Significance
T-cell activation requires cognate antigen encounter plus additional stimulation through B7 costimulatory molecules. This explains why B7 costimulation blockade is therapeutically effective in many autoimmune disorders including diabetes and rheumatoid arthritis. Interestingly, however, intestinal inflammation is uniquely resistant to B7-neutralizing therapies. We show that colonization with commensal bacteria predisposes the intestine to inflammation despite B7 deprivation caused by IL-17–producing T cells that become activated through the acces-sory costimulation molecules ICOSL and OX40L. Reciprocally, intestinal inflammation is silenced when either commensal enteric bacteria or stimulation through ICOSL/OX40L is eliminated. These results suggest that simultaneously neutralizing accessory costimulation molecules may be needed to extinguish inflammation in tissues like the intestine that retain prominent B7-independent pathways for T-cell activation.
Abstract
The costimulatory B7-1 (CD80)/B7-2 (CD86) molecules, along with T-cell receptor stimulation, together facilitate T-cell activation. This explains why in vivo B7 costimulation neutralization efficiently silences a variety of human autoimmune disorders. Paradoxically, however, B7 blockade also potently moderates accumulation of immune-suppressive regulatory T cells (Tregs) essential for protection against multiorgan systemic autoimmunity. Here we show that B7 deprivation in mice overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas peripherally induced Tregs retained in the absence of B7 selectively mitigate intestinal inflammation caused by Th17 effector CD4+ T cells. The need for additional immune suppression in the intestine reflects commensal microbe-driven T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Eradication of commensal enteric bacteria mitigates intestinal inflammation and IL-17 production triggered by Treg depletion in B7-deficient mice, whereas re-establishing intestinal colonization with Candida albicans primes expansion of Th17 cells with commensal specificity. Thus, neutralizing B7 costimulation uncovers an essential role for Tregs in selectively averting intestinal inflammation by Th17 CD4+ T cells with commensal microbe specificity.
Immune activation is stringently controlled to balance mobilization of protective components while simultaneously silencing detrimental responses that cause harm to host tissues. One means of regulation is the additional necessity for B7-1 (CD80)/B7-2 (CD86) costimulatory signals, along with T-cell receptor stimulation, in T-cell activation (1). Reciprocally, soluble recombinant formulations of the natural high-affinity B7 ligand—cytotoxic T-lymphocyte antigen 4 fused with human Ig (CTLA4-Ig), which blocks B7 costimulation—are efficacious in neutralizing aberrant T-cell activation in autoimmune disorders such as rheumatoid arthritis and juvenile idiopathic arthritis (2). Ongoing studies suggest that these therapeutic benefits also extend to many other types of autoimmunity including psoriasis, systemic lupus erthematosus, multiple sclerosis, and type 1 diabetes (3–6). Interestingly, however, the protective benefits of B7 blockade are not universal as CTLA4-Ig is distinctively nonefficacious for inflammatory bowel disease (7) and can induce intestinal inflammation among individuals with unrelated autoimmune disorders (8).
Given that B7 costimulation required for T-cell activation also sustains accumulation of immune-suppressive regulatory T cells (Tregs) essential for averting fatal systemic autoimmunity (9, 10), this discordance in therapeutic efficacy with B7 blockade may reflect tissue-specific differences in necessity for Treg suppression in the absence of B7 costimulation. Here, unique features of the intestine that include high-density commensal bacteria colonization or expression of the accessory costimulatory molecules ICOS ligand (ICOSL) or OX40 ligand (OX40L) may foster susceptibility to inflammation despite B7 deprivation (11–15). To investigate these possibilities, the interplay between Tregs and B7, ICOSL, and OX40L costimulation in autoimmunity was evaluated after targeted ablation of each individually or concurrently. Our results show that B7 deprivation overrides the necessity for Tregs in averting systemic autoimmunity and inflammation in extraintestinal tissues, whereas enteric commensal microbes drive inflammation restricted to the intestine through ICOSL/OX40L stimulation when Tregs and B7 are simultaneously eliminated. These results illustrating persistent intestinal inflammation despite B7 deprivation may explain why inflammatory bowel disease, compared with other forms of autoimmunity, is distinctively resistant to B7 blockade.
Results
Tregs Retained After B7 Deprivation Avert Intestinal Inflammation.
The suppressive Foxp3+ subset of CD4+ T cells, called Tregs, actively suppress autoimmunity, whereas Treg ablation or Foxp3 deficiency causes rapidly fatal multiorgan systemic inflammation (16). In turn, more subtle Tregs defects are increasingly linked with fractured immune tolerance among individuals with various nonfatal autoimmune disorders (17–20). Accordingly, we considered how reduced Treg levels that occur with B7 deprivation may impact autoimmunity. Consistent with findings in diabetes-susceptible NOD mice, CTLA4-Ig B7 blockade induced sharp contraction in peripheral Tregs for mice on the C57BL/6 (B6) background (Fig. 1A) (9, 10). However, despite markedly reduced Tregs, CTLA4-Ig–treated B6 mice remained healthy with no signs of autoimmunity or weight loss despite sustained (100 d) B7 blockade, which is in contradistinction to rapidly progressive insulitis and diabetes that develop with B7 neutralization in NOD mice (9, 10). Thus, diminished Treg levels with B7 costimulation deprivation does not uniformly trigger autoimmunity.
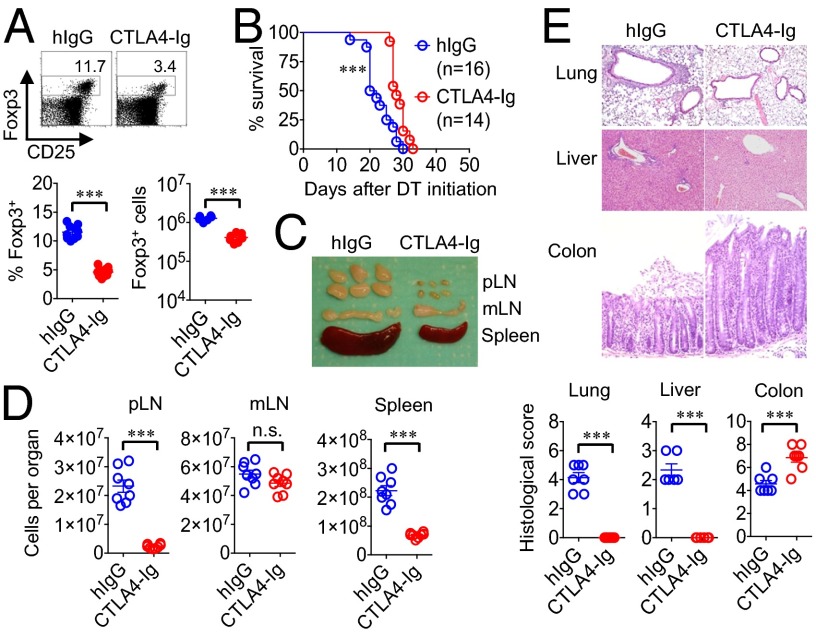
Fig. 1.
Tregs remaining after CTLA4-Ig B7 blockade selectively avert intestinal inflammation. (A) Number and percentage of Foxp3+ among CD4+ splenocytes in B6 mice treated with CTLA4-Ig or human IgG isotype (hIgG) antibody for 10 d. (B) Survival for Foxp3DTR mice treated with CTLA4-Ig or hIgG (500 μg per mouse weekly) beginning 1 d before initiating sustained Treg ablation. (C) Enlargement of peripheral lymph nodes (pLN) (inguinal, axillary, brachial), mesenteric lymph nodes (mLN), and spleen for CTLA4-Ig– or hIgG-treated Foxp3DTR mice day 12 after initiating sustained Treg ablation. (D) Recoverable cell numbers in each tissue for mice described in C. (E) Representative tissue histology after H&E staining and cumulative analysis of inflammation disease score for mice described in C. These data are representative of at least three independent experiments, each with similar results. Error bars indicate mean ± SEM. ***P < 0.001.
To mimic the pathologic defects in immune tolerance among individuals with autoimmunity (17–20), the additional impacts of Treg depletion with B7 blockade were evaluated. Using Foxp3DTR transgenic mice that coexpress the high-affinity human diphtheria toxin receptor with Foxp3, allowing targeted depletion of Tregs with low-dose diphtheria toxin (DT) administration (16), we found fatal autoimmunity induced by sustained Treg ablation among CTLA4-Ig–treated compared with isotype antibody control mice occurred with significantly delayed kinetics, sparing inflammation in peripheral organs (e.g., lung, liver) (Fig. 1 B–E). Interestingly, despite near complete absence of inflammation in other tissues, there was more pronounced colonic glandular elongation, intestinal crypt hyperplasia, and selective enlargement with increased number of mesenteric lymph node (mLN) cells in CTLA4-Ig–treated mice (Fig. 1 C–E). Comparatively, Treg ablation among isotype antibody control mice caused generalized wasting; conjunctivitis; scaly skin; and diffuse inflammation in the lung, liver, and intestine, along with splenomegaly, nonselective enlargement and cell accumulation within all peripheral lymph nodes (Fig. 1 C–E). Thus, although B7 neutralization efficiently prevents extraintestinal autoimmunity induced by Treg depletion, it does not avert, but rather exaggerates, intestinal inflammation.
To investigate if these findings are unique to CTLA4-Ig B7 blockade or are more broadly representative of autoimmunity that occurs in the absence of B7 costimulation, mice with targeted deficiency in B7-1/B7-2 were used (21). Compared with ∼50% diminished Tregs in mice with individual B7-1 or B7-2 defects, more prominent reductions were found with combined B7-1/B7-2 deficiency as Tregs declined by ∼90% compared with B6 control mice (Fig. 2A and SI Appendix, Fig. S1) (22). Interestingly, along with reduced expression of cell-intrinsic activation and proliferation markers by peripheral Tregs remaining among splenocytes in B7-deficient mice or after CTLA4-Ig B7 blockade, Helios and Neuropilin-1 expression were each also diminished among Foxp3+ splenocytes, but not Foxp3+ thymocytes, suggesting the selective retention of peripherally induced Tregs with B7 deprivation (Fig. 2B and SI Appendix, Figs. S2 and S3) (23, 24). These peripheral Treg shifts reflect an environment devoid of B7 costimulation rather than T-cell–intrinsic B7 defects that may also control activation (25) because CD4+ T cells reconstituted from B7-deficient compared with WT bone marrow progenitors each comprise ∼10% phenotypically identical Tregs in sublethally irradiated WT recipients (SI Appendix, Fig. S4). Remarkably however, purified Tregs retained in B7-deficient mice were equally potent on a per-cell basis in suppressing proliferation of responder T cells in coculture (SI Appendix, Fig. S5), suggesting that these Tregs are likely needed for averting residual immune activation that occurs despite B7 deprivation.
Fig. 2.
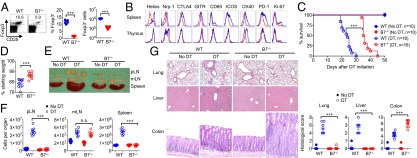
Phenotypically distinct Tregs retained in B7-deficient mice protect against fatal intestinal inflammation. (A) Number and percentage of Foxp3+ among CD4+ splenocytes in WT mice or mice with combined defects in B7-1 and B7-2 (B7-deficient). (B) Histogram plots illustrating expression of each marker by Foxp3+ cells from WT (blue line) compared with B7-deficient (red line) mice among CD4+ splenocytes compared with CD4+ thymocytes. (C) Survival for WT or B7-deficient Foxp3DTR mice after initiating sustained DT treatment or no DT controls. (D) Percentage of starting weight for WT compared with B7-deficient Foxp3DTR mice day 20 after initiating sustained Treg ablation. (E) Enlargement of peripheral lymph nodes (pLN) (inguinal, axillary, brachial), mesenteric lymph nodes (mLN), and spleen for each group of mice day 12 after initiating sustained Treg ablation. (F) Recoverable cell numbers in each tissue for mice described in E. (G) Representative tissue histology after H&E staining and cumulative analysis of inflammation disease score for mice described in E. These data are representative of at least three independent experiments each with similar results. Error bars indicate mean ± SEM. ***P < 0.001; n.s., not significant.
Accordingly, to more definitively investigate the in vivo necessity of Tregs retained in the absence of B7 costimulation, Foxp3DTR mice were intercrossed with B7-deficient mice. After sustained Treg ablation, mortality was significantly delayed in B7-deficient compared with WT mice, and occurred even later compared with Treg depletion in mice administered CTLA4-Ig (Fig. 1B and Fig. 2C). Furthermore, Treg ablation in B7-deficient mice caused diminished weight loss and protection from manifestations of the scurfy phenotype (generalized wasting, conjunctivitis, scaly skin, splenomegaly, diffuse lymphadenopathy), but instead triggered markedly more prominent colonic inflammation and enlargement of the draining mLN while sparing inflammation in extraintestinal tissues (e.g., lung, liver, pancreas, salivary glands) even at more extended time points when mice in each group became moribund (Fig. 2 D–G and SI Appendix, Fig. S6). The focused pathology confined to the intestine after Treg depletion is consistent with enriched Tregs in the lamina propria of B7-deficient mice, where Treg reductions were considerably less prominent compared with other tissues (e.g., liver, lung, blood, thymus) (SI Appendix, Fig. S7). Together, these findings with B7-deficient mice or CTLA4-Ig B7 blockade show that the necessity for Tregs in averting autoimmunity in extraintestinal tissues is bypassed when B7 costimulation is abolished, whereas remaining peripherally induced Tregs selectively protect against colonic inflammation that, with time, is equally fatal.
B7 Deprivation Primes Th17 Effector CD4+ T-Cell Differentiation Through ICOSL and OX40L.
Given the unique pathological features induced by Treg depletion in B7-deficient compared with WT mice, potential differences in differentiation between remaining Foxp3− effector T cells were evaluated. Focusing initially on mLN cells because this tissue becomes comparably enlarged in both groups of mice (Fig. 2 E and F), we found that Treg ablation in B7-deficient mice unleashed robust expansion of IL-17–producing or IL-17/IFN-γ double-positive CD4+ T cells, whereas Treg ablation in WT mice primarily induced IFN-γ–dominated Th1 differentiation (26) (Fig. 3A). This discordance in differentiation was apparent beginning 5 d after initiating Treg ablation and became progressively more pronounced until each group of mice succumbed to autoimmunity (Fig. 3A). Comparatively, other CD4+ T-cell lineage-defining cytokines remained at background levels after Treg ablation in B7-deficient mice, whereas modest but detectable levels of IL-4 and IL-10 production were found among CD4+ T cells in WT mice (SI Appendix, Fig. S8). Importantly, Th17 polarization could not be explained by developmental defects in B7-deficient mice, but rather reflects the necessity for ongoing B7 costimulation as CTLA4-Ig B7 blockade in WT mice triggered similarly exaggerated IL-17 production with reciprocally reduced IFN-γ levels (SI Appendix, Fig. S9).
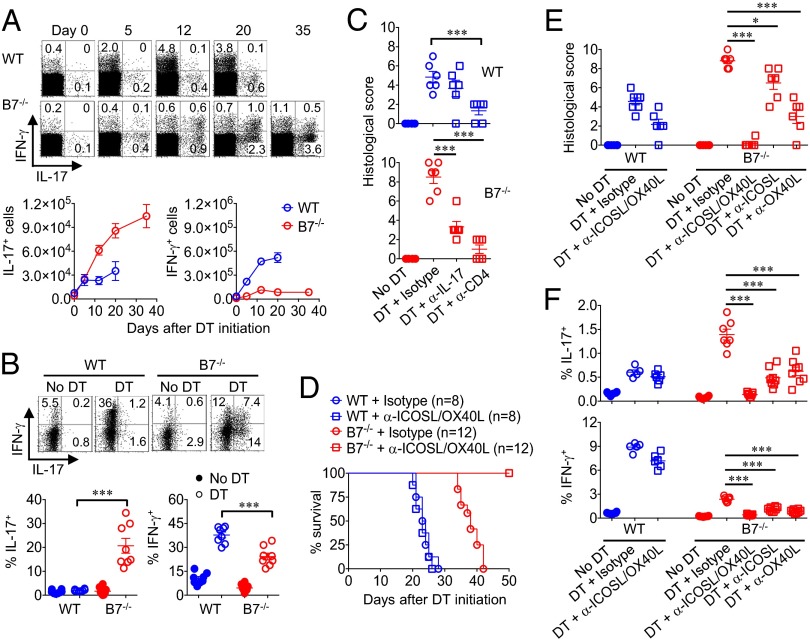
Fig. 3.
B7 deprivation drives expansion of Th17 CD4+ T cells through ICOSL/OX40L stimulation. (A) Percentage and number of IL-17– or IFN-γ–producing mesenteric lymph node CD4+ T cells in WT compared with B7-deficient Foxp3DTR mice at the indicated time points after initiating sustained Treg ablation. (B) Percentage of IL-17– or IFN-γ–producing lamina propria CD4+ T cells in WT compared with B7-deficient Foxp3DTR day 12 after initiating sustained Treg ablation. (C) Colonic inflammation disease score in WT (Upper) compared with B7-deficient Foxp3DTR (Lower) mice treated with either anti–IL-17A, anti-CD4, or isotype control antibody day 12 after concurrent DT administration for Treg ablation. (D) Survival for WT or B7-deficient Foxp3DTR mice after initiating sustained DT treatment and administration of anti-ICOSL/OX40L or isotype control antibodies. (E) Colonic inflammation disease score day 12 after initiating sustained Treg ablation for each group of mice described in D. (F) Percentage of IL-17– or IFN-γ–producing mesenteric lymph node CD4+ T cells day 12 after initiating sustained Treg ablation for each group of mice described in D. These data are representative of at least three independent experiments, each with similar results. Error bars indicate mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Considering that Treg ablation in B7-deficient mice causes inflammation confined to the intestine, the potential for spatial differences in T-cell differentiation were investigated by comparing cytokine production by CD4+ T cells recovered from the lamina prioria and spleen. We found that accumulation of IL-17–producing cells became even more pronounced in the lamina propria where ∼20% of CD4+ T cells produced IL-17, whereas IL-17–producing cells declined to only ∼1% for CD4+ splenocytes (Fig. 3B and SI Appendix, Fig. S10). In turn, IL-17 neutralization or CD4+ cell depletion reduced to near completion colitis induced by Treg depletion in B7-deficient mice, establishing a causative role for these immune components in intestinal inflammation (Fig. 3C). Thus, although Tregs are essential for averting catastrophic Th1 multiorgan systemic autoimmunity with unopposed B7 costimulation, Tregs retained with B7 deprivation selectively restrain colonic inflammation caused by Th17 CD4+ T cells.
To investigate whether Th17 polarization with B7 deprivation represents the default differentiation pathway or is driven by accessory costimulation molecules such as ICOSL or OX40L previously implicated in intestinal inflammation (12–15), expression of each molecule by CD11c+ dendritic cells in the lamina propria, mLN, and spleen were evaluated. ICOSL and OX40L were each constitutively expressed by lamina propria CD11c+ cells regardless of B7 deprivation or Treg ablation, whereas Treg depletion in WT mice selectively induced expression of both by dendritic cells in the mLN and spleen (SI Appendix, Fig. S11). To further address the functional importance of cell activation through ICOSL and OX40L, the impact of neutralizing these molecules on autoimmunity was investigated. We found that mortality, colonic inflammation, and mLN Th17 CD4+ T-cell accumulation induced by Treg ablation in B7-deficient mice were each eliminated by ICOSL/OX40L co-neutralization, whereas anti-ICOSL and anti-OX40L antibodies had no significant effects on Th1-dominated autoimmunity triggered by Treg ablation in WT mice (Fig. 3 D–F and SI Appendix, Fig. S12). Furthermore, in the absence of B7 costimulation, ICOSL and OX40L play additive and nonoverlapping roles because neutralizing each individually caused significant, but only partial, reductions in IL-17 production and intestinal inflammation (Fig. 3 E and F; SI Appendix, Fig. S12). Together, these results demonstrate that Tregs retained with B7 deprivation suppress fatal intestinal inflammation by ICOSL/OX40L-activated Th17 CD4+ T cells.
Th17 Cells with Commensal Specificity Drive Intestinal Inflammation in the Absence of B7 Costimulation.
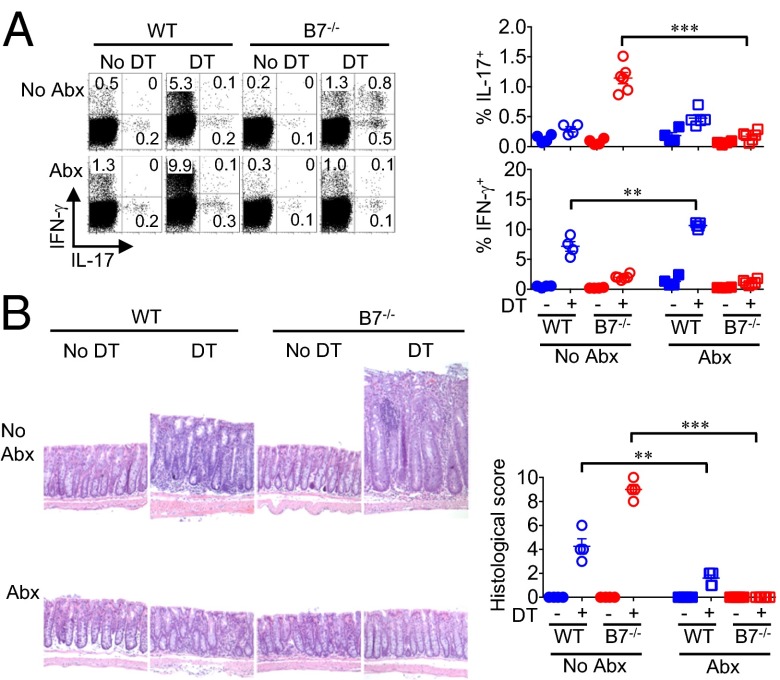
To investigate why the colon is uniquely susceptible to inflammation despite B7 deprivation, the role of commensal microbes that selectively colonize this tissue at very high density was evaluated (11). One week before initiating Treg ablation, an antibiotic mixture that eliminates commensal bacteria to near completion was added to the drinking water (27, 28) (SI Appendix, Fig. S13A). Remarkably, Th17 CD4+ T-cell expansion and intestinal inflammation induced by Treg ablation in B7-deficient mice were each eliminated with concurrent eradication of commensal bacteria (Fig. 4 A and B), demonstrating that commensal enteric bacteria predispose the intestine to Th17 inflammation that requires suppression by Tregs retained with B7 deprivation. Conversely, WT mice succumbed to autoimmunity with significantly increased proportions of IFN-γ–producing CD4+ T cells and reduced colitis after antibiotic treatment, confirming the dominant role that Tregs play in averting multiorgan systemic autoimmunity even under germ-free conditions (Fig. 4 A and B) (29).
Fig. 4.
Eradication of commensal enteric bacteria overrides Th17 intestinal inflammation in B7-deficient mice. (A) Percentage of IL-17– or IFN-γ–producing mesenteric lymph node CD4+ T cells from WT or B7-deficient mice treated with ampicillin, gentamycin, metronidazole, neomycin, and vancomycin in the drinking water compared with no antibiotic treatment control mice day 12 after initiating sustained Treg ablation. (B) Representative colonic tissue histology after H&E staining and cumulative analysis of inflammation disease score for mice described in A. These data are representative of at least three independent experiments, each with similar results. Error bars indicate mean ± SEM. **P < 0.01; ***P < 0.001.
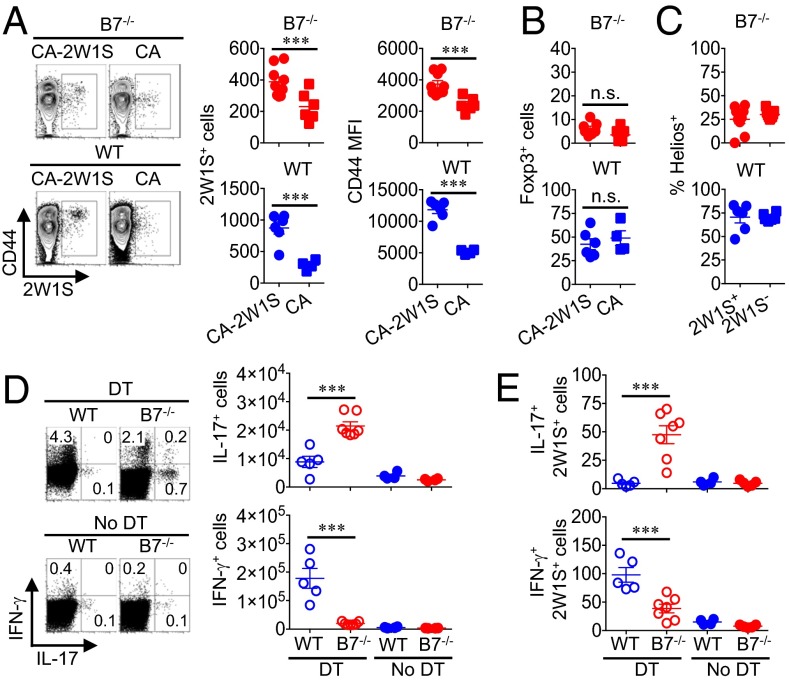
To further address the specificity of CD4+ T cells that cause intestinal inflammation, antibiotic-treated mice were recolonized with Candida albicans, representing a natural commensal microbe not susceptible to antibiotics used to eradicate enteric bacteria (30). We found that a single oral inoculation of C. albicans 1 week after initiating antibiotic supplementation established sustained high-density intestinal colonization (SI Appendix, Fig. S13B). Using recombinant C. albicans expressing the I-Ab:2W1S52–68 peptide (CA-2W1S) that exploits the high precursor frequency of endogenous CD4+ T cells with this specificity (31, 32), CD4+ T cells with 2W1S-surrogate commensal specificity expand and upregulate CD44 expression after colonization with CA-2W1S compared with the nonrecombinant parental strain, illustrating antigen-specific recognition of commensal C. albicans in both B7-deficient and WT mice (Fig. 5A). Interestingly, however, the majority of CD4+ T cells with commensal 2W1S specificity remained Foxp3−, whereas 2W1S+ Tregs did not accumulate appreciably after CA-2W1S colonization in either B7-deficient or WT mice (Fig. 5B). Consistent with the lack of expansion among Tregs with this commensal specificity, Helios expression by 2W1S+ compared with bulk Tregs also did not shift significantly with CA-2W1S intestinal colonization (Fig. 5C). Thus, commensal C. albicans primes expansion of antigen-specific effector, but not Tregs, regardless of B7 costimulation.
Fig. 5.
Tregs retained in B7-deficient mice suppress expansion of Th17 CD4+ T cells with commensal specificity. (A) Expansion and CD44 expression among I-Ab:2W1S52–68 tetramer-positive CD4+ T cells day 20 after recolonization with C. albicans-2W1S or nonrecombinant C. albicans in B7-deficient or WT mice. (B) Number of Foxp3+ CD4+ T cells with I-Ab:2W1S52–68 specificity for each group of mice described in A. (C) Helios expression among Foxp3+ Tregs with 2W1S commensal specificity compared with bulk (2W1S−) Tregs day 20 after C. albicans-2W1S recolonization. (D) Number of IL-17– or IFN-γ cytokine–producing mesenteric lymph node CD4+ T cells day 12 after initiating Treg ablation in WT compared with B7-deficient Foxp3DTR mice, each colonized with C. albicans-2W1S. (E) Number of IL-17– or IFN-γ cytokine–producing mesenteric lymph node CD4+ T cells with I-Ab:2W1S52–68 specificity for mice described in D. These data are representative of at least three independent experiments each with similar results. Error bars indicate mean ± SEM. ***P < 0.001; n.s., not significant.
These studies tracking CD4+ T cells with commensal 2W1S specificity were extended to address the specificity of cytokine-producing effector cells unleashed by Treg ablation. Although CD4+ T-cell IL-17 and IFN-γ production were restored among antibiotic-treated mice after recolonization with CA-2W1S, cytokine production remained under active Treg suppression as only background levels were found without Treg depletion (Fig. 5D). Focusing on cells with commensal 2W1S specificity showed sharply expanded numbers of IL-17–producing CD4+ T cells in B7-deficient mice and reciprocally increased IFN-γ–producing CD4+ T cells in WT mice (Fig. 5E). Interestingly, however, recolonization of antibiotic-treated mice with C. albicans alone did not restore susceptibility to colitis, which most likely reflects antibiotic-induced downregulation of ICOS expression by lamina propria CD4+ T cells that is not restored by commensal C. albicans recolonization alone (SI Appendix, Fig. S14). Nonetheless, these results collectively show that, although eliminating B7 costimulation overrides the necessity for Tregs in averting multiorgan systemic autoimmunity, a ICOSL/OX40L-driven Th17 response to commensal microbes sustains the need for peripherally induced Tregs that protect against localized intestinal inflammation.
Discussion
The B7-1/B7-2 costimulatory molecules play paradoxical and somewhat contradictory roles in immune activation. On one hand, T-cell activation facilitated by B7 costimulation has become widely exploited with B7-neutralizing therapies in a variety of human autoimmune disorders (2–6). On the other hand, B7 costimulation also maintains Tregs at expanded levels that averts multiorgan systemic autoimmunity (9, 10). Thus, B7 blockade either can silence T-cell activation by neutralizing cell-intrinsic positive costimulatory signals or unleash T-cell activation by diminishing immune-suppressive Tregs. To dissect how these discordant properties work together, the interplay between B7 costimulation, Tregs, and autoimmunity was evaluated. We show that the necessity for Tregs in averting multiorgan systemic autoimmunity is bypassed with B7 deprivation using either B7-deficient mice or CTLA4-Ig B7 blockade. Interestingly, however, Treg ablation in B7-deficient mice still caused distinct pathological changes confined to the intestine with delayed fatal inflammation. The unique necessity for Treg suppression in this tissue despite B7 deprivation reflects commensal microbe-induced T-cell activation through the accessory costimulation molecules ICOSL and OX40L. Thus, these findings may provide an explanation for why intestinal inflammation, compared with other types of autoimmunity, is distinctively resistant to therapeutic B7 blockade (7).
Using this model where the interplay between Tregs, effector T cells, and commensal microbes can be evaluated in isolation without complications related to systemic autoimmunity that develop when Tregs are eliminated in a B7-replete environment, we find that C. albicans intestinal colonization primes expansion of CD4+ T cells with commensal specificity that does not require, but occurs more efficiently, with B7 costimulation. After Treg ablation, CD4+ T cells with C. albicans commensal specificity differentiate into Th17 or Th1 effector cells in B7-deficient or B7-sufficient mice, respectively. Thus, Foxp3− CD4+ T cells restrained from activation by Tregs are enriched with specificity to commensal microbes. Interestingly, however, Tregs with C. albicans specificity did not expand significantly in either B7-deficient or control mice. Nonetheless, Foxp3+ cells remain essential for protection against colitis in B7-deficient mice and systemic autoimmunity in B7-sufficient mice because each remained healthy before Treg ablation. At face value, these results suggesting that Tregs with commensal specificity play nonessential roles in mitigating intestinal inflammation may seem inconsistent with the emerging consensus that enteric microbiota heavily influence the Treg repertoire in intestinal tissue that contains unique and nonoverlapping specificities compared with systemic Tregs (33). However, the use of mice with limited polyclonal repertoires required for discriminating specificity based on TCR-α sequencing may limit their broader applicability (34, 35), especially considering the enormous diversity in TCR-α/β chain use among endogenous cells with specificity for the same antigen (32). This limitation is bypassed in our current studies using MHC class II tetramer for identifying CD4+ T cells with commensal specificity.
However, our findings could also be limited by tracking CD4+ T cells exclusively primed with C. albicans that may not be representative of commensal microbes that induce Tregs through production of short-chain fatty acids and other metabolites (36–38). If Treg induction is restricted only to these bacterial species that are eliminated by antimicrobials used in our current studies, it may not be surprising that C. albicans colonization does not prime appreciable expansion of Tregs with commensal fungal specificity (30). This would be analogous to segmented filamentous bacteria that also do not prime appreciable Treg expansion, but Th17 effectors instead (39). Using mice eradicated of commensal bacteria, we also made the curious observation that re-establishing intestinal colonization with C. albicans alone does not induce ICOS expression by lamina propria CD4+ T cells. Whether this reflects discordant cell activation induced by C. ablicans compared with commensal enteric bacteria through TLR4 or NFAT that each promote CD4+ T-cell ICOS expression is uncertain, but represents an important area for future investigation (40, 41).
The shift toward Th17 polarization with reciprocally reduced CD4+ T-cell IFN-γ, IL-4, and IL-10 production we find after Treg ablation in B7-deficient mice is consistent with the more closely linked developmental origins between inducible Tregs and Th17 compared with other effector CD4+ T-cell lineages (42), and the necessity for CD28 in IFN-γ, IL-4, and IL-10 production by CD4+ T cells from scurfy mice with functional Foxp3 defects (43). Thus, selective expansion of IL-17–producing CD4+ T cells with deprivation of B7 or CD28 might suggest that Th17 represents the default differentiation pathway for naive CD4+ T cells when Treg suppression and costimulation are simultaneously eliminated. However, our findings show that Th17 differentiation in the absence of B7 still requires cell activation through the accessory costimulatory molecules ICOSL and OX40L. Applied to the discordant therapeutic efficacy of CTLA4-Ig B7 blockade in organ-specific autoimmunity (2–7), these results suggest that neutralizing ICOSL and OX40L, together with B7, may be needed for extinguishing inflammation in tissues like the intestine that retain prominent B7-independent pathways for T-cell activation.
Materials and Methods
Mice.
C57BL/6 (B6) mice, mice with individual or combined defects in B7-1 and B7-2 (21), or mice expressing the CD45.1+ or CD90.1+ congenic markers were purchased from Jackson Laboratory. Foxp3DTR mice coexpressing DTR plus GFP with Foxp3 were backcrossed over 15 generations to B6 mice (16). B7-deficient Foxp3DTR mice were generated by intercrossing B7-deficient mice with Foxp3DTR mice. For Treg ablation, Foxp3DTR mice were treated with DT (25 μg/kg initial dose followed by 5 μg/kg daily thereafter). For B7 blockade, CTLA4-Ig (Orencia Bristol-Meyers Squibb) or human IgG (BioXcell) was administrated i.p. (500 μg weekly) (44). For in vivo antibody neutralization, anti–IL-17 (17-F3), anti-CD4 (GK1.5), anti-ICOSL (HK5.3), anti-OX40L (RM134L), or rat IgG antibodies (BioXcell) were administrated i.p. (500 μg twice weekly). All experiments were performed under the guidelines of the University of Minnesota or Cincinnati Children’s Hospital institutional animal care and use committee-approved protocols.
Flow Cytometry and Histology.
Antibodies for cell surface and intracellular or intranuclear staining were purchased from eBioscience, BD Biosciences, BioLegend, or R&D Systems, and are more fully described in SI Appendix. Staining with PE-conjugated I-Ab:2W1S52–68 tetramer and enrichment using anti-PE beads were performed as described (32). For cytokine production, cells were stimulated with phorbol myristate acetate/ionomycin in media supplemented with GolgiPlug (BD Biosciences) for 5 h. For histology, each tissue was fixed with paraformaldehyde and embedded in paraffin, cut to 5-μM sections, and stained with H&E. Tissue inflammation scoring was performed in a blinded fashion using previously reported parameters (29, 45–47), which are more fully described in SI Appendix.
Tissue Cell Isolation.
Protocols used for cell isolation from lung, liver, and intestine were performed as described (28), and more are comprehensively outlined in SI Appendix.
In Vitro Treg Suppression Assay.
For enumerating Treg-suppressive potency, GFP+ Tregs were purified among spleen and lymph node cells in Foxp3DTR-GFP WT or B7-deficient mice by initial enrichment for CD4+ cells through negative selection (Miltenyi Biotec) followed by FACS sorting for GFP cells. For each experiment, the Treg purity was verified to be >95% by staining using anti-Foxp3 antibody. Responder T cells isolated from naive CD90.1 mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) and cocultured in triplicate with purified GFP+ Tregs at the indicated ratios in the presence of anti-CD3 antibody (1 μg/mL) for 3 d. The relative suppressive potency of Tregs was calculated by comparing responder cell proliferation (CFSE dilution) as described (48).
Antibiotics and C. albicans.
Autoclaved drinking water was supplemented with ampicillin (0.5 mg/mL), gentamicin (0.5 mg/mL), metronidazole (0.5 mg/mL), neomycin (0.5 mg/mL), and vancomycin (0.25 mg/mL) 7 d before Treg ablation and/or C. albicans inoculation and continued for the duration of the experiment as described (27). Recombinant C. albicans expressing I-Ab:2W1S52–68 peptide or the parental strain (SC5314) (31) were grown in yeast extract peptone dextrose plus acetate (YPAD) media, washed and resuspended in saline, and inoculated by oral gavage (2 × 106 cfu in 20 μL). For some experiments, sustained Treg ablation with DT was initiated 7 d after C. albicans inoculation. For enumerating C. albicans in the feces, the fecal pellet was weighed, dissolved in saline by vigorous vortex, and spread onto YPAD agar plates supplemented with the same antibiotic mixture used for drinking-water supplementation to prevent bacterial growth.
Statistical Analysis.
The differences in survival between groups were analyzed using the log-rank (Mantel–Cox) test. The number and percentage of cells in each group were first analyzed and found to be normally distributed. Thereafter, differences between groups were analyzed using the unpaired Student t test (two groups) or ANOVA with Dunnett correction for multiple comparisons (more than two groups) with statistical significance indicated as *P < 0.05, **P < 0.01, and ***P < 0.001.
Supplementary Material
Acknowledgments
We thank Drs. J. Berman and D. Kaplan (University of Minnesota) for providing recombinant C. albicans-2W1S; Dr. A. Rudensky (Memorial Sloan Kettering) for providing Foxp3DTR mice; and Drs. D. Haslam, S. Hogan, and A. Miethke (Cincinnati Children’s Hospital) for helpful discussions. This research was supported in part by the National Institutes of Health–National Institute of Allergy and Infectious Diseases under Awards R01AI100934, R01AI087830, and R21AI112186, and National Institute of Diabetes and Digestive and Kidney Diseases under Award P30DK078392. S.S.W. holds a Pathogenesis in Infectious Disease award from the Burroughs Wellcome Fund.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402336111/-/DCSupplemental.
References
- 1.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci USA. 1999;96(1):185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kremer JM, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349(20):1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 3.Orban T, et al. Type 1 Diabetes TrialNet Abatacept Study Group Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled trial. Lancet. 2011;378(9789):412–419. doi: 10.1016/S0140-6736(11)60886-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mease P, et al. Abatacept in the treatment of patients with psoriatic arthritis: Results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum. 2011;63(4):939–948. doi: 10.1002/art.30176. [DOI] [PubMed] [Google Scholar]
- 5.Viglietta V, et al. CTLA4Ig treatment in patients with multiple sclerosis: An open-label, phase 1 clinical trial. Neurology. 2008;71(12):917–924. doi: 10.1212/01.wnl.0000325915.00112.61. [DOI] [PubMed] [Google Scholar]
- 6.Merrill JT, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: Results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(10):3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 7.Sandborn WJ, et al. Abatacept for Crohn’s disease and ulcerative colitis. Gastroenterology. 2012;143(1):62–69. doi: 10.1053/j.gastro.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Amezcua-Guerra LM, Hernández-Martínez B, Pineda C, Bojalil R. Ulcerative colitis during CTLA-4Ig therapy in a patient with rheumatoid arthritis. Gut. 2006;55(7):1059–1060. doi: 10.1136/gut.2006.095539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salomon B, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 10.Tang Q, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171(7):3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 11.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 12.Malmström V, et al. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166(11):6972–6981. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 13.Totsuka T, et al. Ameliorating effect of anti-inducible costimulator monoclonal antibody in a murine model of chronic colitis. Gastroenterology. 2003;124(2):410–421. doi: 10.1053/gast.2003.50050. [DOI] [PubMed] [Google Scholar]
- 14.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207(4):699–709. doi: 10.1084/jem.20091618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Jong YP, et al. Blocking inducible co-stimulator in the absence of CD28 impairs Th1 and CD25+ regulatory T cells in murine colitis. Int Immunol. 2004;16(2):205–213. doi: 10.1093/intimm/dxh019. [DOI] [PubMed] [Google Scholar]
- 16.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 17.Carbone F, et al. Regulatory T cell proliferative potential is impaired in human autoimmune disease. Nat Med. 2014;20(1):69–74. doi: 10.1038/nm.3411. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz DA. Regulatory T cells in systemic lupus erythematosus: Past, present and future. Arthritis Res Ther. 2008;10(6):227. doi: 10.1186/ar2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long SA, Buckner JH. CD4+FOXP3+ T regulatory cells in human autoimmunity: More than a numbers game. J Immunol. 2011;187(5):2061–2066. doi: 10.4049/jimmunol.1003224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28(5):687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borriello F, et al. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6(3):303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 22.Zeng M, Guinet E, Nouri-Shirazi M. B7-1 and B7-2 differentially control peripheral homeostasis of CD4(+)CD25(+)Foxp3(+) regulatory T cells. Transpl Immunol. 2009;20(3):171–179. doi: 10.1016/j.trim.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exper Med. 2012;209(10):1713–1722, S1–S19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suscovich TJ, Perdue NR, Campbell DJ. Type-1 immunity drives early lethality in scurfy mice. Eur J Immunol. 2012;42(9):2305–2310. doi: 10.1002/eji.201242391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abt MC, et al. Commensal bacteria calibrate the activation threshold of innate antiviral immunity. Immunity. 2012;37(1):158–170. doi: 10.1016/j.immuni.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elahi S, et al. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504(7478):158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chinen T, Volchkov PY, Chervonsky AV, Rudensky AY. A critical role for regulatory T cell-mediated control of inflammation in the absence of commensal microbiota. J Exp Med. 2010;207(11):2323–2330. doi: 10.1084/jem.20101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iliev ID, et al. Interactions between commensal fungi and the C-type lectin receptor Dectin-1 influence colitis. Science. 2012;336(6086):1314–1317. doi: 10.1126/science.1221789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Igyártó BZ, et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35(2):260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moon JJ, et al. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27(2):203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nutsch KM, Hsieh CS. T cell tolerance and immunity to commensal bacteria. Curr Opin Immunol. 2012;24(4):385–391. doi: 10.1016/j.coi.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lathrop SK, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478(7368):250–254. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cebula A, et al. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature. 2013;497(7448):258–262. doi: 10.1038/nature12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Atarashi K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500(7461):232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 37.Arpaia N, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504(7480):451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furusawa Y, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 39.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shalaby KH, et al. ICOS-expressing CD4 T cells induced via TLR4 in the nasal mucosa are capable of inhibiting experimental allergic asthma. J Immunol. 2012;189(6):2793–2804. doi: 10.4049/jimmunol.1201194. [DOI] [PubMed] [Google Scholar]
- 41.Tan AH, Wong SC, Lam KP. Regulation of mouse inducible costimulator (ICOS) expression by Fyn-NFATc2 and ERK signaling in T cells. J Biol Chem. 2006;281(39):28666–28678. doi: 10.1074/jbc.M604081200. [DOI] [PubMed] [Google Scholar]
- 42.Weaver CT, Hatton RD. Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective. Nat Rev Immunol. 2009;9(12):883–889. doi: 10.1038/nri2660. [DOI] [PubMed] [Google Scholar]
- 43.Singh N, et al. Role of CD28 in fatal autoimmune disorder in scurfy mice. Blood. 2007;110(4):1199–1206. doi: 10.1182/blood-2006-10-054585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ertelt JM, et al. B7-1/B7-2 blockade overrides the activation of protective CD8 T cells stimulated in the absence of Foxp3+ regulatory T cells. J Leukoc Biol. 2013;94(2):367–376. doi: 10.1189/jlb.0313118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harmel-Laws E, Mann EA, Cohen MB, Steinbrecher KA. Guanylate cyclase C deficiency causes severe inflammation in a murine model of spontaneous colitis. PLoS ONE. 2013;8(11):e79180. doi: 10.1371/journal.pone.0079180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rivas MN, et al. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122(5):1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of epithelial RelA results in deregulated intestinal proliferative/apoptotic homeostasis and susceptibility to inflammation. J Immunol. 2008;180(4):2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 48.Ertelt JM, et al. Foxp3+ regulatory T cells impede the priming of protective CD8+ T cells. J Immunol. 2011;187(5):2569–2577. doi: 10.4049/jimmunol.1100374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.