Significance
Natural events in temperate ecosystems are triggered by seasonal temperature changes. Climate change may shift the timing of these events. We use a century of herbarium collections of Himalayan rhododendrons to investigate climate-driven change in flowering time. Although increased annual temperatures are associated with earlier flowering, increased fall temperatures are associated with delayed flowering. Annual warming may advance flowering through positive effects on overwintering bud formation, whereas fall warming may delay flowering through an impact on chilling requirements. These contrasting effects have resulted in opposing changes in flowering time, even as temperatures have warmed rapidly in the past 45 y. This study demonstrates the value of natural history collections to inform ecological questions, especially regarding climate change.
Keywords: phenology, global warming
Abstract
Responses by flowering plants to climate change are complex and only beginning to be understood. Through analyses of 10,295 herbarium specimens of Himalayan Rhododendron collected by plant hunters and botanists since 1884, we were able to separate these responses into significant components. We found a lack of directional change in mean flowering time over the past 45 y of rapid warming. However, over the full 125 y of collections, mean flowering time shows a significant response to year-to-year changes in temperature, and this response varies with season of warming. Mean flowering advances with annual warming (2.27 d earlier per 1 °C warming), and also is delayed with fall warming (2.54 d later per 1 °C warming). Annual warming may advance flowering through positive effects on overwintering bud formation, whereas fall warming may delay flowering through an impact on chilling requirements. The lack of a directional response suggests that contrasting phenological responses to temperature changes may obscure temperature sensitivity in plants. By drawing on large collections from multiple herbaria, made over more than a century, we show how these data may inform studies even of remote localities, and we highlight the increasing value of these and other natural history collections in understanding long-term change.
In an era of ongoing climate change (1), shifts in seasonal timing of life history events (phenology) are among the first and the most important responses seen in biological systems (2–5). Changes in phenology potentially impact organism reproduction, population survival, species boundaries, and ecosystem service (6–8). However, despite the importance of phenological changes (9, 10), data sources are limited (11). Satellite imagery (12), experimental studies (13), and modern observational records of phenology (11, 14) are temporally restricted to the last few decades. Although historical phenological records kept by scientists, amateur naturalists, or for cultural reasons (15–17) may extend much further, these are often limited in geographic range, and tend to focus on North America and Europe (but see ref. 18).
Such records have not been found for the Himalayan region, an area of particular concern when considering climate change. Rapid temperature increases and changes in precipitation, in combination with the importance of Himalayan snowpack and glaciers to water supply and monsoon cycles, make the region one of the most threatened nonpolar areas of the world (1, 19). Recent climate change is impacting Himalayan biological systems, including those upon which humans rely (20–23).
Despite its remoteness, the botanical richness of Yulong Mountain (27°N, 100.2°E), at the eastern limit of the Himalayan region, has made it a center of botanical collection since the late 19th century. Yulong Mountain was home to the prolific plant hunters George Forrest (collecting 1904–1930) and Joseph Rock (collecting 1918–1948). Other early collectors in the area included Jean Marie Delavayi, Heinrich Handel-Mazzetti, Frank Kingdon Ward, George Ludlow and Frank Sheriff, Yu Dejun (T. T. Yu), and Feng Goumei (K. M. Feng). One of the most collected taxa was Rhododendron, a genus of particular ecological, cultural, and economic importance in the Himalaya (24, 25). Especially during the early part of the 20th century, Rhododendron was also of great horticultural value in Europe and North America (26). Originally gathered for species delimitation, historical herbarium collections have been used to impute changes in species ranges (27, 28) and in traits (29, 30). Specimens were usually collected in flower and with data on time and place of collection. Although now dispersed among different herbaria, when compiled, these collections and their associated data constitute a sizable body of knowledge on historical plant distributions and phenologies.
We used 10,295 Rhododendron herbarium specimens from this remote but well-sampled area of the Himalaya to infer flowering time response to temperature from 1884 to 2009.
Methods
We located collections of the 36 Rhododendron species that occur in Lijiang County, preserved in the herbaria of Royal Botanic Garden Edinburgh (E); Royal Botanic Gardens (K); The Natural History Museum (BM); Kunming Institute of Botany, Chinese Academy of Sciences (KUN); Institute of Botany, Chinese Academy of Sciences (PE); Harvard University (A, GH); Missouri Botanical Garden (MO); Muséum National d'Histoire Naturelle (P); and Universität Wien (WU). In all, 10,295 specimens were digitally imaged, and their label information was compiled into databases, including date, elevation, and location of collection. Collection data from duplicate collections (those made at the same time and from the same plant, but held by different herbaria) were combined, and, where available, collection information was supplemented with information from collector field books, diaries, and maps.
We treated duplicates as a single collection, and removed collections without information on date and elevation, collections without flowers, and collections made outside the geographic bounds of our climate data. Finally, because Rhododendron species can exhibit off-season flowering in early winter (remontance), we removed collections made more than 80 d after the species mean flowering date (67 collections) to focus our analysis on peak flowering time. The final data set used for the analyses comprised 1,147 specimens that satisfied all criteria. For a supplementary analysis, we considered an additional 1,199 specimens that lacked information on day of collection but had information on month of collection (Table S1).
Mean elevations and mean flowering seasons differ among Yulong Rhododendron spp. To combine our analysis across species, flowering time (day) and elevation (meters above sea level) of each collection were converted to within-species deviation from the mean as flowering, i.e., days after (+) or before (−) the species mean collection date, and elevation, i.e., meters above (+) or below (−) the species mean elevation. Analyses presented herein are conducted on deviations for all collections.
Two sources of historical weather information are available for Yulong Mountain and the surrounding areas of the eastern Himalaya where Rhododendron specimens were collected. The China Meteorological Administration weather station [i.e., Lijiang weather station (LWS)] in Lijiang, 25 km south of Yulong Mountain, offers measured, daily temperature and precipitation information, but is only available beginning in 1952. The Global Historical Climate Network (GHCN) (31) provides a longer temporal range of climate data over a broader spatial scale: monthly temperature deviations above or below the mean for the observational period are available for 5° gridded cells. For GHCN data, we averaged the three eastern Himalayan grid cells (90°E to 105°E and 25°N to 30°N) that encompassed the specimen collection area. For the period of overlapping data 1952–2009, GHCN data were strongly correlated with LWS data (Pearson r = 0.76).
We used LWS data (1952–2009) and the 460 herbarium collections from the period to look for the effects of recent climate change on phenology. We used linear regression to test for change over year in average annual temperature of the year preceding collection (annual.temp-LWS) and precipitation (precip) and change in flowering over the same period. Simple linear regression analyses were carried out with base R functions (32).
With all 1,146 herbarium collections and GHCN data (1884–2009), we examined year-to-year response of flowering to average annual temperature of the year preceding collection (annual.temp), and average seasonal temperatures. The 12 mo preceding the main Rhododendron flowering peak in May were divided into four 3-mo periods: spring.temp, February to April; winter.temp, November to January; fall.temp, August to October; and summer.temp, May to July. We used backward stepwise selection to test which variables (elevation, annual.temp, spring.temp, winter.temp, fall.temp, and summer.temp) best explained variation in flowering. By using the MASS package in R (32, 33), we selected the multiple linear regression model that minimized Akaike information criterion. The flowering data were then broken into individual species, data from which were tested against the model selected by the stepwise regression.
Additionally, we examined robustness of our model by testing it against an expanded data set for flowering and elevation at a coarser temporal scale (all collections for which month of collection data were available; Table S1) and against a different environmental data set for annual.temp and fall.temp (temperature variables based on LWS data; Table S2). By using the LWS data set, we also tested for precipitation as a significant factor. To avoid problems with inflated P values caused by multiple collections within years, we tested the generic model against average annual flowering, weighted by number of collections (Table S3). We also used linear regression to test for an effect of differential collection intensity across years and across decades on annual and decadal mean flowering (Fig. S1), and for directional change in flowering over year during periods of warming and cooling (Fig. S2).
Results
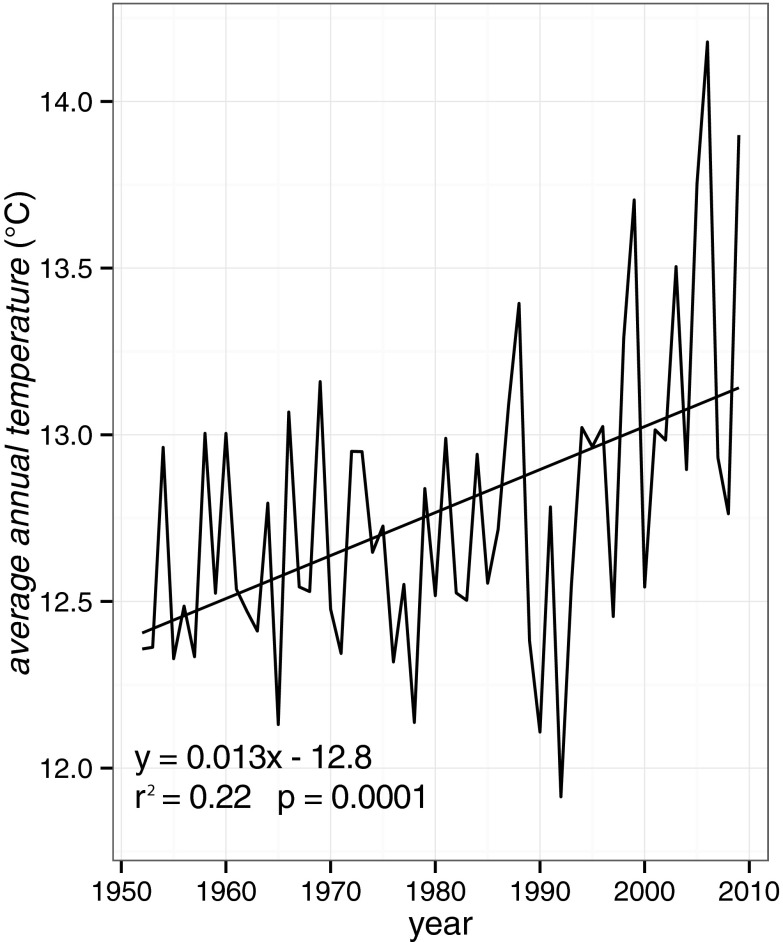
Over the recent period 1952–2009 (LWS data), mean annual temperature (annual.temp-LWS) significantly increased (0.13 °C per decade, P = 0.0001, r2 = 0.22; Fig. 1). Over this period, flowering showed no significant change (P = 0.14). Average annual precipitation (precip) also showed no significant change (P = 0.26).
Fig. 1.
Annual average temperatures recorded by the LWS (annual.temp-LWS) have significantly increased during the past 57 y (0.13 °C per decade, P = 0.0001, r2 = 0.22).
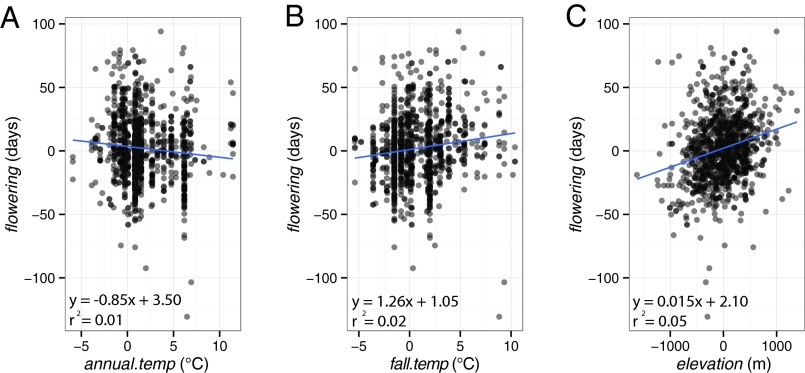
Over the past 125 y (1884–2009, GHCN data), for the full stepwise regression model considering all variables in explaining year-to-year change in flowering, the model that minimized AIC showed that flowering responds to annual.temp (2.27 d earlier per 1 °C), fall.temp (2.54 d later per 1 °C), and elevation (1.4 d later per 100 m); henceforth the “generic model” (Table 1). Considered separately as simple linear regressions, each of these three variables remained similar in effect size, sign, and significance (Fig. 2).
Table 1.
Flowering time significantly responds to weather
| Parameter | Estimate | SE | P value |
| Intercept | 4.11 | 0.84 | 1.23 × 10−06 |
| annual.temp | −2.27 (d/°C) | 0.31 | 6.68 × 10−13 |
| fall.temp | 2.54 (d/°C) | 0.32 | 1.42 × 10−14 |
| elevation | 0.014 (d/m) | 0.001 | 1.46 × 10−15 |
The variable flowering (i.e., day of collection deviation from the species mean) responds significantly to the additive effects of annual.temp (i.e., annual average temperature deviation of the year preceding collection), fall.temp (i.e., temperature deviation of the fall preceding collection) and elevation (i.e., elevation of collection deviation from the species mean) (adjusted r2 = 0.11).
Fig. 2.
Flowering time of Himalayan Rhododendron responds to annual temperature, fall temperature, and elevation. (A) flowering (i.e., day of collection deviation from the species mean) regresses negatively (−0.85 d/°C) and significantly (P = 0.002) with increasing annual.temp (i.e., annual average temperature deviation of the year preceding collection). (B) flowering regresses positively (1.26 d/°C) and significantly (P < 0.001) with increasing fall.temp (i.e., temperature deviation of the fall preceding collection). (C) flowering regresses positively (0.15 d per 100 m) and significantly (P < 0.001) with increasing elevation (i.e., elevation of collection deviation from the species mean). For visual clarity, points are 50% transparent; fully black points represent two or more collections. The model chosen by stepwise selection includes the additive effects of these three variables (Table 1).
The generic model (flowering∼annual.temp+fall.temp+elevation) was applied to collections of each species individually (Table 2). In general, species model term coefficients remained similar in effect size and sign to generic model coefficients (although not always significant given reduced sample size). Only one species, Rhododendron virgatum, showed a significant model coefficient that differed in sign from the generic model coefficients.
Table 2.
Flowering time of Rhododendron spp.
| Species | annual.temp, d/°C | fall.temp, d/°C | elevation, d/m | N |
| Rhododendron adenogynum | −10* | 8* | 0.01 | 35 |
| Rhododendron anthosphaerum | −2.5 | −9 | 0.02 | 36 |
| Rhododendron balfourianum | −4.4 | 5 | 0.04* | 14 |
| Rhododendron beesianum | −2.2* | 2* | 0.01 | 60 |
| Rhododendron bureavii | −0.5 | 1.9 | 0.01 | 25 |
| Rhododendron cephalanthum | 0.57 | 1.4 | 0.02* | 40 |
| Rhododendron cuneatum | −4.4* | 0.56 | 0.02* | 36 |
| Rhododendron decorum | −3.4* | 3.2* | 0.01 | 100 |
| Rhododendron delavayi | −6 | 3.9 | 0.03 | 15 |
| Rhododendron edgeworthii | −1.4 | 4.8* | 0 | 31 |
| Rhododendron fastigiatum | −7.7* | 4.1* | 0.02* | 15 |
| Rhododendron heliolepis | 2.1 | 0.41 | 0 | 23 |
| Rhododendron hippophaeoides | −7* | 3.6 | 0.02 | 33 |
| Rhododendron impeditum | −9.6* | 9.6* | −0.03 | 15 |
| Rhododendron irroratum | −4.6* | 2.3 | −0.01 | 28 |
| Rhododendron lepidotum | −0.54 | 3.3* | 0 | 69 |
| Rhododendron oreotrephes | −1.1 | 1.5 | 0.03* | 44 |
| Rhododendron phaeochrysum | −1.7 | 2.2* | 0.02* | 119 |
| Rhododendron primuliflorum | 0.31 | 3.6* | 0.02* | 75 |
| Rhododendron racemosum | −2.6 | 1.6 | 0.02* | 53 |
| Rhododendron rex | −3.5 | −0.8 | 0.01 | 22 |
| Rhododendron rubiginosum | −8.9* | 8.3* | 0.02 | 46 |
| Rhododendron rupicola | −1.9 | 5* | 0.02* | 49 |
| Rhododendron saluenense | −1.6 | −4 | 0.01 | 24 |
| Rhododendron uvariifolium | −8 | 0.75 | 0.04* | 18 |
| Rhododendron virgatum | 2.1 | −4.5* | 0.03* | 28 |
| Rhododendron yunnanense | −3.8* | 5.8* | −0.01 | 32 |
Flowering time of Rhododendron spp. is determined by the same variables as the generic model (Table 1), flowering∼annual.temp+fall.temp+elevation, where date of collection deviation from the species mean (flowering) is the dependent variable and previous annual average temperature deviation (annual.temp), previous fall average temperature deviation (fall.temp), and elevation of collection deviation from the species mean (elevation) are independent variables. Species coefficients are mostly of the same sign as the generic model. Species with a number of collections <13 included in the generic analysis but not considered separately here are: Rhododendron genestierianum, Rhododendron mollicomum, Rhododendron orthocladum, Rhododendron scabrifolium, Rhododendron tatsienense, Rhododendron telmateium, Rhododendron traillianum, Rhododendron trichostomum, and Rhododendron vernicosum.
Significant coefficients at P < 0.05.
Applied to an expanded data set of coarser temporal scale (all collections for which month of collection data were available), the generic model coefficients remained significant, and similar in effect size and sign (Table S1). A model analogous to the generic model but with temperature terms based on LWS data rather than GHCN data (flowering∼annual.temp-LWS+fall.temp-LWS+elevation) remained significant, and similar in sign (Table S2). The LWS data also included precipitation, but precipitation metrics added to this model were nonsignificant. A second analogous model with flowering and elevation treated as annual averages (floweringYEAR∼annual.temp+fall.temp+elevationYEAR) was significant, and similar in effect size and sign (Table S3), indicating that multiple subsamples (specimens) within year did not affect significance. Additionally, we did not find differential collection intensity across years to be significantly related to annual or decadal mean flowering (Fig. S1), indicating that mean collection time (flowering) is an unbiased estimate.
Discussion
The significant warming trend we found 1952–2009 (Fig. 1) is in accord with global trends (1) and with other studies focused on this area of the eastern Himalaya (20, 21, 34, 35). The lack of significant directional change in Rhododendron flowering time over this warming period might initially suggest that the genus is nonresponsive to temperature. Other multispecies studies have found some taxa not to exhibit response to climate, and a lack of response may lead studies to be discontinued or remain unpublished (14, 36, 37). However, a lack of phenological shift in response to climate may be a real biological effect with important consequences (38-40). In this study, despite the lack of directional change observed in Rhododendron during the warming period 1952–2009, we show that the genus is sensitive to year-to-year temperature changes (Table 1). The opposite and nearly equal effects of annual warming advancing flowering and fall warming delaying flowering may be responsible for this lack of directional change.
The effect of warmer average annual temperature advancing Rhododendron flowering may be caused by positive effects of temperature on the formation and growth of overwintering buds (41), which will become the following year’s flowers, and agrees with a body of literature showing warming resulting in advancing phenology (4, 15, 42, 43). The effect of warmer fall temperatures delaying Rhododendron flowering may be caused by a chilling requirement, which must be reached before overwintering buds will break dormancy and begin their spring growth (12, 14, 44). These results agree with recent landscape-level analysis of the Tibetan Plateau that suggested cold-season warming resulted in delayed phenology (12). Although that study and others (45) found chilling requirements were met in winter, Rhododendron is an alpine genus, and the first sustained freezing temperatures occur in their habitats in the fall. An ecosystem analysis (21) across the Himalayan region found that, even though most locations showed advanced phenology with spring warming, many also showed delayed phenology with fall warming. The role of chilling in driving phenological response to climate change extends beyond the Himalaya (37, 46,47), and it has been suggested to be a factor in taxa that previously were considered to be nonresponsive to climate change (14, 37).
The effect sizes seen in annual average warming on phenology (−2.27 d/°C) and fall warming on phenology (2.54 d/°C) are similar to effect sizes seen in similar studies (10, 48), although some studies have shown considerably greater effect sizes (11). Differences among species in the balance of these two contrasting phenological responses may account for the smaller generic effect size. Although we see general congruence between generic and species analyses, there are also likely to be real differences between species. One species for which we did not find significant effects, Rhododendron delavayi, has also not exhibited a chilling requirement in field studies (24). R. virgatum was the only species for which we found significant effects of a different sign than the generic model, with increasing fall.temp associated significantly with decreasing flowering (advanced phenology). R. virgatum and R. delavayi are among the lowest-elevation species in our sample. As chilling requirements have been proposed as an adaptation to cold climates (19, 24, 37), the lack of chilling requirement we observed in these two species could be related to the milder climatic conditions experienced by low-altitude rhododendron species.
The effects on phenology of warmer temperatures, delayed winter chilling, and other cues such as photoperiod are only beginning to be understood (49, 50). In this context, the validation of phenological models (50) and robust inference through combined methods (39) are important. Our model of contrasting effects in Himalayan Rhododendron is applicable to different data sets for Rhododendron collections (Table S1) and for temperature measurements (Table S2). Although phenological analyses of herbarium data offer unique insights into past responses, they model past responses only, and long-term phenological observation is necessary to fully understand present responses and model those in the future. To complement the herbarium data and test our models, we are directly monitoring Rhododendron phenology on Yulong Mountain, conducting artificial warming experiments, and documenting indigenous peoples’ observations of change.
This study joins other work from the past decade showing the value of herbarium collections to infer long-term phenology (10, 11, 43, 48, 51–63). These have increasingly shown that not only can the “messy” data from herbarium collections be used to infer phenology, but that these data can reveal the complex effects on phenology of geography (43), pollination (62), morphological traits (48, 52), and, in this study, the contrasting response of warming across different seasons. By drawing together historical collections dispersed across many herbaria, we show that herbarium records have the ability to provide information beyond systematics, and further afield than eastern North America and Europe. In addition, our analysis of specimens for which at least month of collection was available yielded similar results to those for which day of collection was available. This suggests that even “incomplete” data such as these, which are often discarded from analysis (11, 54, 56), may merit examination.
Finally, a decline in botanical collection in recent years noted by other authors (10, 54) is also reflected in our data (Fig. S1). In an era of rapid climate change, botanical and other natural history collections hold increasingly valuable data for understanding long-term change and supporting conservation (64). Strengthening specimen collection, curation, and data availability should be a priority.
Supplementary Material
Acknowledgments
We thank staff at all herbaria consulted, and undergraduates at University of Missouri–St. Louis, who provided essential aid to this research. Research was supported by Biodiversity Conservation and Sustainable Development in Southwest China United States National Science Foundation–Integrative Graduate Education and Research Traineeship DGE 0549369, National Key Basic Research Program of China Grant 2014CB954100, the Whitney R. Harris World Ecology Center, and National Science Foundation China Grant 31270524 (to S.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403376111/-/DCSupplemental.
References
- 1.IPCC . Climate Change 2013: The Physical Science Basis. Cambridge, UK: Cambridge Univ Press; 2013. [Google Scholar]
- 2.Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- 3.Bertin RI. Plant phenology and distribution in relation to recent climate change. J Torrey Bot Soc. 2008;135:126–146. [Google Scholar]
- 4.Inouye DW. Effects of climate change on phenology, frost damage, and floral abundance of montane wildflowers. Ecology. 2008;89(2):353–362. doi: 10.1890/06-2128.1. [DOI] [PubMed] [Google Scholar]
- 5.Kudo G. Effects of snow-free period on the phenology of alpine plants inhabiting snow patches. Arct Alp Res. 1991;23:436–443. [Google Scholar]
- 6.Kameyama Y, Kudo G. Flowering phenology influences seed production and outcrossing rate in populations of an alpine snowbed shrub, Phyllodoce aleutica: Effects of pollinators and self-incompatibility. Ann Bot (Lond) 2009;103(9):1385–1394. doi: 10.1093/aob/mcp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chuine II. Why does phenology drive species distribution? Philos Trans R Soc B. 2010;365:3149–3160. doi: 10.1098/rstb.2010.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller-Rushing AJ, Hoye TT, Inouye DW, Post E. The effects of phenological mismatches on demography. Philos Trans R Soc B. 2010;365:3177–3186. doi: 10.1098/rstb.2010.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparks TH, Yates TJ. The effect of spring temperature on the appearance dates of British butterflies 1883-1993. Ecography. 1997;20:368–374. [Google Scholar]
- 10.Houle G. Spring-flowering herbaceous plant species of the deciduous forests of eastern Canada and 20th century climate warming. Can J Res. 2007;37:505–512. [Google Scholar]
- 11.Robbirt KM, Davy AJ, Hutchings MJ, Roberts DL. Validation of biological collections as a source of phenological data for use in climate change studies: A case study with the orchid Ophrys sphegodes. J Ecol. 2010;99(1):235–241. [Google Scholar]
- 12.Yu H, Luedeling E, Xu J. Winter and spring warming result in delayed spring phenology on the Tibetan Plateau. Proc Natl Acad Sci USA. 2010;107(51):22151–22156. doi: 10.1073/pnas.1012490107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molau U. Responses to natural climatic variation and experimental warming in two tundra plant species with contrasting life forms: Cassiope tetragona and Ranunculus nivalis. Glob Change Biol. 1997;3:97–204. [Google Scholar]
- 14.Cook BI, Wolkovich EM, Parmesan C. Divergent responses to spring and winter warming drive community level flowering trends. Proc Natl Acad Sci USA. 2012;109(23):9000–9005. doi: 10.1073/pnas.1118364109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fitter AH, Fitter RS. Rapid changes in flowering time in British plants. Science. 2002;296(5573):1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 16.Miller-Rushing AJ, Primack RB. Global warming and flowering times in Thoreau’s Concord: a community perspective. Ecology. 2008;89(2):332–341. doi: 10.1890/07-0068.1. [DOI] [PubMed] [Google Scholar]
- 17.Meier N, Rutishauser T, Pfister C, Wanner H, Luterbacher J. Grape harvest dates as a proxy for Swiss April to August temperature reconstructions back to AD 1480. Geophys Res Lett. 2007;34:L20705. [Google Scholar]
- 18.Aono Y, Kazui K. Phenological data series of cherry tree flowering in Kyoto, Japan, and its application to reconstruction of springtime temperatures since the 9th century. Int J Climatol. 2008;28:905–914. [Google Scholar]
- 19.Xu J, et al. The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conserv Biol. 2009;23(3):520–530. doi: 10.1111/j.1523-1739.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker BB, Moseley RK. Advancing treeline and retreating glaciers: Implications for conservation in Yunnan, P.R. China. Arct Antarct Alp Res. 2007;39:200–209. [Google Scholar]
- 21.Shrestha UB, Gautam S, Bawa KS. Widespread climate change in the Himalayas and associated changes in local ecosystems. PLoS ONE. 2012;7(5):e36741. doi: 10.1371/journal.pone.0036741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma E, et al. Climate Change Impacts and Vulnerability in the Eastern Himalayas. Kathmandu, Nepal: Centre for Integrated Mountain Development; 2010. [Google Scholar]
- 23.Salick J, Fang Z, Byg A. Eastern Himalayan alpine plant ecology, Tibetan ethnobotany, and climate change. Glob Environ Change. 2009;19:147–155. [Google Scholar]
- 24.Ranjitkar S, Luedeling E, Shrestha KK, Guan K, Xu J. Flowering phenology of tree rhododendron along an elevation gradient in two sites in the Eastern Himalayas. Int J Biometeorol. 2013;57(2):225–240. doi: 10.1007/s00484-012-0548-4. [DOI] [PubMed] [Google Scholar]
- 25.Georgian E, Emshwiller E. Shared and separate knowledge among eight cultural groups based on ethnobotanical uses of Rhododendron (Ericaceae) in Yunnan Province, China. Econ Bot. 2013;67:191–202. [Google Scholar]
- 26.Mueggler E. The Paper Road: Archive and experience in the botanical exploration of west China and Tibet. Berkeley: Univ California Press; 2011. [Google Scholar]
- 27.Loiselle BA, Jørgensen PM, Consiglio T. Predicting species distributions from herbarium collections: Does climate bias in collection sampling influence model outcomes? J Biogeogr. 2008;35:105–116. [Google Scholar]
- 28.Applequist WL, Mcglinn DJ, Miller M, Long QG, Miller JS. How well do herbarium data predict the location of present populations? A test using Echinacea species in Missouri. Biodivers Conserv. 2006;16:1397–1407. [Google Scholar]
- 29.Parkhurst DF. The adaptive significance of stomatal occurrence on one or both surfaces of leaves. J Ecol. 1978;66:367–383. [Google Scholar]
- 30.Law W, Salick J. Human-induced dwarfing of Himalayan snow lotus, Saussurea laniceps (Asteraceae) Proc Natl Acad Sci USA. 2005;102(29):10218–10220. doi: 10.1073/pnas.0502931102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawrimore JH, et al. An overview of the Global Historical Climatology Network monthly mean temperature data set, version 3. J Geophys Res. 2011;116:D19121. [Google Scholar]
- 32.R Core Team . R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 33.Venables WN, Ripley BD. Modern Applied Statistics with S. Berlin: Springer; 2002. [Google Scholar]
- 34.Fan Z-X, Bräuning A, Yang B, Cao K-F. Tree ring density-based summer temperature reconstruction for the central Hengduan Mountains in southern China. Global Planet Change. 2009;65:1–11. [Google Scholar]
- 35.Li Z-S, Zhang Q-B, Ma K. Tree-ring reconstruction of summer temperature for A.D. 1475-2003 in the central Hengduan Mountains, Northwestern Yunnan, China. Clim Change. 2012;110(1–2):455–467. [Google Scholar]
- 36.Willis CG, Ruhfel B, Primack RB, Miller-Rushing AJ, Davis CC. Phylogenetic patterns of species loss in Thoreau’s woods are driven by climate change. Proc Natl Acad Sci USA. 2008;105(44):17029–17033. doi: 10.1073/pnas.0806446105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luedeling E, Gassner A. Partial least squares regression for analyzing walnut phenology in California. Agric Meteorol. 2012;158-159:43–52. [Google Scholar]
- 38.Polgar C, Gallinat A, Primack RB. Drivers of leaf-out phenology and their implications for species invasions: Insights from Thoreau’s Concord. New Phytol. 2014;202(1):106–115. doi: 10.1111/nph.12647. [DOI] [PubMed] [Google Scholar]
- 39.Merilä J, Hendry AP. Climate change, adaptation, and phenotypic plasticity: The problem and the evidence. Evol Appl. 2014;7(1):1–14. doi: 10.1111/eva.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Visser ME, Both C. Shifts in phenology due to global climate change: The need for a yardstick. Proc Biol Sci. 2005;272(1581):2561–2569. doi: 10.1098/rspb.2005.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widrlechner MP, Pellett HM, Ascher PD. The timing of microsporogenesis in deciduous Azalea. J Am Rhododendron Soc. 1983;37:91–94. [Google Scholar]
- 42.Parmesan C. Ecological and evolutionary responses to recent climate change. Annu Rev Ecol Evol Syst. 2009;37:637–669. [Google Scholar]
- 43.Kauserud H, et al. Mushroom fruiting and climate change. Proc Natl Acad Sci USA. 2008;105(10):3811–3814. doi: 10.1073/pnas.0709037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pope KS, et al. Detecting nonlinear response of spring phenology to climate change by Bayesian analysis. Glob Change Biol. 2013;19(5):1518–1525. doi: 10.1111/gcb.12130. [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Dai J, Ranjitkar S, Xu J, Luedeling E. Response of chestnut phenology in China to climate variation and change. Agric Meteorol. 2013;180:164–172. [Google Scholar]
- 46.Luedeling E, Brown PH. A global analysis of the comparability of winter chill models for fruit and nut trees. Int J Biometeorol. 2011;55(3):411–421. doi: 10.1007/s00484-010-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laube J, et al. Chilling outweighs photoperiod in preventing precocious spring development. Glob Change Biol. 2014;20(1):170–182. doi: 10.1111/gcb.12360. [DOI] [PubMed] [Google Scholar]
- 48.Panchen ZA, Primack RB, Anisko T, Lyons RE. Herbarium specimens, photographs, and field observations show Philadelphia area plants are responding to climate change. Am J Bot. 2012;99(4):751–756. doi: 10.3732/ajb.1100198. [DOI] [PubMed] [Google Scholar]
- 49.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327(5972):1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- 50.Richardson AD, et al. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric Meteorol. 2013;169:156–173. [Google Scholar]
- 51.Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am J Bot. 2004;91(8):1260–1264. doi: 10.3732/ajb.91.8.1260. [DOI] [PubMed] [Google Scholar]
- 52.Bolmgren K, Lonnberg K. Herbarium data reveal an association between fleshy fruit type and earlier flowering time. Int J Plant Sci. 2005;166:663–670. [Google Scholar]
- 53.Bowers JE. El Niño and displays of spring-flowering annuals in the Mojave and Sonoran deserts. J Torrey Bot Soc. 2005;132:38–49. [Google Scholar]
- 54.Lavoie C, Lachance D. A new herbarium-based method for reconstructing the phenology of plant species across large areas. Am J Bot. 2006;93(4):512–516. doi: 10.3732/ajb.93.4.512. [DOI] [PubMed] [Google Scholar]
- 55.Miller-Rushing AJ, Primack RB, Primack D, Mukunda S. Photographs and herbarium specimens as tools to document phenological changes in response to global warming. Am J Bot. 2006;93(11):1667–1674. doi: 10.3732/ajb.93.11.1667. [DOI] [PubMed] [Google Scholar]
- 56.MacGillivray F, Hudson IL, Lowe AJ. Herbarium collections and photographic images: alternative data sources for phenological research. In: Hudson IL, Keatley MR, editors. Phenological Research: Methods for Environmental and Climate Change Analysis. Berlin: Springer; 2009. pp. 425–461. [Google Scholar]
- 57.Gallagher R, Hughes L, Leishman M. Phenological trends among Australian alpine species: Using herbarium records to identify climate-change indicators. Aust J Bot. 2009;57:1–9. [Google Scholar]
- 58.Rumpff L, Coates F, Morgan JW. Biological indicators of climate change: Evidence from long-term flowering records of plants along the Victorian coast, Australia. Aust J Bot. 2010;58:428. [Google Scholar]
- 59.Neil KL, Landrum L, Wu J. Effects of urbanization on flowering phenology in the metropolitan phoenix region of USA: Findings from herbarium records. J Arid Environ. 2010;74:440–444. [Google Scholar]
- 60.Gaira KS, Dhar U, Belwal OK. Potential of herbarium records to sequence phenological pattern: A case study of Aconitum heterophyllum in the Himalaya. Biodivers Conserv. 2011;20:2201–2210. [Google Scholar]
- 61.Park IW. Digital herbarium archives as a spatially extensive, taxonomically discriminate phenological record; a comparison to MODIS satellite imagery. Int J Biometeorol. 2012;56(6):1179–1182. doi: 10.1007/s00484-012-0521-2. [DOI] [PubMed] [Google Scholar]
- 62.Molnár AV, et al. Pollination mode predicts phenological response to climate change in terrestrial orchids: A case study from central Europe. J Ecol. 2012;100:1141–1152. [Google Scholar]
- 63.Diskin E, Proctor H, Jebb M, Sparks T, Donnelly A. The phenology of Rubus fruticosus in Ireland: Herbarium specimens provide evidence for the response of phenophases to temperature, with implications for climate warming. Int J Biometeorol. 2012;56(6):1103–1111. doi: 10.1007/s00484-012-0524-z. [DOI] [PubMed] [Google Scholar]
- 64.Hart R, Law W, Wyse Jackson P. Biocultural collections for conservation. In: Konchar K, Salick J, Nesbitt M, editors. Curating Biocultural Collections: A Handbook. London: Royal Botanic Gardens, Kew; 2014. pp. 319–336. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.