Significance
Axons within the mammalian central nervous system must navigate with high accuracy over long distances. Tissue polarity (also called planar cell polarity) signaling is emerging as a major axon guidance system. Frizzled3 (Fz3), a core polarity gene, is shown here to be essential for a wide variety of axon guidance decisions in the mouse brain and spinal cord beginning with the earliest axon trajectories in the day 12 embryo. In particular, this work shows that axon guidance defects in Fz3 mutant embryos involve erroneous directions of growth rather than axon elongation per se. In adult mice in which the spinal cord is missing Fz3, anatomic and behavioral analyses show that sensory information from the limbs does not reach the brain.
Keywords: planar cell polarity, Cre/loxP
Abstract
Targeted mutation of the Frizzled3 (Fz3) gene in mice has been shown to disrupt the growth and guidance of a subset of peripheral and central axons. Here we used conditional deletion of Fz3 to explore the forebrain territories in which Fz3 action is required for the development of the anterior commissure and the corticothalamic, corticospinal, and thalamocortical tracts. Experiments with region-specific deletion of Fz3 using a variety of Cre lines show that proper routing of corticothalamic and thalamocortical axons in the internal capsule requires Fz3 expression in the ventral telencephalon. The pattern of defects among forebrain axon tracts that are induced by conditional deletion of Fz3 conforms closely to the pattern previously observed with analogous conditional deletion of Celsr3, implying a close mechanistic link between Fz3 and Celsr3 in axon guidance. We further found that several central nervous system axon tracts require Fz3 function as early as embryonic day 11.5, and that Fz3 is required for pathfinding by dopaminergic and serotonergic axons in the brain and by a subset of optic tract axons. In addition, conditional deletion of Fz3 in all tissues caudal to the neck eliminates the spinothalamic tract and the transmission of somatosensory information from the spinal cord to the brain, as determined by neuroanatomic tracing and behavioral testing.
Complexity within the mammalian nervous system can be roughly divided into two categories: cell type identity and morphology/connectivity. On its largest scale, the second category is exemplified by the many distinct patterns of long-range axonal trajectories within the adult CNS. The guidance mechanisms responsible for these axonal trajectories have been an object of longstanding interest to neurobiologists, and, over the past 20 y, a wide variety of attractive and repulsive axon guidance systems have been identified (1). For a few well-defined axon growth and guidance decisions—such as dorsal vs. ventral growth of limb motor axons and midline crossing of retinal ganglion cell (RGC) and spinal sensory axons—the roles of several guidance systems, including Slit/Robo, Ephrin/Eph, and Semaphorin/Plexin, have been intensively investigated (2, 3).
One recently discovered axon growth and guidance system uses some of the same molecular components that were identified in the context of epithelial polarity determination, a process referred to as tissue polarity or planar cell polarity (PCP). As first defined in Drosophila, PCP controls polarity within the plane of the epithelium and is mediated by a small number of integral membrane or membrane-associated proteins (4). Within the responsive epithelium, several PCP proteins are localized asymmetrically within each cell in a pattern that matches the large-scale vectorial asymmetry of the epithelium. In mice, two integral membrane PCP proteins, Frizzled3 (Fz3) and Celsr3, control a nearly identical set of axon growth and guidance processes, with loss-of-function mutations in either gene producing severe disruptions in the anterior commissure and the corticothalamic, thalamocortical, and nigrostriatal tracts (5–7). In the midgestation spinal cord, where the primary defect in Fz3−/− and Celsr3−/− axon development has been examined in detail, commissural sensory axons appear incapable of sensing the rostrocaudal vector after midline crossing (8). Recent work has revealed additional defects in Fz3−/− mice, including defective migration of a subset of neural crest cells, a failure of some cranial motor axons to reach their targets, and stalling of spinal motor axons that are destined to innervate the dorsal limb (9).
The present study addresses several open questions regarding the role of Fz3 in CNS axon guidance. What is the effect of selectively deleting Fz3 in the thalamus, cortex, striatum, retina, or spinal cord? What are the earliest defects in CNS axon guidance referable to loss of Fz3? Are there additional Fz3−/− axon growth/guidance phenotypes outside of the spinal cord that permit a distinction to be made between defective polarity and defective growth? Can selective disruption of particular axon tracts by regional deletion of Fz3 be used to study the functional role of those pathways? We address these questions by visualizing defined axon tracts at early and late gestational ages and by deleting Fz3 in selected CNS territories.
Results
Overview of Axon Tract Defects in the Fz3−/− Brain.
As noted earlier, previous experiments demonstrated that Fz3 is required for the development of several major axon tracts in the mouse forebrain (5, 6). These earlier experiments primarily used methods for visualizing axons, such as Neurofilament-M (NF) immunostaining, diffusion tensor MRI, and 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate tracing, that identify axons based on their locations but do not distinguish among axons based on biochemical or other molecular properties of the cell of origin. In regions where axons from different tracts are closely apposed or interdigitated—as seen, for example, with the corticothalamic and thalamocortical tracts—these methods do not distinguish the different classes of axons. To circumvent this limitation and obtain a more detailed and selective visualization of axon defects in Fz3−/− mice, we have supplemented these nonspecific methods of axon visualization with tract-specific methods, including Cre/loxP-based labeling of axons in a cell type- or region-specific manner, and immunostaining for the neurotransmitter-specific markers tyrosine hydroxylase (TH) and serotonin/5-hydroxytryptamine (5-HT) to visualize aminergic and serotonergic axons, respectively.
Fig. 1 compares the brain defects in Fz3−/− mice visualized by NF immunostaining with the trajectories of dorsal thalamic axons visualized with a RORα-IRES-Cre knock-in allele and a Cre-controlled human placental alkaline phosphatase (hPLAP; hereafter “AP”) reporter, R26iAP (10, 11). In the prenatal mouse brain, RORα is expressed in the dorsal thalamus (12). As Fz3−/− mice die shortly after birth, we performed these analyses on WT (Fz3+/− or Fz3+/+) and Fz3−/− brains at embryonic day (E) 18.5. The NF immunostaining shown in Fig. 1 A–G′ confirms and extends previous descriptions of the Fz3−/− brain phenotype (5, 6). In addition to the defects noted in the Introduction, the corticospinal tract was greatly reduced, the fasciculus retroflexus and the mammillothalamic tract were nearly completely absent, the medial lemniscus was less compact (Fig. 1 E and E′), and the regular matrix of axon bundles in the reticular formation was disorganized (Fig. 1 F–G′).
Fig. 1.
In the brain, multiple axon tracts are affected by loss of Fz3. (A–G′) NF immunostaining of E18.5 coronal brain sections showing the normal position of axon tracts in the Fz3+/− brain (arrows) and aberrant (white arrowheads) or missing axon tracts (orange arrowheads) in the Fz3−/− brain. These include the absence of subcortical and striatal axons (A and A′); absence of the anterior commissure (B and B′); misrouting of thalamocortical axons (C–D′); a nearly complete absence of the corticospinal tract, the fasciculus retroflexus, and the mammillothalamic tract; and poor fasciculation of the medial lemniscus (E and E′). (G and G′) Enlarged views of boxed regions in F and F′, respectively, showing disorganization of reticular formation axons. Arrows and arrowheads designated by numbers point to different axons/tracts: (1) axons in the cortical intermediate zone, (2) fibers in the striatum, (3) anterior commissure, (4) thalamocortical axons, (5) fibers in the tectum, (6) medial lemniscus, (7) fasciculus retroflexus, (8) mammillothalamic tract, (9) corticospinal tract, (10) aberrant fibers surrounding the globus pallidus, and (11) aberrant fibers within the external capsule. Throughout this study, orange arrowheads indicate missing axons/tracts; white arrowheads (in contrast with white arrows) and red arrows (in contrast with green arrows) indicate fiber tracts with defects [misrouted or diminutive or, in rare cases, poorly fasciculated (arrow 6 in E vs. arrowhead 6 in E′)]. GP, globus pallidus. (Scale bars: E′, 1 mm; G′, 200 µm.) (H–N′) Thalamocortical axons labeled by AP staining of consecutive coronal sections from E18.5 RORα-IRES-Cre;R26iAP;Fz3+/− and RORα-IRES-Cre;R26iAP;Fz3−/− brains. In the Fz3−/− brain, most thalamocortical axons descend ventrally and then form a U-shaped axon tract to innervate the contralateral thalamus (M′ and N′) or project laterally to the cortical marginal zone (J′–L′). Complete brain sections in M–N′ show the U-shaped thalamocortical tracts in the Fz3−/− brain. (Scale bars, 1 mm.) (O and P) Planes of sections in A–N′. (Q) Schematics showing trajectories of thalamocortical axons in WT (Left) and Fz3−/− (Right) brains. In the Fz3−/− brain, thalamocortical axons from the left and right hemibrains are drawn in red and blue, respectively, for better illustration of the reciprocal innervation from the contralateral thalamus.
AP histochemical staining of RORα-IRES-Cre;R26iAP;Fz3+/− and RORα-IRES-Cre;R26iAP;Fz3−/− brain sections showed strong and selective labeling of thalamocortical neurons and axons (Fig. 1 H–N′). In the RORα-IRES-Cre;R26iAP;Fz3−/− brain, virtually none of the thalamic axons enters the striatum as they do in the WT control. Instead, most of the Fz3−/− thalamic axons project ventrally and then either create a novel tract along the floor of the midbrain to innervate the contralateral thalamus or project laterally for a short distance along the surface of the cortex. A small subset of the Fz3−/− thalamic axons project dorsally and posteriorly to the colliculus (Fig. 1 M′ and Q).
Fz3 Is Required in Ventral Telencephalic Neurons for the Development of Corticothalamic, Corticospinal, and Thalamocortical Axons.
The major axon tracts in the mouse brain, including those affected by loss of Fz3, extend over long distances and are guided by extracellular attractive and repulsive cues, guidepost cells, and/or preexisting axons (13). Fz3 is widely expressed in the developing mouse brain (6), but it is not known which of its expression domains is required for the growth and guidance of particular axon tracts. To address this question, we asked whether Fz3 expression is required in cortical pyramidal, thalamic, or striatal neurons for the development of the anterior commissure, and the corticothalamic, thalamocortical, and corticospinal tracts. For these experiments, we combined a conditional Fz3 allele (Fz3CKO) (9) with transgene or knock-in alleles that express Cre in: (i) the dorsal thalamus (RORα-IRES-Cre) (10); (ii) the midbrain and hindbrain (Dbx1-IRES-Cre) (14); (iii) the dorsal telencephalon, including the neocortex (Emx1-IRES-Cre) (15); (iv) the ventral telencephalon, including a large region of the pallium (Dlx5/6-Cre) (16); and (v) the telencephalon but not the thalamus (Foxg1-Cre) (17). A detailed description of the topography of Cre action in several of these lines can be found in the work of Zhou et al. (18).
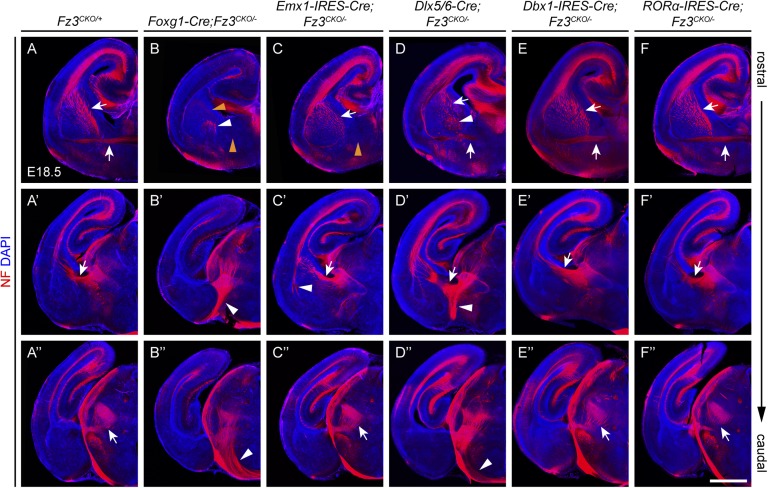
When Fz3 was inactivated throughout the telencephalon (Foxg1-Cre;Fz3CKO/−), the forebrain exhibited the full spectrum of axon defects seen in the Fz3−/− forebrain (Fig. 2 B–B″ and Fig. S1 A′–E′). In contrast, when Fz3 was deleted exclusively in the dorsal thalamus (RORα-IRES-Cre;Fz3CKO/−) or in the midbrain and hindbrain (Dbx1-IRES-Cre;Fz3CKO/−), no defects in axon tracts were observed (Fig. 2 E–F″). We note that, in these and other experiments in which Cre-mediated deletion of the Fz3CKO allele failed to produce a phenotype, any inferences regarding the dispensability of Fz3 action in the region in question must be considered tentative because (i) the onset of Cre expression could be delayed relative to the time window of Fz3 action or (ii) the level of Cre expression could be insufficient to eliminate Fz3 from a large enough fraction of the relevant cells.
Fig. 2.
The effect of region-specific inactivation of Fz3 on the development of major axon tracts in prenatal mouse brains. (A–F″) NF immunostaining of coronal sections from E18.5 brains in which Fz3 has been inactivated in the entire forebrain (Foxg1-Cre; B–B″), in the cerebral cortex (Emx1-IRES-Cre; C–C″), in the striatum (Dlx5/6-Cre; D–D″), in the midbrain and hindbrain (Dbx1-IRES-Cre; E–E″), and in the dorsal thalamus (RORα-IRES-Cre; F–F″). (A–A″) Phenotypically normal control sections. (A–F) Anterior commissure (lower arrows and arrowheads), axon tracts in the striatum (upper arrows and arrowhead), and aberrant axons surrounding the globus pallidus (middle white arrowheads in B and D). (A′–F″) More caudal images mainly show thalamocortical axons, except that the arrowhead in C′ points to aberrant axon tracts within the external capsule. (Scale bar, 1 mm.)
When Fz3 was ablated in the neocortex (Emx1-IRES-Cre;Fz3CKO/−), the posterior part of the anterior commissure was completely missing and axons with aberrant trajectories appeared in the external capsule (Fig. 2 C–C″ and Fig. S1 A″–E″). As other CNS axon tracts appear to be unaffected in Emx1-IRES-Cre;Fz3CKO/− mice, it is likely that cortical axons that would normally have formed the posterior part of the anterior commissure constitute the aberrant axons in the external capsule. With the caveats noted earlier regarding the timing and efficiency of Cre-mediated recombination, these observations suggest that Fz3 expression may not be required in all cortical and thalamic neurons for the development of the corticothalamic, corticospinal, and thalamocortical tracts.
The results described thus far are consistent with the possibility that corticothalamic, corticospinal, and thalamocortical axon pathfinding might require Fz3 function in the structures through which these tracts pass. We tested this possibility by eliminating Fz3 in the ventral telencephalon (Dlx5/6-Cre;Fz3CKO/−) and observed that the corticothalamic, corticospinal, and thalamocortical tracts showed defects to various extents, with aberrant axon trajectories around the globus pallidus, disorganized axon bundles in the internal capsule, a subset of thalamocortical axons descending ventrally, and a diminished corticospinal tract (Fig. 2 D–D″). The most severely affected brains showed few or no axons linking the neocortex and the thalamus (Fig. 3 A and B). Many Dlx5/6-Cre;Fz3CKO/− mice survived to adulthood but were runted. In adult Dlx5/6-Cre;Fz3CKO/− brains, the lateral ventricles were markedly enlarged, and, in the striatum, presumptive corticothalamic and/or thalamocortical axons were bundled together and associated with activated astrocytes (Fig. 3 C–D′).
Fig. 3.
Defects in embryonic and adult Dlx5/6-Cre;Fz3CKO/− brains and the effect of Fz3 loss of function on corridor cells. (A and B) E13.5 Dlx5/6-Cre;Fz3CKO/− brain with no axonal connection between the thalamus and cerebral cortex (arrow in A vs. arrowhead in B), the most severe phenotype seen in Dlx5/6-Cre;Fz3CKO/− brains. (Scale bar, 500 µm.) (C–D′) Control vs. Dlx5/6-Cre;Fz3CKO/− brains at postnatal day (P) 16 showing NF and glial fibrillary acid protein immunostaining in coronal sections. The Dlx5/6-Cre;Fz3CKO/− brain shows an enlarged lateral ventricle (arrow in C vs. arrowhead in D), closely bundled axons (right arrowhead in D′), and activated (i.e., glial fibrillary acid protein-expressing) astrocytes (left arrowhead in D′) in the striatum. Dashed lines delineate the edge of the brain (C and D); the continuous white lines encircle the lateral ventricle (C and D). (C′ and D′) Magnified views of boxed regions in C and D, respectively. (Scale bars: D, 1 mm; D′, 500 µm.) (E–F″) NF and Ebf1 immunostaining of E13.5 brain sections showing in the Fz3−/− brain Ebf1+ corridor cells encircling the globus pallidus (arrows in E′ and E″ vs. arrowheads in F′ and F″) and thalamocortical axons missing from the internal capsule (arrow in E vs. arrowhead in F). (Scale bar, 500 µm.) (G and H) Planes of sections in A, B, E, and F.
Taken together, these conditional ablation experiments showed that the most severe axon pathfinding phenotypes were produced when Fz3 was deleted in all forebrain neurons (Foxg1-Cre) or only in ventral forebrain neurons (Dlx5/6-Cre), rather than in the cortical and thalamic neurons from which the axons originate. This pattern of phenotypes is strikingly similar to that obtained upon inactivation of a conditional allele of Celsr3 with the same Cre drivers (18). This similarity argues for an intimate association between Fz3 and Celsr3 in forebrain axon pathfinding.
Corridor cells, a neuronal population derived from the lateral ganglionic eminence, form a permissive bridge for the growth of thalamocortical axons (19). The presence of corridor cells between the neocortex and the thalamus together with the essential role of Fz3 in the ventral telencephalon raised the possibility that loss of Fz3 might affect corridor cell development, migration, survival, or function. By immunostaining for Ebf1, a transcription factor expressed in corridor cells, we found that the distribution and abundance of corridor cells were very similar in E13.5 WT and Fz3−/− brains (Fig. 3 E–F″). The only apparent difference between genotypes was the presence of Ebf1+ cells at the medioventral face of the Fz3−/− globus pallidus, with the result that the Fz3−/− globus pallidus was completely encircled by corridor cells. Whether this difference in cellular distribution plays a causal role in, is a consequence of, or is unrelated to the misrouting thalamocortical axons in the Fz3−/− brain is unknown.
Fz3 Is Required for the Normal Development of the Earliest Axon Tracts in the Mouse Brain.
In the vertebrate CNS, the first axon tracts develop in a stereotyped spatiotemporal pattern and serve as scaffolds for later growing axons (13). As a first step in determining whether Fz3 plays a role in the development of the earliest CNS axon tracts, we examined their arrangement by NF immunostaining of whole-mount E12.5 heads (Fig. 4 A and A′). Although axon tracts in the brain were not as strongly labeled as were peripheral nerves, they could still be clearly identified. As seen in Fig. 4 A and A′, three major axon tracts (labeled I, II, and III) were arrested, greatly reduced in intensity, or absent in the Fz3−/− brain. In addition, the ventral branch of the trigeminal nerve (i.e., V3) was markedly thinned or completely missing in Fz3−/− embryos.
Fig. 4.
Several early developing axon tracts are disrupted in the Fz3−/− brain. (A and A′) NF immunostaining of E12.5 whole-mount heads in lateral view showing a complete or nearly complete absence of the three most prominent axon tracts in the brain (numbered I, II, and III; upper three arrows in A vs. arrowheads in A′) and the ventral branch of the trigeminal nerve (V3) in Fz3−/− embryos (lower pair of arrows in A vs. arrowhead in A′). Axon tract I is the medial forebrain bundle originating from the midbrain and ascending to the basal forebrain; axon tract II is the calretinin+ fiber tract in the thalamic eminence; and axon tract III originates from the brainstem and projects rostrally. The axons are color coded to represent depth within the Z-stack, with red representing superficial axons on the near side, green representing axons in the center, and blue representing superficial axons on the far side. A, anterior; P, posterior. (Scale bar: 500 µm.) (B–D) Planes of sections in E–H′ (B), I and I′ (C), and J–R′ (D), and summary comparisons of axon tracts (Lower): axon tracts in WT (colored arrows and dot, left side of drawings) vs. defective counterparts in Fz3−/− brains (gray dashed or orange arrows and orange dot, right side of drawings). The green dot in B and green arrow in D represent tract II; the lower blue arrows in B and D represent tract I; the red arrow in D represents tract III; and the cyan arrow in C represents calretinin+ thalamocortical axons. In all schematic drawings, gray dashed arrows represent diminutive axon tracts, and orange arrows and dots represent completely missing axon tracts. (E–H′) NF and calretinin immunostaining of consecutive E11.5 brain sections showing the diminutive medial forebrain bundle (axon tract I, lower arrows vs. arrowheads in E–H′) and the absence of the calretinin+ axon tract in the thalamic eminence (axon tract II, top arrows vs. arrowheads in E–G′) in the Fz3−/− brain. (Scale bar, 500 µm.) (I and I′) Calretinin+ thalamocortical axons fail to enter the internal capsule in the E13.5 Fz3−/− brain (arrow vs. arrowhead), as seen in coronal sections. (Scale bar, 500 µm.) (J–R′) NF and calbindin immunostaining of consecutive E12.5 brain sections shows an unaltered distribution of calbindin+ neurons in the Fz3−/− brain, the diminutive medial forebrain bundle (axon tract I, lower arrows in J–M vs. lower arrowheads in J′–M′), the absence of the axon tract in the thalamic eminence (axon tract II, top arrows in K–M vs. top arrowheads in K′–M′), and the diminutive ascending fiber tract from the brainstem to the midbrain (axon tract III, arrows in P–R vs. arrowheads in P′–R′). (Scale bar, 500 µm.)
To more precisely identify the locations of the earliest axon tracts, serial sections of E11.5 and E12.5 heads were costained for NF and calbindin or calretinin, each of which labels distinctive populations of early born neurons that serve as useful landmarks (20). Calbindin+ neurons showed the same spatial distribution in Fz3+/− and Fz3−/− brains at E12.5 (Fig. 4 J–R vs. Fig. 4 J′–R′). In calbindin and NF double-stained sections, several axon tracts—along the lateral wall of the diencephalon (axon tract II; Fig. 4 K–M; upper arrows), connecting the diencephalon and the ventrolateral telencephalon (axon tract I; Fig. 4 J–M, lower arrows), and ascending from the brainstem to the midbrain (axon tract III; Fig. 4 P–R, arrows)—were missing or dramatically attenuated in the Fz3−/− brain.
In contrast to the unchanged distribution of calbindin+ neurons, the spatial distribution of calretinin+ immunoreactivity shows two clear differences between Fz3+/− and Fz3−/− brains at E11.5, both of which are associated with differences in axon trajectories. The first of these is shown in the center of Fig. 4 E–H′ and in Fig. S2 E–L′, in which two different planes of section through the same region of the Fz3−/− midbrain show a bundle of rostrally growing axons abutting a contiguous zone of calretinin+ cells (axon tract I, medial forebrain bundle). The corresponding region of the Fz3+/− midbrain shows the same axon bundle passing between two separated zones of calretinin+ neurons (Fig. 4H). The spatial relationship between axon bundles and calretinin+ neurons in the Fz3+/− brain suggests that the territories marked by calretinin+ neurons are nonpermissive for axon growth. In an extension of this hypothesis, the presence of the contiguous zone of calretinin+ neurons in the Fz3−/− midbrain may have blocked the growth of the axon bundle (Fig. 4H′). We note that the images are also consistent with a model in which the cessation of axon growth in the Fz3−/− midbrain occurred independently and permitted the separate territories of calretinin+ neurons to subsequently coalesce.
The second example of a difference in axon growth and calretinin+ immunoreactivity at E11.5 is seen at the top of Fig. 4 E–G′ and in Fig. S2 A–D′, where a cluster of calretinin+ neurons in the developing diencephalon is associated with a rostrally directed axon bundle in the Fz3+/− control brain (axon tract II, calretinin+ fiber tract in the thalamic eminence) that is absent in the Fz3−/− brain. The dependence of this axon bundle on Fz3 was confirmed by selectively labeling it with AP in horizontal sections of E13.5 Calb2-IRES-Cre;R26iAP;Fz3−/− and Calb2-IRES-Cre;R26iAP;Fz3+/− brains (Calb2 is the gene encoding calretinin; Fig. S3 A–F′). The failure of the diencephalic calretinin+ neurons to produce this axon bundle in the Fz3−/− brain at E11.5 implies an essential role for Fz3 at the earliest stage in the development of diencephalic projections. At E13.5, calretinin+ neurons occupy the anterior thalamus (Fig. S3 E–G′). In the Fz3+/− brain these neurons are the source of calretinin+ thalamocortical axons (Fig. 4I). By contrast, no calretinin+ axons are observed in the internal capsule in the Fz3−/− brain (Fig. 4I′), consistent with the generalized defect in thalamocortical projections illustrated in Fig. 1.
To test whether deletion of Fz3 selectively in calretinin+ neurons alters thalamocortical axon development, we generated Calb2-IRES-Cre;Fz3CKO/− and Calb2-IRES-Cre;Fz3CKO/+ embryos. As seen in Fig. S3 G–I′, K, and L, embryos of both genotypes have calretinin+ and NF+ thalamocortical axons that are correctly positioned within the internal capsule at E13.5, and there was no difference between genotypes in the overall appearance of the thalamocortical projections at E15.5. With the caveats noted earlier regarding the timing and efficiency of Cre-mediated recombination, these observations suggest that Fz3 expression may not be required in calretinin+ thalamic neurons for the development of the thalamocortical tract.
Autonomous and Nonautonomous Roles of Fz3 in RGC Axon Guidance.
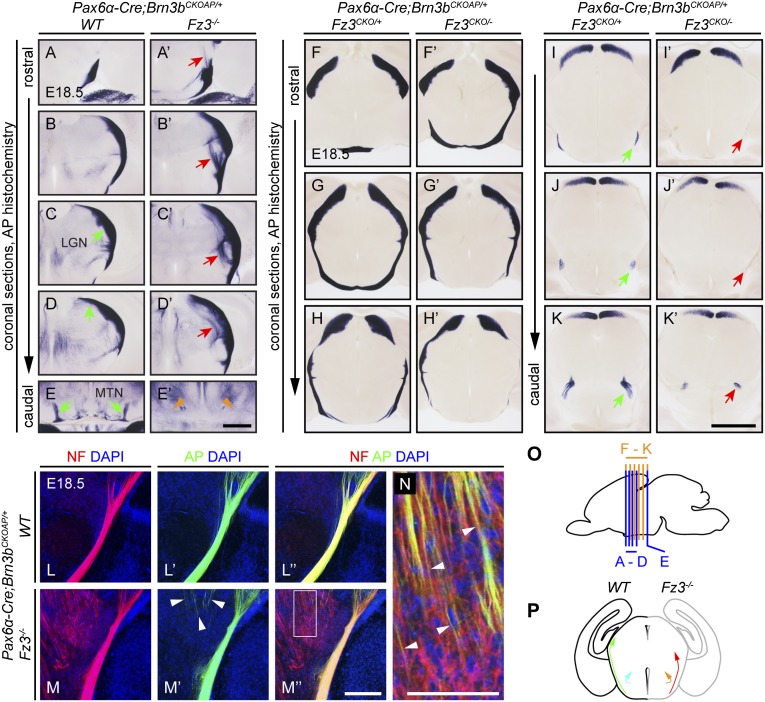
Fz3 is expressed in the developing mouse retina (6), but it is not known whether Fz3 plays a role in retinal development or RGC axon growth and guidance. To assess the trajectories of RGC axons, we combined a retina-specific Pax6α-Cre transgene, which efficiently recombines target loci beginning at E9.5 (21), with a Brn3bCKOAP allele in which an AP reporter inserted 3′ of the Brn3b transcription termination site is expressed following Cre-mediated deletion of both Brn3b exons (22). Brn3b is expressed by the majority of RGCs, and therefore the majority of RGC axons can be visualized by AP histochemistry in Pax6α-Cre;Brn3bCKOAP/+ brains.
As seen in Pax6α-Cre;Brn3bCKOAP/+ (i.e., WT) brains, central targets of RGC axons include the lateral geniculate nucleus and the medial terminal nucleus (MTN; Fig. 5 A–E). In Pax6α-Cre;Brn3bCKOAP/+;Fz3−/− brains, the optic nerves and optic chiasm were unaffected, but two defects were observed: (i) caudal to the optic chiasm, a subset of optic tract axons diverged from their normal trajectories and entered the medial thalamus and (ii) projections to the MTN were missing (Fig. 5 A′–E′). As shown in Fig. 1D′, in the Fz3−/− thalamus, misrouted thalamocortical axons form a massive U-shaped bundle that courses adjacent to the optic tract along the ventral and lateral boundaries of the thalamus. To determine whether some of the divergent optic tract axons were cofasciculating with these misrouted intrathalamic axons, we visualized RGC axons by immunostaining for AP and all axons by immunostaining for NF in coronal sections from Pax6α-Cre;Brn3bCKOAP/+;Fz3+/− and Pax6α-Cre;Brn3bCKOAP/+;Fz3−/− brains. This analysis shows some RGC axons in the Pax6α-Cre;Brn3bCKOAP/+;Fz3−/− brain in close contact with misrouted thalamocortical axons (Fig. 5 L–N).
Fig. 5.
Defects in the central projections of Fz3−/− RGC axons. (A–E′) AP+ RGC axons from Pax6α-Cre;Brn3bCKOAP/+ mice at E18.5. In the Fz3−/− brain, some RGC axons in the thalamus diverge from the optic tract (red arrows in A′–D′), and the MTN is not innervated (green arrows in E vs. orange arrowheads in E′). LGN, lateral geniculate nucleus. (Scale bar, 500 µm.) (F–K′) AP+ RGC axons in Pax6α-Cre;Brn3bCKOAP/+;Fz3CKO/− mice at E18.5 exhibit normal trajectories except for a reduced projection to the inferior fasciculus and MTN (green vs. red arrows in I–K′). (Scale bar, 1 mm.) (L–N) NF and AP immunostaining of coronal sections of an E18.5 Pax6α-Cre;Brn3bCKOAP/+;Fz3−/− thalamus showing misrouted AP+/NF+ RGC axons cofasciculating with aberrant and ventrally directed NF+/AP− thalamic axons (arrowheads in M′ and N). (N) Enlarged view of the boxed region in M″. (Scale bars: M″, 200 µm; N, 100 µm.) (O) Planes of sections in A–K′. (P) The green arrow (Left) shows the trajectory of the optic tract in the WT brain, and the red arrow (Right) shows the medial misrouting of some optic tract axons in the Fz3−/− brain; the cyan arrow at left shows the innervation of the MTN by optic tract axons in the WT brain, and the orange arrow at right shows the absence of MTN innervation by optic tract axons in the Fz3−/− brain.
To address the question of whether the aberrant trajectories of Fz3−/− RGC axons reflect (i) an autonomous requirement for Fz3 function in RGCs, (ii) an interaction between RGC axons and misrouted thalamic axons, or (iii) a combination of the two, we generated Pax6α-Cre;Brn3bCKOAP/+;Fz3CKO/− embryos in which Fz3 was deleted in hPLAP-expressing RGCs but the rest of the CNS remained WT (Fig. 5 F–K′). In these mice, AP+ projections to the lateral geniculate nucleus and superior colliculus showed no pathfinding defects, implying that the misrouting of optic tract axons within the Fz3−/− thalamus is not a cell-autonomous process, but most likely arises from inappropriate fasciculation with misrouted intrathalamic axons. In contrast, AP+ projections to the MTN were greatly attenuated in Pax6α-Cre;Brn3bCKOAP/+;Fz3CKO/− brains, implying that MTN targeting requires Fz3 function within RGC axons or growth cones. The small number of RGC axons in Pax6α-Cre;Brn3bCKOAP/+;Fz3CKO/− brains that project to the MTN could derive from RGCs that have recombined the Brn3bCKOAP allele but not the Fz3CKO allele, a plausible scenario given that Pax6α-Cre expression progressively declines within the lateral and nasal retina (21).
We next asked whether Fz3 is involved in retinal lamination, a major organizing feature for retinal circuitry. To address this question, we analyzed retinas from adult Six3-Cre;Fz3CKO/− mice. The Six3-Cre transgene is expressed throughout the retina starting at approximately E11 (23), but its expression in the brain is sufficiently limited that Six3-Cre;Fz3CKO/− mice survive to adulthood. In cross-sections of Six3-Cre;Fz3CKO/− retinas, the overall organization of the retina appeared unaltered, and, as shown in Fig. S4, GABAergic, dopaminergic, cholinergic, calbindin+, and calretinin+ amacrine cells were present in their correct abundances and locations and exhibited normal patterns of dendritic stratification within the inner plexiform layer. These observations rule out a major role for Fz3 in retinal development.
Effects of Fz3 KO on the Growth and Guidance of Catecholaminergic and Serotonergic Axons.
In the mammalian brain, neuromodulatory systems that use low molecular weight neurotransmitters—acetylcholine, dopamine, epinephrine, norepinephrine, histamine, and serotonin—are characterized by diffuse projections from the ventral forebrain and/or brainstem to multiple target areas. The initial description of the Fz3−/− axon guidance phenotype noted the failure of midbrain dopaminergic axons to arrive at the striatum, and subsequent experiments have demonstrated defects in cell body orientation and axon trajectories among dopaminergic and serotonergic neurons (6, 24). To assess these defects in more detail, we have characterized dopaminergic and serotonergic projections at E13.5 and E16.5 or E18.5 by using anti-TH (adrenergic, noradrenergic, and dopaminergic) and anti–5-HT (serotonergic) immunolabeling.
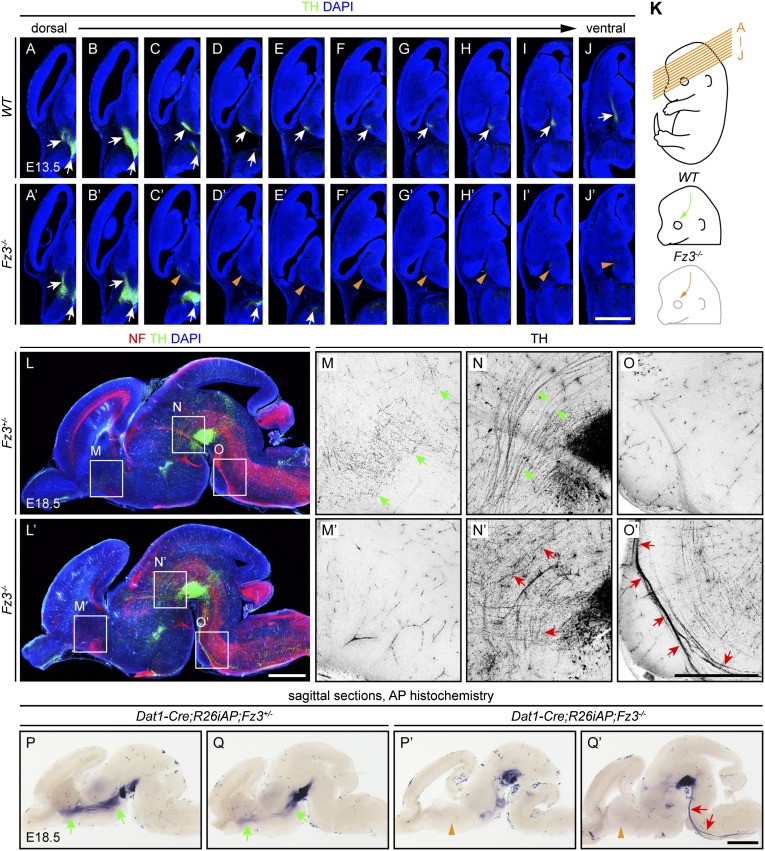
In WT brains, TH+ axons extend ∼2 mm anteriorly from their origin in the midbrain at E13.5 (Fig. 6 A–J), and are present at high density in the striatum at E18.5 (Fig. 6 L and M). In contrast, in Fz3−/− brains, TH+ axons extend only ∼0.5 mm anteriorly at E13.5 (Fig. 6 A′–J′) and are present at low density in the striatum at E18.5 (Fig. 6 L′ and M′). In WT and Fz3−/− embryos, numerous anteriorly directed TH+ axons are seen at E18.5 in the midbrain and diencephalon (Fig. 6 N and N′), with many axons appearing less well organized along the rostrocaudal axis in the Fz3−/− brain (Fig. 6N′, red arrows). Strikingly, in Fz3−/− brains, there is a large and aberrant TH+ axon tract coursing caudally from the midbrain along the ventral surface of the medulla (Fig. 6 O and O′). These observations were confirmed with the use of a more specific genetic marking system based on Cre expression from the Dopamine transporter-1 (Dat1) locus (25). As Dat1 is expressed exclusively in dopaminergic neurons, analyses based on Dat1-Cre are not confounded by signals from adrenergic and noradrenergic axons. Fig. 6 P–Q′ and Fig. S5 show the full trajectories of dopaminergic axons in Dat1-Cre;R26iAP;Fz3+/− (WT) and Dat1-Cre;R26iAP;Fz3−/− brains at E18.5. These data demonstrate that, in the Fz3−/− brain, few if any midbrain dopaminergic axons reach the striatum and numerous dopaminergic axons project to the spinal cord.
Fig. 6.
Defects in the orientation and growth of midbrain dopaminergic axons in Fz3−/− embryos. (A–J′) In consecutive sections of WT and Fz3−/− brains at E13.5, midbrain TH+ neurons are present (lower arrows in A–D and A′–E′), but, in the Fz3−/− brain, TH+ axons do not project to the basal forebrain (top white arrows in C–J vs. orange arrowheads in C′–J′). (Scale bar: 1 mm.) (K) Planes of sections for A–J′ (Top), trajectories of TH+ axons in the E13.5 WT brain (green arrow; Middle), and missing axons in the E13.5 Fz3−/− brain (orange arrow; Bottom). (L–O′) NF and TH immunostaining of E18.5 sagittal brain sections showing that, in the Fz3−/− brain, the striatum is devoid of TH+ axons (M vs. M′), some TH+ axons project rostrally from the substantia nigra (N vs. N′), and other TH+ axons descend to the spinal cord (O vs. O′). (M–O′) Enlarged views of boxed regions in L and L′ showing only the anti-TH signal with intensity inverted. Background immunostaining is present in the vasculature. (Scale bars: L′, 1 mm; O′, 500 µm.) (P–Q′) In the Dat1-Cre;R26iAP;Fz3−/− brain, AP+ dopaminergic axons do not innervate the striatum (green arrows in P and Q vs. orange arrowheads in P′ and Q′) and form an aberrant tract that descends to the spinal cord (red arrows in Q′). (Scale bar, 1 mm.)
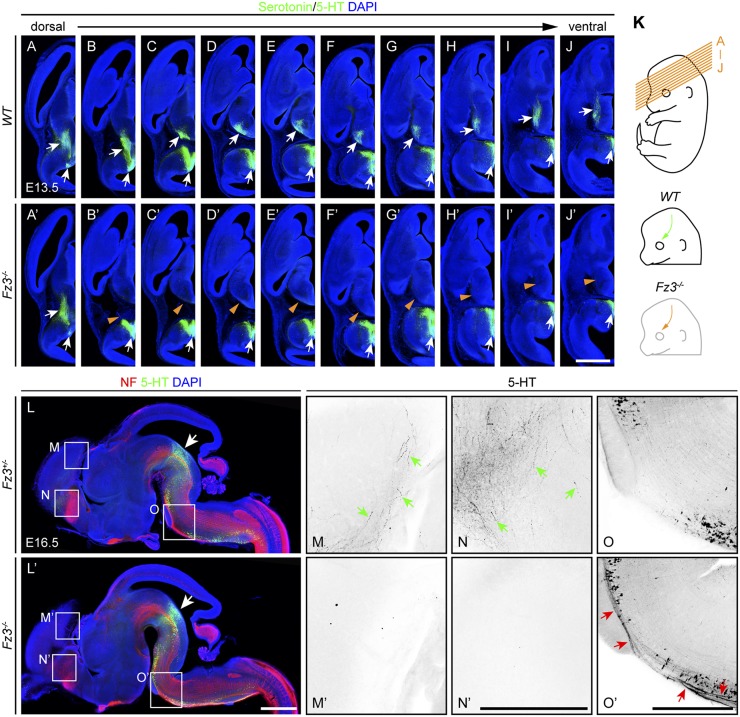
Anti–5-HT immunostaining of E13.5 WT and Fz3−/− embryos showed that serotonergic axons in the Fz3−/− brainstem fail to project rostrally beyond the midbrain (Fig. 7 A–J′). A sagittal view of the entire brain at E16.5 shows normal 5-HT+ axons in the tegmentum (Fig. 7 L and L′, arrows), but a complete absence of 5-HT+ axons in the cerebral cortex and striatum (Fig. 7 M–N′) and an aberrant 5-HT+ axon tract coursing caudally along the ventral medulla (Fig. 7 O and O′), a pattern that closely resembles the defects described above for dopaminergic axons in the Fz3−/− brain. These data confirm and extend the earlier work of Wang et al. (6) and Fenstermaker et al. (24), and show that loss of Fz3 leads to a severe defect in the asymmetric rostrocaudal orientation of dopaminergic and serotonergic axons.
Fig. 7.
Defects in the orientation and growth of serotonergic axons in Fz3−/− embryos. (A–J′) In WT and Fz3−/− brains, brainstem serotonergic neurons are present (lower arrows in A–J and A′–J′), but, in the Fz3−/− brain, serotonergic axons do not project rostrally (upper white arrows in B–J vs. orange arrowheads in B′–J′), shown by immunostaining for 5-HT on consecutive E13.5 brain sections. (Scale bar, 1 mm.) (K) Planes of sections for A–J′ (Top), trajectories of serotonergic axons in the E13.5 WT brain (green arrow; Middle), and missing axons in the E13.5 Fz3−/− brain (orange arrow; Bottom). (L–O′) NF and 5-HT immunostaining of E16.5 sagittal brain sections showing that in the Fz3−/− brain the cortex and striatum are devoid of serotonergic axons (arrows in M and N vs. M′ and N′), and numerous serotonergic axons descend along the ventral medulla (arrows in O′). (M–O′) Enlarged views of boxed regions in L and L′ showing only the anti-5-HT signal with intensity inverted. Arrows in L and L′ point to the tegmentum. (Scale bars: L′, 1 mm; N′ and O′, 500 µm.)
Absence of the Spinothalamic Tract in Fz3−/− Mice: Functional Consequences.
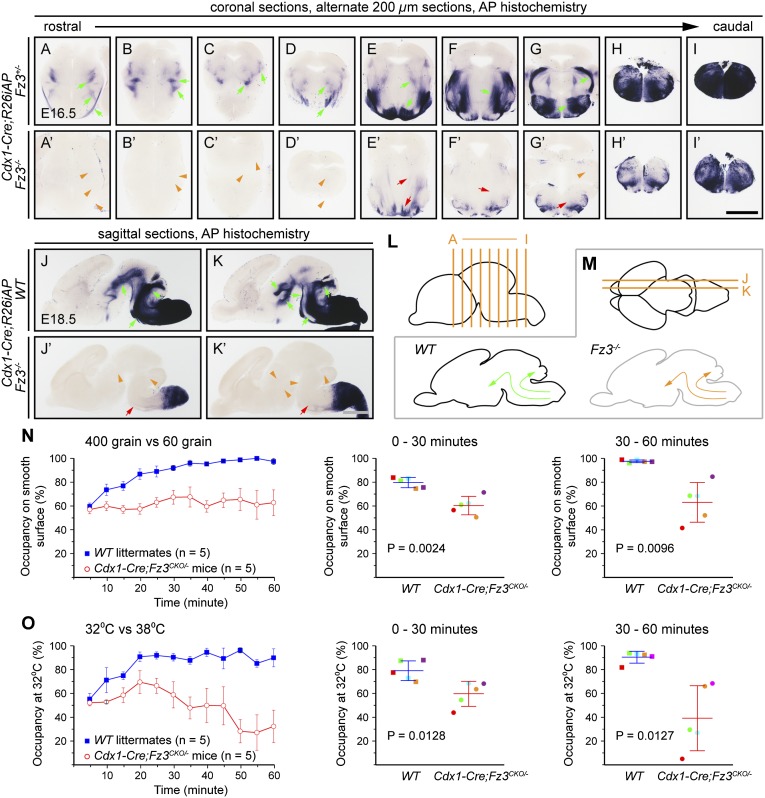
Loss of Fz3 leads to impaired rostral turning by growth cones of spinal cord commissural sensory axons at midgestation (8). To define the neuroanatomic consequences of this guidance defect later in gestation, we visualized ascending axons in WT and Fz3−/− brains by combining the R26iAP reporter and a Caudal-1 (Cdx1)-Cre transgene, which recombines target alleles in all tissues caudal to the upper thorax before midgestation (9, 26). As seen in Fig. 8 A–M, there is a large reduction in ascending axons in the brainstem and a complete absence of ascending axons in the midbrain and thalamus in Fz3−/− brains.
Fig. 8.
Ascending axon tracts from the spinal cord are missing in the Fz3−/− brain, and Cdx1-Cre;Fz3CKO/− mice show no texture or temperature preferences when stimuli are delivered to their feet. (A–K′) AP+ ascending axon tracts from the spinal cord to the brain in E16.5 coronal (A–I′) and E18.5 sagittal (J–K′) brain sections from embryos carrying the R26iAP reporter recombined by the Cdx1-Cre transgene (green arrows in A–G, J, and K). These axons are largely missing in Fz3−/− brains (orange arrowheads in A–D′, G′, J′, and K′ show missing axon tracts, and red arrows show diminutive tracts). (Scale bars: 1 mm.) (L) Planes of sections in A–I′. (M) Planes of sections in J–K′ (Upper), and ascending spinal cord axon tracts in the WT brain (green arrows; Left) are missing in the Fz3−/− brain (orange arrows; Right). (N) Texture preference test results for WT (blue) and Cdx1-Cre;Fz3CKO/− mice (red) for smooth (400-grain sandpaper) vs. rough (60-grain sandpaper) surfaces over 60 min. For each genotype, five age-matched mice consisting of three males and two females were tested. Each WT mouse was tested twice, and each Cdx1-Cre;Fz3CKO/− mouse was tested three times. (Left) The percent of the time spent on the smooth surface is shown as the mean ± SEM for each 5-min interval. (Right) The percent of the time spent on the smooth surface is shown for the first and second 30-min intervals as the mean ± SD; each of the five colored dots represents a different mouse. For statistical analyses, the test results for each mouse were first averaged. WT mice show a strong preference for the smooth surface. Cdx1-Cre;Fz3CKO/− mice show no preference. (O) Thermal preference test results for WT (blue) and Cdx1-Cre;Fz3CKO/− mice (red) for 32 °C vs. 38 °C surfaces over 60 min (mean ± SEM). Data are displayed as described for the texture preference data in N. Each WT mouse was tested twice, and each Cdx1-Cre;Fz3CKO/− mouse was tested three times. WT mice show a strong preference for the 32 °C surface. Cdx1-Cre;Fz3CKO/− mice show no preference.
In previous work, we used Cdx1-Cre to eliminate Fz3 in all cells in the trunk and limbs and found that, except for a curled tail and an odd gait—the result of a failure of motor axons to innervate the anterior compartment musculature of the lower hindlimb—adult Cdx1-Cre;Fz3CKO/− mice appear completely healthy (9). However, the absence of ascending spinal cord axons—and, in particular, the absence of the spinothalamic tract—in the Fz3−/− CNS suggested the interesting possibility that, despite their grossly normal appearance, Cdx1-Cre;Fz3CKO/− mice might lack the ability to transmit sensory information from the trunk and limbs to the brain.
Before embarking on a test of somatosensation in Cdx1-Cre;Fz3CKO/− mice, we first asked whether loss of Fz3 affects the structure of peripheral sensory nerves. To selectively visualize sensory axons, we studied WT and Fz3−/− mice carrying an AP reporter knocked into the Brn3a gene, which is expressed in all or nearly all neurons in dorsal root ganglia and cranial sensory ganglia (22, 27). AP staining of horizontal sections from E15.5 Brn3aAP/+;Fz3+/− and Brn3aAP/+;Fz3−/− embryos showed no abnormalities in sensory nerve development or target innervation (Fig. S6). We also tested ambulation by using a rotarod. Although Cdx1-Cre;Fz3CKO/− mice walk awkwardly compared with WT controls, they are able to maintain their balance on a 6-cm-diameter rod rotating at 8 rpm (Movies S1 and S2).
On the assumption that primary somatosensation at the level of dorsal root ganglia neurons was largely or completely intact in Cdx1-Cre;Fz3CKO/− mice, we then asked whether somatosensory information from the spinal cord could be communicated to the brain by testing 1–3-mo-old Cdx1-Cre;Fz3CKO/− mice and their WT littermates in a pair of thermal and texture preference tasks. When WT mice are presented with a choice between surfaces held at 32 °C and 38 °C, they show a marked preference for the 32 °C surface. Similarly, when WT mice are presented with a choice between rough and smooth surfaces (60-grain and 400-grain sandpaper, respectively), they show a marked preference for the smooth surface. (Before performing the texture preference task, the mice had their whiskers trimmed; under these conditions, we presume that texture is sensed through their feet.) Independent experiments, performed in the dark with WT mice, demonstrated that texture preference does not depend on visual cues. These preferences are presumed to involve integration of sensory inputs at the level of the thalamus and higher cognitive centers. Strikingly, in these behavioral tests, Cdx1-Cre;Fz3CKO/− mice appeared to be largely or completely oblivious to differences in temperature and texture, whereas control littermates showed the expected preference for lower temperature and smoother texture (Fig. 8 N and O and Fig. S7). In comparing 10 trials with WT controls (two trials per mouse × five mice) and 15 trials with Cdx1-Cre;Fz3CKO/− mice (three trials per mouse × five mice), the P values for the preference differences between genotypes during each 30-min half of the 60-min trial were P < 0.01 and P < 0.02 for the texture and temperature tasks, respectively. Although we cannot rule out the possibility that the indifference shown by Cdx1-Cre;Fz3CKO/− mice to the different stimuli reflects an information-processing defect within the spinal cord, the most straightforward explanation for the anatomic and behavioral data is that temperature and texture preferences require supraspinal information processing. If correct, this explanation would imply that Cdx1-Cre;Fz3CKO/− mice inhabit a mental world without brain representations of somatosensory information from the trunk and limbs.
Discussion
The experiments reported here substantially extend earlier studies of Fz3 action in the CNS. In particular, they show that ubiquitous loss of Fz3 disrupts the growth and correct orientation of several axon tracts in the developing brain from the earliest times in their development (E11.5), produces large-scale anterior/posterior misrouting of midbrain dopaminergic and brainstem serotonergic axons, and leads to aberrant cofasciculation of optic and intrathalamic axon tracts. These experiments also show that loss of Fz3 in cell populations along the route taken by thalamocortical axons perturbs the trajectories of these axons in a manner that is identical or nearly identical to the perturbations induced by loss of Celsr3 in the same cell populations. Finally, these experiments show that selective deletion of Fz3 in the trunk and limbs results in an adult nervous system in which most, if not all, ascending spinal sensory axons are missing, permitting an analysis of brain structure and function in the absence of sensory input from the lower body.
PCP Genes and the Control of Axon Growth and Guidance.
As noted earlier, the identity or near-identity of phenotypes in comparisons of constitutive Fz3 and Celsr3 mutations and of conditional Fz3 and Celsr3 mutations in the cortex (Emx1-Cre) or ventral telencephalon (Dlx5/6-Cre) argues that these two integral membrane PCP proteins are likely to be involved in the same axon signaling mechanism(s) (5, 6, 18, 28). In epithelia, Frizzled and Celsr/Fmi/Stan proteins are partially colocalized, with Frizzled localized to one face of each epithelial cell and Celsr/Fmi/Stan localized to that face and to the opposite face of the cell (29). A critical and still open question is whether axon guidance involves homophilic Celsr/Fmi/Stan complexes between neighboring cells, as appears to be the case for the transfer of epithelial polarity information.
In several regions in the CNS, axons that are dependent on Fz3 exhibit alternate trajectories when Fz3 is missing. Thus, thalamic axons are rerouted to the contralateral thalamus and the ventral telencephalon, and many midbrain dopaminergic and brainstem serotonergic axons are rerouted caudally to the medulla and spinal cord. These observations suggest that there is a hierarchy of default trajectories, and they also emphasize the point that loss of Fz3 does not affect axon growth per se. A different axon phenotype was recently described in the Fz3−/− dorsal limb in which motor axons stall at a discrete location within the nerve plexus at the base of the limb (9). Motor axon growth proximal to the stalling point was essentially normal, so this phenotype also is unlikely to represent a defect in axon growth. These distinct patterns of defective axon growth and guidance indicate that different axons respond differently to defects in Fz3 signaling, perhaps because interactions with other guidance cues modify the response to Fz3 signals.
The defect in thalamocortical axon guidance in Dlx5/6-Cre;Fz3CKO/− mice implies that Fz3/Celsr3 signaling plays an essential role in cell populations, such as corridor cells, that line the route through which these axons pass. Although more refined Cre drivers will be required to precisely define the relevant cell population in which Fz3 and Celsr3 act in the ventral telencephalon, these data already reveal an added layer of complexity, as earlier work using CNS-specific deletion of Fz3 in the spinal cord indicated that motor axon growth in the dorsal limb does not require Fz3 expression in peripheral tissues along the route through which motor axons pass in WT limbs and within which they stall in the Fz3−/− limb (9).
Brain Function Without Ascending Sensory Information.
Somatosensory information from the limbs and trunk is conveyed to the brain via anatomically distinct spinal pathways (30). Pain, temperature, and crude touch are conveyed by the anterolateral pathway, which projects to the brainstem reticular formation, midbrain tectal nuclei, and thalamus. Tactile and proprioceptive information is conveyed by the dorsal column-medial lemniscal system, which projects to the thalamus. Additional pathways, such as the spinocerebellar pathway, convey information to subcortical structures to regulate reflex responses and fine tune voluntary movements. The temperature and texture preferences displayed by WT mice (Fig. 8 N and O) require (i) a differentiation of two innocuous stimuli based on afferent information from the soles of the feet, (ii) a decision to seek out one of the stimuli and/or avoid the other, and (iii) a set of motor commands to execute the decision. It is very likely that successful completion of this task requires information processing by the somatosensory and frontal regions of cortex, and therefore a relay of sensory information from spinal cord to thalamus to cortex. Based on the behavioral and anatomic data, it seems unlikely that any somatosensory information from the trunk and limbs is being transmitted to the Cdx1-Cre;Fz3CKO/− thalamus and cortex. However, we cannot rule out the possibility that a small minority of ascending sensory axons in the Cdx1-Cre;Fz3CKO/− spinal cord are impervious to the effects of Fz3 inactivation or that some neurons that relay ascending information escape Cre-mediated recombination. Because the medulla straddles the boundary of Cre+ and Cre− territories (Fig. 8 A–K′), the first consideration is most relevant for axons that project to the medulla and the second consideration is most relevant for neurons that reside in the medulla.
Despite their somatosensory deficiencies, the ambulatory ability of Cdx1-Cre;Fz3CKO/− mice—although partially compromised by a lower hindlimb motor innervation defect—appears to be substantially intact. This might seem surprising, as the defect in ascending spinal projections likely blocks the transmission of proprioceptive information to the brain. However, a large body of work on intrinsic spinal cord locomotor circuits demonstrates that for many reflex responses somatosensory information is integrated into locomotor programs at the level of spinal circuits with little or no involvement of supraspinal mechanisms (31, 32). Moreover, the sparing of sensory axons in the head in Cdx1-Cre;Fz3CKO/− mice implies that vestibular, whisker, and visual inputs should be integrated normally to modulate descending motor commands to the spinal cord.
It would be interesting to test Cdx1-Cre;Fz3CKO/− mice for their responses to other somatosensory stimuli, such as itch and surface-borne vibration. It would also be interesting to examine the anatomy and physiology of neurons in the thalamus and somatosensory cortex in Cdx1-Cre;Fz3CKO/− mice to explore the fate of territories that normally process somatosensory information from the trunk and limbs. One might anticipate that there would be a large scale remapping of these territories analogous to the remapping of visual cortex in humans or animals that are congenitally blind (33–35).
Materials and Methods
The following mouse lines were used: Brn3aCKOAP (22), Brn3bCKOAP (22), Cdx1-Cre (26), Fz3−/− (6), Fz3CKO/CKO (9), Calb2-IRES-Cre (010774; Jackson Laboratory) (36), Dat1-Cre (25), Dbx1-IRES-Cre (031751-MU; Mutant Mouse Regional Resource Centers), Dlx5/6-Cre (16), Emx1-IRES-Cre (15), Foxg1-Cre (17), Pax6α-Cre (21), RORα-IRES-Cre (10), R26iAP (11), and Sox2-Cre (37). Mice were handled and housed in accordance with institutional animal care and use committee guidelines of the Johns Hopkins Medical Institutions.
Alkaline phosphatase histochemistry, immunohistochemistry, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate anterograde tracing of cortical axons, microscopy, image analysis and videotaping, two-texture and two-temperature preference assay, and number of mice analyzed and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Drs. Andreas Hierholzer and Rolf Kemmler for sharing their Cdx1-Cre mouse line; Zixuan Pang for assistance with the thermal preference assay; Dr. Randy Reed for the gift of anti-Ebf1 antibodies; and Drs. Hao Chang, Amir Rattner, Hao Wu, and the two reviewers for helpful comments on the manuscript. This work was supported by the Howard Hughes Medical Institute, the Neurosurgery Pain Research Institute, and National Institute of Dental and Craniofacial Research Grant DEO22750.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406399111/-/DCSupplemental.
References
- 1.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: A primer. Cold Spring Harb Perspect Biol. 2011;3(6) doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonanomi D, Pfaff SL. Motor axon pathfinding. Cold Spring Harb Perspect Biol. 2010;2(3):a001735. doi: 10.1101/cshperspect.a001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldheim DA, O’Leary DD. Visual map development: Bidirectional signaling, bifunctional guidance molecules, and competition. Cold Spring Harb Perspect Biol. 2010;2(11):a001768. doi: 10.1101/cshperspect.a001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138(10):1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang J, Mori S, Nathans J. Axonal growth and guidance defects in Frizzled3 knock-out mice: A comparison of diffusion tensor magnetic resonance imaging, neurofilament staining, and genetically directed cell labeling. J Neurosci. 2006;26(2):355–364. doi: 10.1523/JNEUROSCI.3221-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci. 2002;22(19):8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tissir F, Bar I, Jossin Y, De Backer O, Goffinet AM. Protocadherin Celsr3 is crucial in axonal tract development. Nat Neurosci. 2005;8(4):451–457. doi: 10.1038/nn1428. [DOI] [PubMed] [Google Scholar]
- 8.Lyuksyutova AI, et al. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302(5652):1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- 9.Hua ZL, Smallwood PM, Nathans J. Frizzled3 controls axonal development in distinct populations of cranial and spinal motor neurons. eLife. 2013;2:e01482. doi: 10.7554/eLife.01482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu CS, et al. Requirement of cannabinoid CB(1) receptors in cortical pyramidal neurons for appropriate development of corticothalamic and thalamocortical projections. Eur J Neurosci. 2010;32(5):693–706. doi: 10.1111/j.1460-9568.2010.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Badea TC, et al. New mouse lines for the analysis of neuronal morphology using CreER(T)/loxP-directed sparse labeling. PLoS ONE. 2009;4(11):e7859. doi: 10.1371/journal.pone.0007859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa Y, O’Leary DD. Dynamic patterned expression of orphan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci. 2003;25(2-4):234–244. doi: 10.1159/000072271. [DOI] [PubMed] [Google Scholar]
- 13.Chédotal A, Richards LJ. Wiring the brain: The biology of neuronal guidance. Cold Spring Harb Perspect Biol. 2010;2(6):a001917. doi: 10.1101/cshperspect.a001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NIH Neuroscience Blueprint Cre Driver Network . Cre Recombinase-Expressing Mice Generated for the NIH Neuroscience Blueprint Cre Driver Network. MGI Direct Data Submission. Bar Harbor, ME: Jackson Laboratory; 2009. [Google Scholar]
- 15.Gorski JA, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenman J, Toresson H, Campbell K. Identification of two distinct progenitor populations in the lateral ganglionic eminence: implications for striatal and olfactory bulb neurogenesis. J Neurosci. 2003;23(1):167–174. doi: 10.1523/JNEUROSCI.23-01-00167.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hébert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222(2):296–306. doi: 10.1006/dbio.2000.9732. [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, et al. Early forebrain wiring: Genetic dissection using conditional Celsr3 mutant mice. Science. 2008;320(5878):946–949. doi: 10.1126/science.1155244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.López-Bendito G, et al. Tangential neuronal migration controls axon guidance: A role for neuregulin-1 in thalamocortical axon navigation. Cell. 2006;125(1):127–142. doi: 10.1016/j.cell.2006.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jacobowitz DM, Abbott LC. Chemoarchitectonic Atlas of the Developing Mouse Brain. Boca Raton, FL: CRC; 1998. [Google Scholar]
- 21.Marquardt T, et al. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/s0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 22.Badea TC, Cahill H, Ecker J, Hattar S, Nathans J. Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron. 2009;61(6):852–864. doi: 10.1016/j.neuron.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuta Y, Lagutin O, Hogan BL, Oliver GC. Retina- and ventral forebrain-specific Cre recombinase activity in transgenic mice. Genesis. 2000;26(2):130–132. [PubMed] [Google Scholar]
- 24.Fenstermaker AG, et al. Wnt/planar cell polarity signaling controls the anterior-posterior organization of monoaminergic axons in the brainstem. J Neurosci. 2010;30(47):16053–16064. doi: 10.1523/JNEUROSCI.4508-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143(1):27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Hierholzer A, Kemler R. Cdx1:Cre allele for gene analysis in the extraembryonic ectoderm and the three germ layers of mice at mid-gastrulation. Genesis. 2009;47(3):204–209. doi: 10.1002/dvg.20484. [DOI] [PubMed] [Google Scholar]
- 27.Eng SR, et al. Defects in sensory axon growth precede neuronal death in Brn3a-deficient mice. J Neurosci. 2001;21(2):541–549. doi: 10.1523/JNEUROSCI.21-02-00541.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tissir F, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13(6):700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: New insights and new questions. Development. 2007;134(4):647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 30.Gardner EP, Martin JH, Jessell TM. The bodily senses. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4th Ed. New York: McGraw-Hill; 2000. pp. 430–450. [Google Scholar]
- 31.Whelan PJ. Control of locomotion in the decerebrate cat. Prog Neurobiol. 1996;49(5):481–515. doi: 10.1016/0301-0082(96)00028-7. [DOI] [PubMed] [Google Scholar]
- 32.Stuart DG, Hultborn H. Thomas Graham Brown (1882–1965), Anders Lundberg (1920-), and the neural control of stepping. Brain Res Brain Res Rev. 2008;59(1):74–95. doi: 10.1016/j.brainresrev.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Charbonneau V, Laramée ME, Boucher V, Bronchti G, Boire D. Cortical and subcortical projections to primary visual cortex in anophthalmic, enucleated and sighted mice. Eur J Neurosci. 2012;36(7):2949–2963. doi: 10.1111/j.1460-9568.2012.08215.x. [DOI] [PubMed] [Google Scholar]
- 34.Klinge C, Eippert F, Röder B, Büchel C. Corticocortical connections mediate primary visual cortex responses to auditory stimulation in the blind. J Neurosci. 2010;30(38):12798–12805. doi: 10.1523/JNEUROSCI.2384-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins KE, et al. Early auditory processing in area V5/MT+ of the congenitally blind brain. J Neurosci. 2013;33(46):18242–18246. doi: 10.1523/JNEUROSCI.2546-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi H, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: A tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.