Abstract
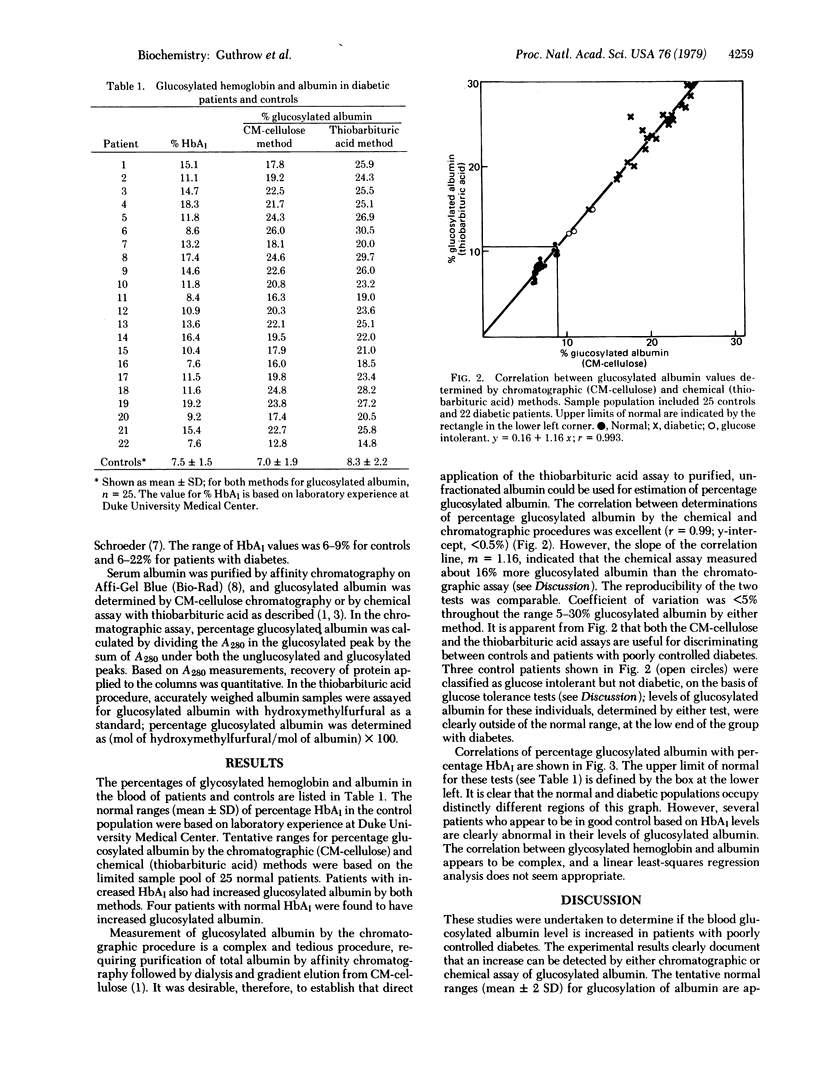
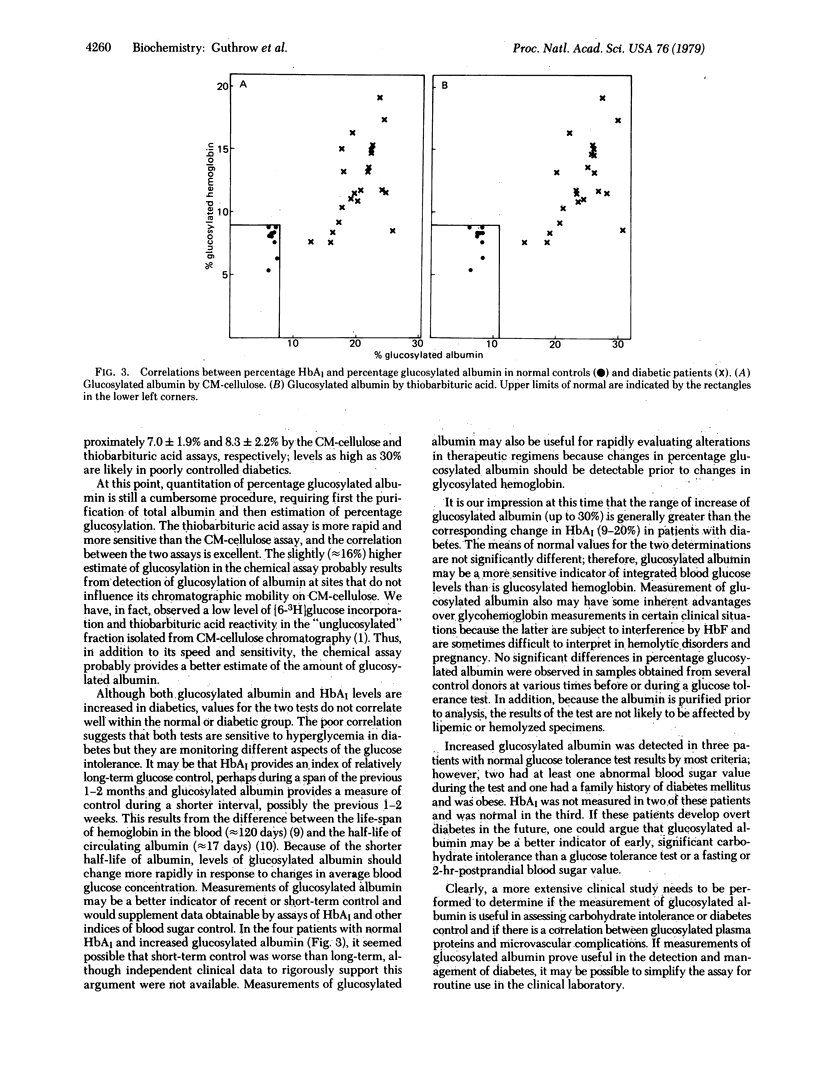
Use of an ion exchange chromatographic method and a colorimetric method with thiobarbituric acid showed that levels of nonenzymatically glucosylated serum albumin were increased in patients with poorly controlled diabetes mellitus compared to controls. The two methods correlated well (r = 0.99) and clearly discriminated between normal and poorly controlled diabetic populations. The levels of glycosylated hemoglobin were also measured in both populations. Several patients apparently in good control based on glycosylated hemoglobin measurements were found to have increased levels of glycosylated albumin. Because albumin has a shorter circulating half-life than does the human erythrocyte, the plasma concentration of glucosylated albumin should be expected to reflect short-term control of hyperglycemia in diabetes. The studies reported here suggest that the level of glucosylated albumin may indeed be a sensitive indicator of moderate hyperglycemia and of early glucose intolerance.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Robins S. P., Tanner M. J. Reducible components in the proteins of human erythrocyte membrane. Biochim Biophys Acta. 1976 May 20;434(1):51–57. doi: 10.1016/0005-2795(76)90034-9. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Gabbay K. H., Gallop P. M. The glycosylation of hemoglobin: relevance to diabetes mellitus. Science. 1978 Apr 7;200(4337):21–27. doi: 10.1126/science.635569. [DOI] [PubMed] [Google Scholar]
- Day J. F., Thorpe S. R., Baynes J. W. Nonenzymatically glucosylated albumin. In vitro preparation and isolation from normal human serum. J Biol Chem. 1979 Feb 10;254(3):595–597. [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Gonen B., Rubenstein A., Rochman H., Tanega S. P., Horwitz D. L. Haemoglobin A1: An indicator of the metabolic control of diabetic patients. Lancet. 1977 Oct 8;2(8041):734–737. doi: 10.1016/s0140-6736(77)90237-9. [DOI] [PubMed] [Google Scholar]
- Travis J., Pannell R. Selective removal of albumin from plasma by affinity chromatography. Clin Chim Acta. 1973 Nov 23;49(1):49–52. doi: 10.1016/0009-8981(73)90341-0. [DOI] [PubMed] [Google Scholar]



