The mitochondrial permeability transition pore (MPTP) describes an inducible activity that regulates solute exchange between mitochondrial matrix contents and the surrounding cytoplasm, which acutely leads to loss of mitochondrial inner membrane potential but eventually organelle swelling and rupture. Mitochondrial rupture due to prolonged MPTP engagement, which is often the result of ischemic cellular injury due to elevated intracellular Ca2+ levels and reactive oxygen species, underlies regulated necrotic cell death. An understanding of the molecular constituents that generate the MPTP, as well as the other proteins that can affect it, is of profound disease relevance. However, the molecular identity of the MPTP has been the subject of a protracted scientific debate for nearly three decades. In PNAS, Alavian et al. make a strong case that the c-subunit of the F1FO ATP synthase forms the inner mitochondrial membrane pore of the MPTP (1).
The MPTP is a highly evolutionarily conserved pore within the inner membrane of the mitochondrion that is permeable to molecules less than 1.5 kDa in size. MPTP opening results in loss of inner membrane potential, the proton gradient and ATP production, eventually leading to mitochondrial dysfunction and cell death (2). The existence of the MPTP was first proposed in the mid-20th century with the observations that high levels of Ca2+ could lead to mitochondrial swelling and dysfunction (3, 4). It was not until 1988 when the first pharmacological inhibitor, cyclosporine A (CsA), was shown to inhibit MPTP opening (5). CsA is an immunosuppressant that binds to cyclophilin proteins and inhibits their activity. Two years later, the adenine nucleotide translocator (ANT) within the inner mitochondrial membrane was hypothesized to be the pore-forming component of the MPTP (Fig. 1A). Not only could ANT form pore-like properties in reconstituted membranes, but the ANT binding agents bongkrekic acid and atractyloside, which are effectors of ADP/ATP translocation across the ANT, can either block or induce mitochondrial swelling, respectively (6).
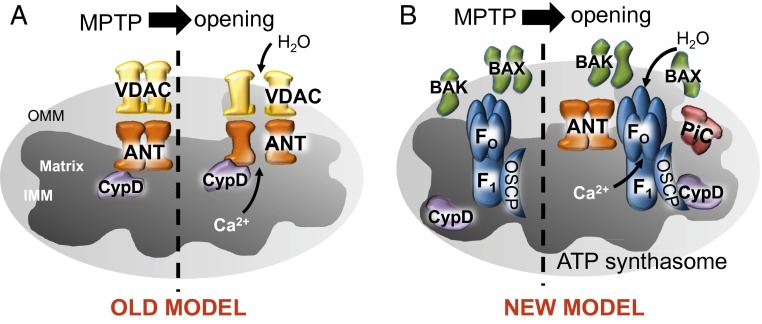
Fig. 1.
(A) Schematic of the original model of the MPTP. The MPTP was proposed to be one contiguous pore composed of VDAC and ANT regulated by CypD, which formed at contact sites between the inner and outer membranes. (B) Schematic of the new model of the MPTP consisting of Bax/Bak on the outer mitochondrial membrane to impart a level of necessary permeability and the F1FO ATP synthase on the inner mitochondrial membrane regulated by CypD in the matrix as the component that responds to stimuli to initiate opening, efflux of Ca2+, loss of membrane potential, and mitochondrial swelling.
The next suggested component of the MPTP was the voltage-dependent anion channel (VDAC), which is highly abundant within the outer mitochondrial membrane, where it enables conductance of most solutes into the intermembrane space (7). In 1997, the specific cyclophilin that regulated the MPTP was identified as cyclophilin D (CypD), the only known mitochondrial localized cyclophilin protein, which functions as a peptidylprolyl isomerase (8). With these proposed components, the model predicted that the MPTP was generated as one contiguous pore spanning the intermembrane space by apposition of VDAC within the outer membrane and ANT within the inner membrane, regulated by CypD within the mitochondrial matrix (Fig. 1A) (9). This model would go untested until 2004, when Kokoszka et al. determined through genetic ablation of two genes encoding ANT isoforms that it was not required for MPTP opening (10). However, mitochondria from these null mice held twofold more Ca2+ than WT control mitochondria, suggesting that although ANT was not the pore itself, it was still an important regulator. This report largely disproved the long-standing model of the MPTP shown in Fig. 1A, although 1 y later deletion of the gene encoding CypD (Ppif) in mice supported the basic tenet of the entire field that the MPTP was dependent on cyclophilin protein function (11, 12); hence, this aspect of the original model was proven correct. Indeed, mitochondria lacking CypD are desensitized to MPTP opening and mice lacking the gene encoding CypD are desensitized to various MPTP-dependent pathologies, such as ischemia reperfusion injuries across multiple tissues, as well as degenerative disorders associated with Ca2+ overload such as muscular dystrophy (11–13). Another suggested inner membrane pore-forming component of the MPTP was the mitochondrial phosphate carrier (PiC). PiC was initially hypothesized to be a component of the MPTP due to its ability to form a pore and bind to CypD and due to the fact that phosphate greatly influences MPTP opening (14). However, similar to ANT, PiC has also recently been disproven as being required for MPTP opening using a genetic approach in the mouse and mitochondria lacking the PiC protein (15, 16).
In 2007, gene-targeted mice lacking various isoforms of the VDAC were analyzed, which further invalidated the original model of the MPTP (Fig. 1A), because mitochondria deficient in the VDAC protein still showed MPTP functionality (17). These collective results spurred renewed interest in attempts to discover new candidates that constitute the inner and outer membrane pore-forming components of the MPTP.
Recently, the proapoptotic Bcl-2 family members Bax and Bak were suggested to function as the outer membrane component of the MPTP in permitting mitochondrial swelling and dysfunction (18). Mitochondria from Bax and Bak double-deleted fibroblasts, liver, or heart were resistant to Ca2+-induced swelling and lacked permeability/conductivity as measured by mito-patching (18). Hence, Bax and Bak are required as the pore-forming components within the outer membrane of the mitochondria, but this activity need not form in apposition with the inner mitochondrial membrane to generate a contiguous pore (Fig. 1B). Thus, the new model predicts that the inner and outer mitochondrial membranes function separately in MPTP opening and that the outer membrane, in the presence of Bax or Bak, is largely permissive, whereas the inner membrane is the site of regulation (Fig. 1B).
With respect to the final component of the MPTP, that being the identity of the inner membrane pore-forming entity, Alavian et al. present data that the c-subunit of the F1FO ATP synthase creates the regulated pore (Fig. 1B). In previous supportive reports, Bonora et al. and Giorgio et al. also made a strong case for the pore residing somewhere within the F1FO ATP synthase (19, 20). This concept was first proposed in 2009 due to the ability of the F1FO ATP synthase to bind with CypD (21). Recently, it was shown that gene silencing of isomers of the c-subunit of the ATP synthase inhibited MPTP opening (19). In addition, CypD was shown to bind to oligomycin sensitivity-conferring protein (OSCP), the oligomycin-sensitive component of the F1FO ATP synthase, which triggers MPTP opening through dimerization of the F1FO ATP synthase (20). Alavian et al. go even further in forming this newly proposed model in that the c-subunit itself can generate pore-forming activity in proteoliposomes, although in this state, it lacks regulation by CypD. However, when the whole ATP synthase is isolated and reconstituted, it then creates a pore that is regulated by CypD, Ca2+, and CsA (1). The authors propose that previously reported work showing the interaction between OSCP and CypD may be how CypD regulates the c-subunit pore. Using
Alavian et al. make a strong case that the c-subunit of the F1FO ATP synthase forms the inner mitochondrial membrane pore of the MPTP.
shRNAs for c-subunit isomers (ATP5G1 and ATP5G3), the authors show reduced MPTP opening and decreased neuronal cell death induced by glutamate or H2O2 (1). They go on to conclude that that the F1 β-subunit portion of the ATP synthase plays an inhibitory role on the c-subunit pore. Therefore, the authors suggest the F1 subunit must become removed from the FO ATP synthase to induce MPTP opening. Indeed, Ca2+-induced swelling released c-subunit oligomers from F1, whereas MPTP inhibitors (CsA and ADP) blocked this release (1).
Atlhough these exciting results support a novel model for the MPTP, a caveat is that the F1FO ATP synthase cannot explain regulation by all known MPTP effectors, such as the ANT binding agents bongkrekic acid or atractyloside, which can dramatically effect MPTP activity through altering the conformational states of ANT. One possibility is that ANT is known to be part of the ATP synthasome complex (along with PiC) in association with the F1FO ATP synthase, and hence ANT or PiC (phosphate regulates the MPTP) might regulate the pore-forming aspects of the c-subunit of the ATPase (Fig. 1B). If this was indeed the case, it could suggest a secondary mechanism for CsA regulation of the presumed F1FO ATP synthase pore activity through ANT or even PiC, both of which bind CypD. Another minor issue for future investigation is that part of the authors’ proof for the F1FO ATP synthase c-subunit as being the pore itself involves an shRNA-mediated knockdown of the isoforms of the c-subunit, but this manipulation could also elevate matrix ADP, which could inhibit the actual pore itself if it were not the F1FO ATP synthase. A final issue is the uncertain therapeutic potential of the F1FO ATP synthase as a target because agents that block the c-subunit pore activity should also reduce ATP production, although in certain disease situations this later effect might also be a benefit (such as with select neurodegenerative disorders). Finally, genetically testing this newly hypothesized role of the F1FO ATP synthase as the MPTP will be very challenging for similar reasons, but will be absolutely critical in fully validating this model.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
See companion article on page 10580.
References
- 1.Alavian KN, et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci USA. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion—A target for cardioprotection. Cardiovasc Res. 2004;61(3):372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 3.Raaflaub J. [Swelling of isolated mitochondria of the liver and their susceptibility to physicochemical influences] Helv Physiol Pharmacol Acta. 1953;11(2):142–156. [PubMed] [Google Scholar]
- 4.Hunter FE, Jr, Ford L. Inactivation of oxidative and phosphorylative systems in mitochondria by preincubation with phosphate and other ions. J Biol Chem. 1955;216(1):357–369. [PubMed] [Google Scholar]
- 5.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255(1):357–360. [PMC free article] [PubMed] [Google Scholar]
- 6.Halestrap AP, Davidson AM. Inhibition of Ca2(+)-induced large-amplitude swelling of liver and heart mitochondria by cyclosporin is probably caused by the inhibitor binding to mitochondrial-matrix peptidyl-prolyl cis-trans isomerase and preventing it interacting with the adenine nucleotide translocase. Biochem J. 1990;268(1):153–160. doi: 10.1042/bj2680153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szabó I, De Pinto V, Zoratti M. The mitochondrial permeability transition pore may comprise VDAC molecules. II. The electrophysiological properties of VDAC are compatible with those of the mitochondrial megachannel. FEBS Lett. 1993;330(2):206–210. doi: 10.1016/0014-5793(93)80274-x. [DOI] [PubMed] [Google Scholar]
- 8.Woodfield KY, Price NT, Halestrap AP. cDNA cloning of rat mitochondrial cyclophilin. Biochim Biophys Acta. 1997;1351(1-2):27–30. doi: 10.1016/s0167-4781(97)00017-1. [DOI] [PubMed] [Google Scholar]
- 9.Baines CP. The molecular composition of the mitochondrial permeability transition pore. J Mol Cell Cardiol. 2009;46(6):850–857. doi: 10.1016/j.yjmcc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kokoszka JE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427(6973):461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434(7033):652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 12.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434(7033):658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 13.Millay DP, et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat Med. 2008;14(4):442–447. doi: 10.1038/nm1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46(6):821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 15.Kwong JQ, et al. Genetic deletion of the mitochondrial phosphate carrier desensitizes the mitochondrial permeability transition pore and causes cardiomyopathy. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutiérrez-Aguilar M, et al. Genetic manipulation of the cardiac mitochondrial phosphate carrier does not affect permeability transition. J Mol Cell Cardiol. 2014;72(0):316–325. doi: 10.1016/j.yjmcc.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9(5):550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karch J, et al. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. eLife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonora M, et al. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12(4):674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giorgio V, et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci USA. 2013;110(15):5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giorgio V, et al. Cyclophilin D modulates mitochondrial F0F1-ATP synthase by interacting with the lateral stalk of the complex. J Biol Chem. 2009;284(49):33982–33988. doi: 10.1074/jbc.M109.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]