Significance
Many neurotransmitters dampen excitability in the heart and brain by activating G-protein–gated inwardly rectifying K+ (GIRK) channels. The lack of selective pharmacological tools for GIRK channels has hindered investigations into their physiological and pathophysiological relevance. Here, we examined the mechanisms underlying the activation of GIRK channels by ML297, the prototypical member of a new family of small molecule GIRK channel modulators. ML297 activates GIRK channels via a unique mechanism that requires two amino acids specific to the GIRK1 subunit. In addition, ML297 reduces anxiety-related behavior in mice, in a GIRK1-dependent manner, without triggering sedation or addiction-related behavior. Thus, ML297 is a new tool for probing the therapeutic potential of GIRK channel modulation, which may benefit individuals with anxiety-related disorders.
Keywords: electrophysiology, structure–activity relationship
Abstract
ML297 was recently identified as a potent and selective small molecule agonist of G-protein–gated inwardly rectifying K+ (GIRK/Kir3) channels. Here, we show ML297 selectively activates recombinant neuronal GIRK channels containing the GIRK1 subunit in a manner that requires phosphatidylinositol-4,5-bisphosphate (PIP2), but is otherwise distinct from receptor-induced, G-protein–dependent channel activation. Two amino acids unique to the pore helix (F137) and second membrane-spanning (D173) domain of GIRK1 were identified as necessary and sufficient for the selective activation of GIRK channels by ML297. Further investigation into the behavioral effects of ML297 revealed that in addition to its known antiseizure efficacy, ML297 decreases anxiety-related behavior without sedative or addictive liabilities. Importantly, the anxiolytic effect of ML297 was lost in mice lacking GIRK1. Thus, activation of GIRK1-containing channels by ML297 or derivatives may represent a new approach to the treatment of seizure and/or anxiety disorders.
Signal transduction involving inhibitory (Gi/o) G proteins titrates the excitability of neurons, cardiac myocytes, and endocrine cells, influencing behavior, cardiac output, and energy homeostasis (1). G-protein–gated inwardly rectifying potassium (K+) (GIRK/Kir3) channels are a common effector for Gi/o-dependent signaling pathways in the heart and nervous system (2, 3). Polymorphisms and mutations in human GIRK channels have been linked to arrhythmias, hyperaldosteronism (and associated hypertension), schizophrenia, sensitivity to analgesics, and alcohol dependence (1).
GIRK channels are activated by binding of the G protein Gβγ subunit (1–3). Gβγ binding strengthens channel affinity for phosphatidylinositol-4,5-bisphosphate (PIP2), a necessary cofactor for channel gating (4, 5). GIRK channels are also activated in a G-protein–independent manner by ethanol (6, 7), volatile anesthetics (8, 9), and naringin (10). Many psychoactive and clinically relevant compounds with other primary molecular targets inhibit GIRK channels, albeit at relatively high doses (1, 11). The lack of selective GIRK channel modulators, and in particular, drugs that discriminate among GIRK channel subtypes, has hampered investigation into their physiological relevance and therapeutic potential.
GIRK channels are homo- and heterotetramers formed by GIRK1, GIRK2, GIRK3, and GIRK4 subunits (2, 3). GIRK subunits exhibit overlapping but distinct cellular expression patterns, potentially yielding multiple channel subtypes (1). Although it cannot form functional homomers (12), GIRK1 is an integral subunit of the cardiac GIRK channel and most neuronal GIRK channels (13, 14). GIRK1 confers robust basal and receptor-dependent activity to GIRK heteromers, attributable in part to unique residues in the pore and second transmembrane domain (15–17). The intracellular C-terminal domain also contributes to the potentiating influence of GIRK1 on channel activity, likely due to the presence of unique structures that modify the interaction between the channel and Gβγ, Gα, and PIP2 (1–3).
Recently, we identified a class of small molecule GIRK channel modulators (18). The prototype (ML297) is a potent agonist selective for GIRK1-containing channels. At present, however, the selectivity of ML297 in vivo is untested and mechanisms underlying its selective activation of GIRK1-containing channels are unclear. The goals of this study were to identify the structural basis of ML297 efficacy and selectivity for GIRK1-containing channels, explore the mechanisms underlying channel activation, and probe further its therapeutic potential. We report that ML297 activates GIRK1-containing channels in unique fashion, requiring only two amino acids specific to GIRK1, and suggest that ML297 or derivatives might represent a class of anxiolytic compounds with limited sedative and addictive liabilities.
Results
We began by comparing whole-cell currents evoked by ML297 and the GABAB receptor (GABABR) agonist baclofen in transfected HEK293 cells. ML297 evoked concentration-dependent inward currents in cells expressing GABABR and the prototypical neuronal GIRK channel (GIRK1/2; Fig. 1A). The EC50 for ML297-induced activation of GIRK1/2 channels was 233 ± 38 nM; 10 μM ML297 evoked a maximal response (Fig. S1A). Activation and deactivation kinetics of the ML297-induced current were concentration dependent, increasing and decreasing, respectively, with higher ML297 concentrations (Fig. S1B). Maximal ML297-induced currents were larger than those evoked by a saturating concentration of baclofen (100 μM; Fig. 1B). Importantly, ML297 did not induce currents in cells expressing GABABR and GIRK2 alone, whereas baclofen evoked reliable responses in these cells (Fig. 1B). Reversal potentials measured for basal, baclofen-induced, and ML297-induced currents carried by GIRK1/2 channels were comparable and close to the predicted value for a K+-selective channel as measured in a high-K+ bath solution (EK = −43 mV; Fig. 1C). Inward rectification, however, was markedly stronger for basal and baclofen-induced currents than for ML297-induced current (Fig. 1 C and D).
Fig. 1.
ML297- and baclofen-induced GIRK currents. (A) Trace showing the effect of increasing concentrations of ML297 on holding current (Vhold = −70 mV; 25 mM K+ bath solution) in a cell expressing GABABR and GIRK1/2. Horizontal bars denote the duration of ML297 application. (B) Peak current densities evoked by vehicle (V, 0.1% DMSO), baclofen (B, 100 μM), and ML297 (M, 10 μM) in cells expressing GABABR and GIRK1/2 (F2,26 = 12.2, P < 0.001, n = 4–15 per group; **P < 0.01), or GABABR and GIRK2 (t10 = 3.1, n = 6 per group; *P < 0.05). (C) I-V plot for basal GIRK current (black circles), together with plots for GIRK currents evoked by baclofen (100 μM; red squares) and ML297 (10 μM; blue triangles) (n = 3 per group). (D) Rectification index (ratio of current measured at 0 mV and −80 mV) for basal GIRK current, or GIRK currents evoked by baclofen (B, 100 μM) or ML297 (M, 10 μM). A significant impact of group on rectification index was observed (F2,11 = 5.5, P < 0.05). *P < 0.05 vs. basal and baclofen groups.
We next measured the effect of ML297 on GIRK1/2 channels in outside-out patches from transfected cells (Fig. S2). ML297 evoked a concentration-dependent increase in gating (NPo) of GIRK1/2 channels (Fig. S2 A and B), but had no effect on GIRK2 homomers (Fig. S2B). At the highest ML297 concentration tested (1 μM), GIRK1/2 channel activity was enhanced eightfold over basal levels, complicating accurate extraction of unitary channel properties. At lower concentrations (100 nM), however, we observed that ML297 promoted the occurrence of longer opening events without altering single channel conductance (29.3 ± 0.5 pS before vs. 31.3 ± 1.5 pS after; P = 0.3) (Fig. S2 A and C). Baclofen (100 μM) also increased channel activity without altering single-channel conductance (29.3 ± 0.5 pS before vs. 29.1 ± 1.6 pS after; P = 0.9). In contrast to ML297, however, baclofen primarily increased the frequency of shorter-lived events (Fig. S2D). Collectively, data from whole-cell and single-channel experiments suggested that baclofen and ML297 activate GIRK channels in distinct manners.
Whereas ML297 activates GIRK1-containing channels in the presence of the Gi/o G-protein inhibitor pertussis toxin (18), the dependence of ML297 efficacy on Gβγ is uncertain. To address this issue, we compared baclofen- and ML297-induced currents carried by GIRK1/2 channels in the absence or presence of a coexpressed C-terminal fragment of the G-protein–coupled receptor kinase-3 (GRK3ct), a freely diffusible scavenger that can bind free Gβγ dimers and inhibit Gβγ-dependent signaling (19, 20). Whereas baclofen-induced currents were suppressed in cells expressing GRK3ct, ML297-induced currents were unaffected (Fig. 2 A and B). Thus, ML297-induced activation of GIRK1-containing GIRK channels, unlike receptor-induced channel activation, does not require Gβγ.
Fig. 2.
ML297- and baclofen-induced GIRK currents: Gβγ and PIP2 dependence. (A) Currents evoked by baclofen (100 μM) and ML297 (10 μM) in cells expressing GABABR and GIRK1/2, in the absence (Upper) or presence (Lower) of the Gβγ scavenger, GRK3ct. (B) Summary of GRK3ct effects on responses induced by baclofen (t9 = 4.2; **P < 0.01) and ML297 (t9 = 0.001; P = 1.0) (n = 5–6 per group). (C) Trace showing the effect of PIP2 depletion on currents evoked by baclofen (100 μM) and ML297 (10 μM) in a cell expressing GABABR, GIRK1/2, and Dr-VSP. Black dashes denote the cell being held at −70 mV (inactive Dr-VSP). Orange step symbols denote the Dr-VSP activation protocol (alternating 500-ms voltage steps between −70 and +100 mV). At least 120 steps to +100 mV were made before the second application of baclofen and ML297. (D) Responses evoked by baclofen (100 μM) and ML297 (10 μM) in cells expressing GABABR, GIRK1/2, and Dr-VSP. Peak amplitude of the second response was normalized to the amplitude of the first response. Dr-VSP activation significantly decreased baclofen-induced (t16 = 4.6; ***P < 0.001) and ML297-induced (t16 = 2.3; *P < 0.05) currents (n = 9 per group). (E) Responses evoked by baclofen and ML297 in cells expressing GABABR and GIRK1/2, but not Dr-VSP. No difference in peak current density was observed between first and second applications of either baclofen (t6 = 0.7; P = 0.5) or ML297 (t6 = 0.1; P = 0.9) groups (n = 4 per group).
To determine whether the activation of GIRK1-containing channels by ML297 requires PIP2, we next measured the impact of the Danio rerio voltage-sensitive phosphatase (Dr-VSP) on baclofen- and ML297-induced GIRK currents. Dr-VSP is activated by strong depolarization, leading to depletion of membrane-bound PIP2 (21). GIRK currents induced by baclofen and ML297 were recorded twice in each cell, once before and once after Dr-VSP activation (Fig. 2C). GIRK currents induced by baclofen and ML297 were attenuated following Dr-VSP activation (Fig. 2 C and D). In contrast, no attenuation of GIRK currents was seen in cells lacking Dr-VSP (Fig. 2E). Thus, ML297-induced activation of GIRK1-containing channels, like other modes of GIRK channel activation, requires PIP2.
To test whether ML297 can activate GIRK1-containing channels in cells that normally express GIRK channels, we measured ML297-induced currents in cultured hippocampal neurons, which express GIRK1–3 (22). ML297 evoked a concentration-dependent inward current in large pyramidal-shaped hippocampal neurons from wild-type mice (Fig. S3A). The EC50 (377 ± 70 nM) and impact of ML297 concentration on current activation and deactivation kinetics was comparable to that seen with recombinant GIRK1/2 channels (Fig. S3 B and C). Currents evoked by 10 μM ML297 were comparable in magnitude to those evoked by a saturating concentration of baclofen (100 μM; Fig. 3 A and B). Unlike baclofen-induced responses, however, ML297-induced currents showed little acute desensitization (Fig. 3 A and C), and the kinetics were slower than those measured for baclofen (Fig. 3 A and D). Importantly, whereas ML297 had negligible effects on holding current in neurons from Girk1−/− mice, baclofen evoked reliable (albeit small) currents in these neurons (Fig. 3 A and B), likely attributable to activation of residual GIRK1-lacking channels (17).
Fig. 3.
ML297- and baclofen-induced GIRK currents in hippocampal neurons. (A) Traces showing the effects of vehicle (0.1% DMSO), baclofen (100 µM), and ML297 (10 µM) on holding currents in neurons from wild-type (Upper) and Girk1−/− (Lower) mice. (B) Summary of peak current densities evoked by vehicle, baclofen, and ML297 in neurons from wild-type (WT) and Girk1−/− (Girk1) mice; a significant genotype × drug interaction was observed (F2,43 = 16.4; P < 0.001). ***P < 0.001 (within drug); ###P < 0.001 vs. ML297 (within genotype). (C) Acute desensitization of currents induced by baclofen (bac, 100 μM) and ML297 (10 μM), measured by comparing peak drug-induced currents with currents measured 20 s after drug application. Baclofen-induced currents showed modest acute desensitization (∼20%), whereas ML297-induced currents did not (t12 = 4.2; **P < 0.01). (D) Activation (t8 = 12.8; ***P < 0.001) and deactivation (t8 = 6.5; ***P < 0.001) kinetics of currents induced by baclofen and ML297 in hippocampal neurons from wild-type mice.
To identify structural elements in GIRK1 required for ML297-induced channel activation, we used a thallium flux assay to compare responses induced by ML297 and the short-chain alcohol and nonselective GIRK channel agonist methyl pentanediol (MPD) in cells coexpressing GIRK2 and either GIRK1 or one of a series of chimeras harboring discrete GIRK1 subdomains on a GIRK2 backbone (Fig. S4A). We showed previously that these chimeras interact with wild-type GIRK2 and promote levels of GIRK2-containing channels on the cell surface comparable to wild-type GIRK1 (17). Here, we found that, whereas MPD activated GIRK-dependent responses in all transfected cells, ML297 sensitivity required the P–M2 domain of GIRK1, which contains the pore helix/K+ selectivity filter and second membrane-spanning domain (Fig. S4B).
We next mutated residues in GIRK1 to match the corresponding residues in GIRK2, within the P–M2 domain (Fig. S4A), in an effort to identify specific amino acids required for the ML297-induced activation of GIRK1-containing channels. Two GIRK1 residues (F137 and D173) were identified using this approach (Fig. S4 A and B). In cells expressing GIRK2 and either GIRK1F137S or GIRK1D173N, ML297-induced responses were minimal, whereas MPD sensitivity was preserved. Introduction of either of these GIRK1 residues individually into GIRK2 (S148F or N184D) failed to confer ML297 sensitivity. In cells expressing GIRK2 and the GIRK2 mutant harboring both GIRK1 residues (GIRK2FD), however, ML297-induced sensitivity was restored.
To extend these findings, ML297-induced whole-cell currents were compared in cells expressing GIRK2 and either GIRK1 or GIRK1 mutant (Fig. 4). ML297-induced currents were strongly attenuated or undetectable in cells expressing GIRK2 and either GIRK1F137S, GIRK1D173N, or GIRK1SN (Fig. 4C, Upper). ML297-induced currents were also small or undetectable in cells expressing GIRK2 together with GIRK2, GIRK2S148F, or GIRK2N184D (Fig. 4C, Lower). ML297-induced current amplitudes were normal, however, in cells coexpressing GIRK2 and GIRK2FD (Fig. 4 B and C). Moreover, the current/voltage (I-V) profiles of basal, ML297-induced, and baclofen-induced currents carried by GIRK2FD/GIRK2 channels were comparable to those observed for GIRK1/2 channels, with ML297 significantly weakening channel inward rectification (Fig. S5 A and B). Thus, GIRK1 residues F137 and D173 are necessary for the ML297-induced activation of GIRK1-containing channels, and are sufficient to confer ML297 sensitivity to GIRK2. Importantly, however, ML297-induced responses were small to nonexistent in cells expressing only GIRK2FD (Fig. 4C, Lower), indicating that robust GIRK channel activation by ML297 requires heteromeric contributions at two critical positions (F137/S148 and D173/N184) within the channel core.
Fig. 4.
Structural elements in GIRK1 required for ML297 activation. (A) Alignment of the P–M2 regions of GIRK1 and GIRK2: pore helix, K+-selectivity filter, second extracellular domain (ex-2), and M2 (second transmembrane spanning domain). Shaded boxes highlight the unique GIRK1 residues identified as necessary and sufficient for ML297 activation of GIRK channels. (B) Currents evoked by ML297 (10 µM) in cells expressing GABABR and either GIRK1/2 (Upper) or GIRK2FD/GIRK2 (Lower). (C) ML297-induced peak current density in cells expressing GIRK2 and either GIRK1 or GIRK1 mutant (Upper series, black), or GIRK2 or GIRK2 mutant (Lower series, white) (n = 4–6 per group). A significant impact of channel type was found for the GIRK1 mutant series (F3,20 = 18.9, P < 0.001), and for the GIRK2 mutant series (F3,19 = 45.4, P < 0.001). **P < 0.01 vs. GIRK1; ++P < 0.01 vs. GIRK2. Cells expressing only GIRK2FD exhibited small ML297-induced currents (gray; t9 = 6.4, n = 5–6 per group; ###P < 0.001). Significant differences were found for (D) activation (t12 = 4.3; **P < 0.01) and (E) deactivation kinetics (t10 = 3.6; **P < 0.01) of ML297-induced currents carried by cells expressing GIRK2 and either GIRK1 or GIRK2FD (n = 6–8 per group).
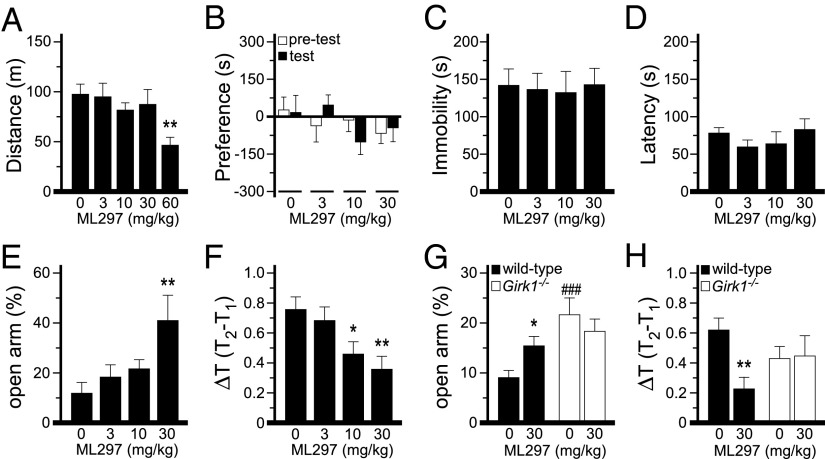
Previously, we reported that ML297 was protective in rat epilepsy models (18). To further investigate the behavioral effects of ML297, we probed its efficacy in tests of motor activity, reward, depression, and anxiety. We began with an open-field motor activity test involving wild-type C57BL/6J mice. We found that at the highest dose tested (60 mg/kg i.p.), ML297 suppressed motor activity (Fig. 5A). Lower doses (3, 10, and 30 mg/kg), however, had no impact on motor activity. Thus, to avoid the potentially confounding effect of ML297 on motor activity, 30 mg/kg was selected as the maximum dose used in subsequent behavioral tests.
Fig. 5.
Behavioral impact of ML297. (A) Total distance traveled (m) by C57BL/6J mice in an open-field following i.p. injection of ML297 (0/vehicle, 3, 10, 30, 60 mg/kg; n = 11–12 mice per dose). A significant effect of dose was observed (F4,58 = 3.2, P < 0.05). **P < 0.01 vs. 0/vehicle. (B) Difference in time spent by C57BL/6J mice between the drug-paired and unpaired sides of a CPP chamber (preference), measured on the first day of testing (pretest, white bars), and after four conditioning sessions with ML297 (0, 3, 10, or 30 mg/kg i.p.; n = 12–13 mice per dose). No group differences were observed for the pretest (F3,48 = 0.5, P = 0.65) or on test day (F3,48 = 1.5, P = 0.24). (C and D) Total immobility time (C) (F3,43 = 0.5, P = 0.70) and latency to first immobility period (D) (F3,43 = 0.9, P = 0.46) measured during a 6-min forced swim test conducted 30 min after injection of ML297 (0, 3, 10, or 30 mg/kg i.p.; n = 11 mice per dose). (E) Percentage of time spent in the open arms (F3,43 = 3.8, P < 0.05) in a 5-min elevated plus maze test performed 30 min after injection of ML297 (0/vehicle, 3, 10, or 30 mg/kg i.p.; n = 10–12 mice per dose). **P < 0.01 vs. 0/vehicle. (F) Effect of ML297 on stress-induced hyperthermia. The change in temperature (ΔT, in °C) from the point of stress (rectal temperature measurement 1, T1) to 10 min after stress (rectal temperature measurement 2, T2), measured 30 min after injection of ML297 (0, 3, 10, or 30 mg/kg i.p.; n = 12 mice per dose). A significant effect of dose was detected (F3,47 = 4.9, P < 0.01). *P < 0.05, **P < 0.01, respectively, vs. 0/vehicle. (G) Percent time spent in the open arms by wild-type and Girk1−/− mice in a 5-min elevated plus maze test performed 30 min after injection of ML297 (0 or 30 mg/kg i.p.; n = 11–16 mice per group). A significant drug × genotype interaction (F1,52 = 7.0, P < 0.01) was observed. *P < 0.05 vs. 0 mg/kg (within genotype); ###P < 0.001 vs. wild type (within dose). (H) Effect of ML297 (0 or 30 mg/kg i.p.; n = 11–16 mice per group) on stress-induced hyperthermia in wild-type and Girk1−/− mice. A significant drug × genotype interaction (F1,36 = 4.2, P < 0.05) was observed. **P < 0.01 vs. 0 mg/kg (within genotype).
ML297 did not exhibit significant reinforcing effects in wild-type mice as measured using a conditioned place preference (CPP) test (Fig. 5B), nor did it exhibit antidepressant efficacy in the forced swim test (FST) (Fig. 5 C and D). ML297 did evoke a dose-dependent decrease in anxiety-related behavior in the elevated plus maze (EPM) test, increasing time spent in the open arms of the maze (Fig. 5E). Notably, motor activity measured during the EPM test did not differ as a function of ML297 dose (Fig. S6). Moreover, ML297 produced a dose-dependent suppression of stress-induced hyperthermia (SIH) (Fig. 5F), a motor-activity–independent physiological stress response blunted by anxiolytic drugs (23).
We repeated EPM and SIH tests using a cohort of wild-type and Girk1−/− siblings. Consistent with published data for Girk2−/− mice (24, 25), Girk1−/− mice exhibited less anxiety-related behavior than wild-type controls in the EPM test (Fig. 5G). No additional anxiolytic effect of ML297, however, was observed in Girk1−/− mice, whereas ML297 increased time spent in the open arm in the wild-type group. Similarly, no anxiolytic effect of ML297 was observed in Girk1−/− mice during the SIH test (Fig. 5H). Collectively, these results argue that the anxiolytic effect of ML297 observed in wild-type mice is attributable to activation of GIRK1-containing channels.
Discussion
The classical mode of GIRK channel activation involves the receptor-induced activation of Gi/o G proteins, which facilitates an interaction between channel and Gβγ. Three key lines of evidence argue that ML297-induced activation of GIRK channels differs mechanistically from this mode of channel activation. First, ML297 activation of GIRK1-containing channels is not impacted by pertussis toxin (18), which prevents the receptor-induced activation of Gi/o G proteins. Second, receptor-induced activation of GIRK channels is precluded by overexpression of a Gβγ scavenger (Fig. 2), whereas ML297-induced channel activation is unaltered. Third, ML297 selectively activates GIRK1-containing channels, whereas Gβγ activates both GIRK1-containing and GIRK1-lacking channels (e.g., ref. 26).
Clear resolution of the channel–Gβγ interaction was obtained with the cocrystallization of Gβγ and a GIRK2 homomer (27). Gβγ binds to an outward-facing surface created by two adjacent GIRK cytoplasmic domains (the βK, βL, βM, and βN sheets from one subunit, and the βD and βE sheets from the adjacent subunit; Fig. S7A). GIRK2 residues mediating the GIRK–Gβγ interaction include Q248 and F254 in βD–βE, and L342-T343-L344 in βL–βM. The GIRK–Gβγ interaction is electrostatic, facilitated by glutamic and aspartic acid residues found in the βL–βM loop that attract the electropositive binding face on Gβγ. Importantly, key elements of this interaction interface are conserved across all GIRK subunits, including GIRK1.
ML297-induced activation of GIRK channels also differs from channel modulation by other known channel activators. Intracellular Na+ (EC50 30–40 mM) activates neuronal and cardiac GIRK channels in a manner dependent on an aspartic acid residue found in the βC–βD loop of GIRK2 and GIRK4, respectively (28, 29). This residue, which contributes to the binding site for Na+ (30) (Fig. S7A), is not found in GIRK1. Alcohols activate both GIRK1-containing and -lacking channels in a G-protein–independent manner, without altering the strong rectification profile (6, 7). The hydrophobic alcohol-binding pocket in GIRK2 homomers is formed by a residue in the N terminus (Y58) and two residues in the βL–βM sheet from one subunit (L342 and Y349), together with three residues in the βD–βE sheet from the adjacent subunit (I244, P256, and L257) (31) (Fig. S7A). As is the case for structures involved in mediating channel interactions with Gβγ, the structures mediating channel–alcohol interactions are largely conserved in GIRK1 (6, 7). Furthermore, selective GIRK1 mutations can yield channels (e.g., GIRK1SN/GIRK2) that retains alcohol (MPD) sensitivity but are ML297 insensitive.
Whereas channel activation via Gβγ, ethanol, and Na+ involves unique structural determinants, these agents (and ML297) require membrane-bound PIP2 to activate GIRK channels. PIP2 interacts with lysine residues found at the interface between the transmembrane and cytoplasmic domains of GIRK subunits (Fig. S7A). Binding of Gβγ, ethanol, and Na+ to GIRK channels strengthens channel affinity for PIP2 (4, 32, 33). PIP2 binding triggers a rotation of the inner transmembrane helices, displacing the inner helical gate found at the junction of the transmembrane and cytoplasmic domains. With PIP2 present, GIRK channels are “primed” for activation. Indeed, Gβγ binding (in the presence of PIP2) leads to opening of the inner-helical gate and the G-loop gate, which is formed by the inner face of the cytosolic domains; Gβγ in the absence of PIP2 can only open the G-loop gate (27, 30). Our data suggest that ML297, like other channel agonists, ultimately activates GIRK channels by opening inner-helical and G-loop gates.
Our data also argue that ML297 interacts directly with GIRK1-containing channels. Indeed, the observations that one of the GIRK1 residues (D173) required for ML297 agonism has been linked to inward rectification (34), and that ML297 weakens the inward rectification of the channel, are difficult to reconcile with an indirect mechanism of action for ML297. Moreover, the relatively close spatial proximity of the two GIRK1 residues that are necessary and sufficient for ML297 agonism suggests the possibility that ML297 binds in a pocket formed by one or both residues (30) (Fig. S7B). We cannot exclude the possibility, however, that ML297 binds to other domains of GIRK1 or to a domain(s) conserved across all GIRK subunits. ML297 may bind to both GIRK1/2 heteromers and GIRK2 homomers, for example, but residues F137 and D173 in GIRK1 translate ML297 binding to enhanced channel activity better than their counterparts in GIRK2. Moreover, the deactivation rate of ML297-induced current carried by GIRK2FD/GIRK2 heteromers was substantially slower than that observed for GIRK1/2 heteromers (Fig. 4E). Because deactivation rate for a direct-acting agonist should largely reflect agonist-channel affinity, this finding supports the contention that structures in GIRK2 influence the ML297–GIRK channel interaction. This contention is also supported by the observation that some ML297 derivatives show differential selectivity for GIRK1/2 and GIRK1/4 channels (35).
ML297 reduced anxiety-related behavior in mice in a GIRK1-dependent manner, without displaying rewarding or sedative effects. Interestingly, genetic ablation of Girk1 (this study) or Girk2 also correlated with reduced anxiety-related behavior in mice (24, 25). The similarity in behavioral outcome for Girk ablation and acute pharmacologic GIRK activation could indicate that either too much or too little GIRK activity is anxiogenic. Alternatively, molecular adaptations occurring secondary to constitutive Girk gene ablation may underlie these seemingly disparate observations. Indeed, enhanced glutamatergic signaling has been documented in multiple neuron populations in Girk1−/− and Girk2−/− mice (36, 37).
The alcohol sensitivity of GIRK channels suggests that they are relevant molecular targets for ethanol (6, 7). Indeed, wild-type but not Girk2−/− mice developed a conditioned place preference to ethanol (38). ML297, however, did not evoke a conditioned place preference in wild-type mice. A possible explanation for the differential reward liability of ethanol and ML297 is that the reward-related, GIRK-dependent effects of ethanol are mediated by GIRK channels lacking GIRK1. In this context, it is noteworthy that dopamine neurons in the ventral tegmental area, a key anatomic substrate of addictive drugs, express GIRK2/3 heteromers (39). It is also possible, however, that the level of ML297 in the brain achieved following a 30 mg/kg systemic injection is sufficient to trigger anxiolysis, but insufficient to trigger reward-related or other behaviors. In support of this contention, the 60 mg/kg dose of ML297, which was efficacious in the seizure models, yielded a maximal brain concentration of 130 nM, just below the EC50 for activation of GIRK1/2 channels (18). Thus, the 30 mg/kg dose used in our studies should yield a lower maximal brain concentration of ML297, and correspondingly limited activation of GIRK1-containing channels. Future studies with ML297 derivatives that more effectively penetrate the blood–brain barrier will shed light on this important issue.
The lack of potent and selective pharmacologic tools for studying GIRK channels has limited progress on understanding their physiological and pathophysiological relevance. Translational benefits associated with inhibiting or enhancing GIRK signaling are unlikely to be achieved without an ability to manipulate GIRK signaling in a region and/or subunit-selective manner. Here, we show that ML297 selectively activates native GIRK1-containing channels, decreasing anxiety-related behavior at doses without associated reward or motor liabilities. Thus, ML297—or perhaps its next-generation derivatives—represents an important step toward realizing the full therapeutic potential of GIRK channel manipulation.
Materials and Methods
Animal experiments were approved by the Institutional Animal Care and Use Committee at the University of Minnesota, Minneapolis. Detailed descriptions of animals and reagents, and methods for cell culture and transfection studies, the thallium flux assay, electrophysiological techniques, and behavioral experiments, are available in SI Materials and Methods. Data are presented throughout as mean ± SEM.
Supplementary Material
Acknowledgments
The authors thank Jennifer Kutzke for maintaining the mouse colony, Ian Romaine (Vanderbilt Institute of Chemical Biology’s Chemical Synthesis Core and the Vanderbilt Molecular Libraries Probe Production Centers Network and Specialized Chemistry Center for ML297 Synthesis), and Charles A. Herring for assistance with generating the image in Fig. S7. This work was supported by National Institutes of Health Grants DA007234 (to N.W.), DA007097 (to M.C.H.), P30 NS062158 (to M.J.T.), HL105550, MH061933, and DA034696 (to K.W.), and CA068485 (to C.D.W.).
Footnotes
Conflict of interest statement: C.D.W. receives royalties from the sale of the thallium-sensitive dye, Thallos, through a licensing agreement between Vanderbilt University and TEFlabs.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405190111/-/DCSupplemental.
References
- 1.Luján R, Marron Fernandez de Velasco E, Aguado C, Wickman K. New insights into the therapeutic potential of Girk channels. Trends Neurosci. 2014;37(1):20–29. doi: 10.1016/j.tins.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11(5):301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hibino H, et al. Inwardly rectifying potassium channels: Their structure, function, and physiological roles. Physiol Rev. 2010;90(1):291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 4.Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391(6669):803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- 5.Sui JL, Petit-Jacques J, Logothetis DE. Activation of the atrial KACh channel by the betagamma subunits of G proteins or intracellular Na+ ions depends on the presence of phosphatidylinositol phosphates. Proc Natl Acad Sci USA. 1998;95(3):1307–1312. doi: 10.1073/pnas.95.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi T, et al. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2(12):1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- 7.Lewohl JM, et al. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2(12):1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- 8.Weigl LG, Schreibmayer W. G protein-gated inwardly rectifying potassium channels are targets for volatile anesthetics. Mol Pharmacol. 2001;60(2):282–289. doi: 10.1124/mol.60.2.282. [DOI] [PubMed] [Google Scholar]
- 9.Yamakura T, Lewohl JM, Harris RA. Differential effects of general anesthetics on G protein-coupled inwardly rectifying and other potassium channels. Anesthesiology. 2001;95(1):144–153. doi: 10.1097/00000542-200107000-00025. [DOI] [PubMed] [Google Scholar]
- 10.Yow TT, et al. Naringin directly activates inwardly rectifying potassium channels at an overlapping binding site to tertiapin-Q. Br J Pharmacol. 2011;163(5):1017–1033. doi: 10.1111/j.1476-5381.2011.01315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi T, Ikeda K. G protein-activated inwardly rectifying potassium channels as potential therapeutic targets. Curr Pharm Des. 2006;12(34):4513–4523. doi: 10.2174/138161206779010468. [DOI] [PubMed] [Google Scholar]
- 12.Ma D, et al. Diverse trafficking patterns due to multiple traffic motifs in G protein-activated inwardly rectifying potassium channels from brain and heart. Neuron. 2002;33(5):715–729. doi: 10.1016/s0896-6273(02)00614-1. [DOI] [PubMed] [Google Scholar]
- 13.Krapivinsky G, et al. The G-protein-gated atrial K+ channel IKACh is a heteromultimer of two inwardly rectifying K(+)-channel proteins. Nature. 1995;374(6518):135–141. doi: 10.1038/374135a0. [DOI] [PubMed] [Google Scholar]
- 14.Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16(22):7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slesinger PA, Reuveny E, Jan YN, Jan LY. Identification of structural elements involved in G protein gating of the GIRK1 potassium channel. Neuron. 1995;15(5):1145–1156. doi: 10.1016/0896-6273(95)90102-7. [DOI] [PubMed] [Google Scholar]
- 16.Chan KW, Sui JL, Vivaudou M, Logothetis DE. Control of channel activity through a unique amino acid residue of a G protein-gated inwardly rectifying K+ channel subunit. Proc Natl Acad Sci USA. 1996;93(24):14193–14198. doi: 10.1073/pnas.93.24.14193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wydeven N, Young D, Mirkovic K, Wickman K. Structural elements in the Girk1 subunit that potentiate G protein-gated potassium channel activity. Proc Natl Acad Sci USA. 2012;109(52):21492–21497. doi: 10.1073/pnas.1212019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufmann K, et al. ML297 (VU0456810), the first potent and selective activator of the GIRK potassium channel, displays antiepileptic properties in mice. ACS Chem Neurosci. 2013;4(9):1278–1286. doi: 10.1021/cn400062a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollins B, Kuravi S, Digby GJ, Lambert NA. The c-terminus of GRK3 indicates rapid dissociation of G protein heterotrimers. Cell Signal. 2009;21(6):1015–1021. doi: 10.1016/j.cellsig.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie K, Masuho I, Brand C, Dessauer CW, Martemyanov KA. The complex of G protein regulator RGS9-2 and Gβ(5) controls sensitization and signaling kinetics of type 5 adenylyl cyclase in the striatum. Sci Signal. 2012;5(239):ra63. doi: 10.1126/scisignal.2002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura Y, Murata Y, Iwasaki H. Voltage-sensing phosphatase: Actions and potentials. J Physiol. 2009;587(Pt 3):513–520. doi: 10.1113/jphysiol.2008.163097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leaney JL. Contribution of Kir3.1, Kir3.2A and Kir3.2C subunits to native G protein-gated inwardly rectifying potassium currents in cultured hippocampal neurons. Eur J Neurosci. 2003;18(8):2110–2118. doi: 10.1046/j.1460-9568.2003.02933.x. [DOI] [PubMed] [Google Scholar]
- 23.Van der Heyden JA, Zethof TJ, Olivier B. Stress-induced hyperthermia in singly housed mice. Physiol Behav. 1997;62(3):463–470. doi: 10.1016/s0031-9384(97)00157-1. [DOI] [PubMed] [Google Scholar]
- 24.Blednov YA, Stoffel M, Chang SR, Harris RA. GIRK2 deficient mice. Evidence for hyperactivity and reduced anxiety. Physiol Behav. 2001;74(1-2):109–117. doi: 10.1016/s0031-9384(01)00555-8. [DOI] [PubMed] [Google Scholar]
- 25.Pravetoni M, Wickman K. Behavioral characterization of mice lacking GIRK/Kir3 channel subunits. Genes Brain Behav. 2008;7(5):523–531. doi: 10.1111/j.1601-183X.2008.00388.x. [DOI] [PubMed] [Google Scholar]
- 26.Jelacic TM, Kennedy ME, Wickman K, Clapham DE. Functional and biochemical evidence for G-protein-gated inwardly rectifying K+ (GIRK) channels composed of GIRK2 and GIRK3. J Biol Chem. 2000;275(46):36211–36216. doi: 10.1074/jbc.M007087200. [DOI] [PubMed] [Google Scholar]
- 27.Whorton MR, MacKinnon R. X-ray structure of the mammalian GIRK2-βγ G-protein complex. Nature. 2013;498(7453):190–197. doi: 10.1038/nature12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sui JL, Chan KW, Logothetis DE. Na+ activation of the muscarinic K+ channel by a G-protein-independent mechanism. J Gen Physiol. 1996;108(5):381–391. doi: 10.1085/jgp.108.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho IH, Murrell-Lagnado RD. Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J Biol Chem. 1999;274(13):8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- 30.Whorton MR, MacKinnon R. Crystal structure of the mammalian GIRK2 K+ channel and gating regulation by G proteins, PIP2, and sodium. Cell. 2011;147(1):199–208. doi: 10.1016/j.cell.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aryal P, Dvir H, Choe S, Slesinger PA. A discrete alcohol pocket involved in GIRK channel activation. Nat Neurosci. 2009;12(8):988–995. doi: 10.1038/nn.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns(4,5)P2 interactions. Nat Cell Biol. 1999;1(3):183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- 33.Bodhinathan K, Slesinger PA. Molecular mechanism underlying ethanol activation of G-protein-gated inwardly rectifying potassium channels. Proc Natl Acad Sci USA. 2013;110(45):18309–18314. doi: 10.1073/pnas.1311406110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kofuji P, Doupnik CA, Davidson N, Lester HA. A unique P-region residue is required for slow voltage-dependent gating of a G protein-activated inward rectifier K+ channel expressed in Xenopus oocytes. J Physiol. 1996;490(Pt 3):633–645. doi: 10.1113/jphysiol.1996.sp021173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wen W, et al. Discovery of ‘molecular switches’ within a GIRK activator scaffold that afford selective GIRK inhibitors. Bioorg Med Chem Lett. 2013;23(16):4562–4566. doi: 10.1016/j.bmcl.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arora D, et al. Altered neurotransmission in the mesolimbic reward system of Girk mice. J Neurochem. 2010;114(5):1487–1497. doi: 10.1111/j.1471-4159.2010.06864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hearing M, et al. Repeated cocaine weakens GABA(B)-Girk signaling in layer 5/6 pyramidal neurons in the prelimbic cortex. Neuron. 2013;80(1):159–170. doi: 10.1016/j.neuron.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hill KG, Alva H, Blednov YA, Cunningham CL. Reduced ethanol-induced conditioned taste aversion and conditioned place preference in GIRK2 null mutant mice. Psychopharmacology (Berl) 2003;169(1):108–114. doi: 10.1007/s00213-003-1472-4. [DOI] [PubMed] [Google Scholar]
- 39.Cruz HG, et al. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7(2):153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.