus system (Expression Systems). Receptor was extracted and purified as described previously (24, 29) (details are provided in SI Appendix, SI Materials and Methods). For crystallographic experiments, a construct of human β2AR fused to an N-terminal T4 lysozyme (30) containing an H93C mutation was expressed and purified from Sf9 insect cells. After purification, the receptor was incubated for 60 min at room temperature with a stoichiometric excess of compound 2. A 1.3-fold molar excess of Nb6B9 was then added and incubated for an additional 30 min. The sample was then concentrated using a 50-kDa spin concentrator and purified over a Sephadex S200 size-exclusion column (GE Healthcare) (details are provided in SI Appendix, SI Materials and Methods).
Expression and Purification of Nb6B9.
Nanobody was expressed in Escherichia coli and purified as described previously (5) (details are provided in SI Appendix, SI Materials and Methods).
Crystallization, Data Collection, and Refinement of the 2-β2ARH93C–Nb6B9 Complex.
Crystals were grown in lipid mesophase using 35 nL of protein/lipid drops with 600 nL of overlay solution, which consisted of 24–27% PEG 400, 100 mM Tris (pH 7.8–8.4), and 5–10 mM sodium formate. The X-ray diffraction data were collected at the General Medical Sciences and Cancer Institutes Structural Biology Facility at the Advanced Photon Source GM/CA at APS beamline 23ID-B (further details are provided in SI Appendix, SI Materials and Methods). Data from 10 crystals have been merged. Diffraction data were processed in HKL-2000 (HKL Research, Inc.) (31), and the structure was solved using molecular replacement with the structures of active β2AR bound to Nb6B9 (24) (Protein Data Bank ID code 4LDO) as search models in Phaser (32). The resulting structure was iteratively refined in Phenix (33) and manually rebuilt in Coot (34). Final refinement statistics are summarized in SI Appendix, Table S1.
Radioligand Depletion Assay and G Protein Activation Assay for β2AR.
Purified WT β2AR or β2ARH93C reconstituted into recombinant HDL particles (22) was used as described (20) (details are provided in SI Appendix, SI Materials and Methods).
Radioligand Depletion Assay for the D2 and 5-HT2A Receptor Ligands.
Membranes from HEK 293 cells transiently expressing the human D2RL94C or human 5-HT2AS131G-T134C were prepared and incubated with the covalent agonists 3 and 4 and the reversible ligands quinpirole and serotonin, respectively (details are provided in SI Appendix, SI Materials and Methods). Membranes were then used for radioligand binding experiments with [3H]spiperone (specific activity = 81 Ci/mmol) or [3H]ketanserin (specific activity = 53 Ci/mmol) (both from PerkinElmer) to determine specific binding at the D2R and the 5-HT2A receptor, respectively, as described (35) (details are provided in SI Appendix, SI Materials and Methods and Table S2).
Determination of Covalent D2, 5-HT2A, and H1 Receptor Activation via IP Assays.
Agonist-induced activation of the human D2RL94C, 5-HT2AS131G-T134C, and H1Y87C receptors was studied using IP accumulation assays as described (6, 23). For activation studies with Gi-coupled GPCRs, HEK 293 cells were transiently cotransfected with cDNA encoding for D2RL94C and the hybrid G protein Gαqi5 (Gαq protein with the last five amino acids at the C terminus replaced by the corresponding sequence of Gαi; a gift from The J. David Gladstone Institutes) (36). Cells were preincubated with myo-[3H]inositol (specific activity = 22.5 Ci⋅mmol−1; PerkinElmer) for 15 h. After incubation with compound 3 for 30 min, the antagonist haloperidol was added to half of the samples (buffer was added to the other half). Incubation was continued for additional 150 min, and total IP was determined (details are provided in SI Appendix, SI Materials and Methods). Activation studies with a Gq-coupled GPCR were done with HEK 293 cells, which were transiently transfected with cDNA encoding for the 5-HT2AS131G-T134C or H1Y87C receptor, respectively, similar to the described method (details are provided in SI Appendix, SI Materials and Methods). After incubation of 5-HT2AS131G-T134C with compound 4 and serotonin for 90 min, ketanserin was added to half of the samples to inhibit further receptor activation. After further incubation for 150 min, total IP was determined as described. Likewise, the H1Y87C receptor was incubated with compound 5 and histamine for 90 min, and diphenhydramine was then added to half of the samples (details are provided in SI Appendix, SI Materials and Methods).
Supplementary Material
Acknowledgments
We thank T. S. Kobilka for preparation of affinity chromatography reagents and F. S. Thian for help with cell culture. We also thank Manuel Plomer for work on biological assays and Daniela Huber and Stefan Löber for helpful discussions. This work was supported by grants from the German Research Foundation (Deutsche Forschungsgemeinschaft Gm 13/10, GRK 1910), the Bayerische Forschungsstiftung, the BioMedTec International Graduate School of Science, and the Elitenetzwerk Bayern.
Footnotes
The authors declare no conflict of interest.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4QKX).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410415111/-/DCSupplemental.
References
- 1.Nygaard R, et al. The dynamic process of β(2)-adrenergic receptor activation. Cell. 2013;152(3):532–542. doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kobilka B, Schertler GF. New G-protein-coupled receptor crystal structures: Insights and limitations. Trends Pharmacol Sci. 2008;29(2):79–83. doi: 10.1016/j.tips.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Granier S, Kobilka B. A new era of GPCR structural and chemical biology. Nat Chem Biol. 2012;8(8):670–673. doi: 10.1038/nchembio.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda S, Schertler GF. Production of GPCR and GPCR complexes for structure determination. Curr Opin Struct Biol. 2013;23(3):381–392. doi: 10.1016/j.sbi.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469(7329):175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kruse AC, et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504(7478):101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newman AH. Irreversible ligands as probes for drug receptors. NIDA Res Monogr. 1991;112:256–283. [PubMed] [Google Scholar]
- 8.Baker SP, Deyrup MD. Development of novel irreversible ligands. Neuroprotocols. 1994;4(1):66–75. [Google Scholar]
- 9.Nijmeijer S, et al. Design and pharmacological characterization of VUF14480, a covalent partial agonist that interacts with cysteine 98(3.36) of the human histamine H₄ receptor. Br J Pharmacol. 2013;170(1):89–100. doi: 10.1111/bph.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsotinis A, Afroudakis PA, Davidson K, Prashar A, Sugden D. Design, synthesis, and melatoninergic activity of new azido- and isothiocyanato-substituted indoles. J Med Chem. 2007;50(25):6436–6440. doi: 10.1021/jm7010723. [DOI] [PubMed] [Google Scholar]
- 11.Picone RP, Fournier DJ, Makriyannis A. Ligand based structural studies of the CB1 cannabinoid receptor. J Pept Res. 2002;60(6):348–356. doi: 10.1034/j.1399-3011.2002.21069.x. [DOI] [PubMed] [Google Scholar]
- 12.Milecki J, et al. Carbostyril derivatives having potent β-adrenergic agonist properties. J Med Chem. 1987;30(9):1563–1566. doi: 10.1021/jm00392a006. [DOI] [PubMed] [Google Scholar]
- 13.Xiang Z, et al. Adding an unnatural covalent bond to proteins through proximity-enhanced bioreactivity. Nat Methods. 2013;10(9):885–888. doi: 10.1038/nmeth.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan F, et al. Structure-based design, synthesis, and biochemical and pharmacological characterization of novel salvinorin A analogues as active state probes of the kappa-opioid receptor. Biochemistry. 2009;48(29):6898–6908. doi: 10.1021/bi900605n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kung M-P, Mu M, Zhuang Z-P, Kung HF. NCS-MPP (4-(2′-methoxy-phenyl)-1-[2′-(N-2′′-pyridyl)-p-isothiocyanobenz amido]-ethyl-piperazine): A high affinity and irreversible 5-HT1A receptor ligand. Life Sci. 1996;58(3):177–186. doi: 10.1016/0024-3205(95)02275-9. [DOI] [PubMed] [Google Scholar]
- 16.Baker SP, Liptak A, Pitha J. Irreversible inactivation of the β-adrenoreceptor by a partial agonist. Evidence for selective loss of the agonist high affinity binding sites. J Biol Chem. 1985;260(29):15820–15828. [PubMed] [Google Scholar]
- 17.Buck E, Bourne H, Wells JA. Site-specific disulfide capture of agonist and antagonist peptides on the C5a receptor. J Biol Chem. 2005;280(6):4009–4012. doi: 10.1074/jbc.C400500200. [DOI] [PubMed] [Google Scholar]
- 18.Buck E, Wells JA. Disulfide trapping to localize small-molecule agonists and antagonists for a G protein-coupled receptor. Proc Natl Acad Sci USA. 2005;102(8):2719–2724. doi: 10.1073/pnas.0500016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy JA. A link means a lot: Disulfide tethering in structure-based drug design. In: Stroud J, Finer-Moore J, editors. Comput Struct Approaches Drug Discovery. Cambridge, UK: RSC Publishing; 2008. pp. 319–348. [Google Scholar]
- 20.Rosenbaum DM, et al. Structure and function of an irreversible agonist-β(2) adrenoceptor complex. Nature. 2011;469(7329):236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Ischia M, Palumbo A, Prota G. Adrenalin oxidation revisited. New products beyond the adrenochrome stage. Tetrahedron. 1988;44(20):6441–6446. [Google Scholar]
- 22.Whorton MR, et al. A monomeric G protein-coupled receptor isolated in a high-density lipoprotein particle efficiently activates its G protein. Proc Natl Acad Sci USA. 2007;104(18):7682–7687. doi: 10.1073/pnas.0611448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chee MJS, et al. The third intracellular loop stabilizes the inactive state of the neuropeptide Y1 receptor. J Biol Chem. 2008;283(48):33337–33346. doi: 10.1074/jbc.M804671200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ring AM, et al. Adrenaline-activated structure of β2-adrenoceptor stabilized by an engineered nanobody. Nature. 2013;502(7472):575–579. doi: 10.1038/nature12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caffrey M, Cherezov V. Crystallizing membrane proteins using lipidic mesophases. Nat Protoc. 2009;4(5):706–731. doi: 10.1038/nprot.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westfield GH, et al. Structural flexibility of the G α s α-helical domain in the β2-adrenoceptor Gs complex. Proc Natl Acad Sci USA. 2011;108(38):16086–16091. doi: 10.1073/pnas.1113645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasmussen SG, et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steyaert J, Kobilka BK. Nanobody stabilization of G protein-coupled receptor conformational states. Curr Opin Struct Biol. 2011;21(4):567–572. doi: 10.1016/j.sbi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobilka BK. Amino and carboxyl terminal modifications to facilitate the production and purification of a G protein-coupled receptor. Anal Biochem. 1995;231(1):269–271. doi: 10.1006/abio.1995.1533. [DOI] [PubMed] [Google Scholar]
- 30.Zou Y, Weis WI, Kobilka BK. N-terminal T4 lysozyme fusion facilitates crystallization of a G protein coupled receptor. PLoS ONE. 2012;7(10):e46039. doi: 10.1371/journal.pone.0046039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276(Macromolecular Crystallography, Part A):307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 32.McCoy AJ, et al. Phaser crystallographic software. J Appl Cryst. 2007;40(Pt 4):658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afonine PV, et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr. 2012;68(Pt 4):352–367. doi: 10.1107/S0907444912001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60(Pt 12 Pt 1):2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 35.Hübner H, Haubmann C, Utz W, Gmeiner P. Conjugated enynes as nonaromatic catechol bioisosteres: Synthesis, binding experiments, and computational studies of novel dopamine receptor agonists recognizing preferentially the D(3) subtype. J Med Chem. 2000;43(4):756–762. doi: 10.1021/jm991098z. [DOI] [PubMed] [Google Scholar]
- 36.Broach JR, Thorner J. High-throughput screening for drug discovery. Nature. 1996;384(6604) Suppl:14–16. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.