Abstract
Background
Eosinophilic Esophagitis (EE) is now a commonly encountered disorder that was rarely diagnosed a decade ago.
Objective
We aimed to determine the epidemiologic and histologic features of retrospective pediatric esophageal eosinophilia before the first case of EE at our institution was recognized.
Methods
Esophageal biopsies obtained between 1982–1999 with reflux esophagitis were re-examined and re-organized into 2 groups based on peak esophageal eosinophil number (<15 eos/hpf, ≥ 15 eos/hpf). The epidemiology and histology of the entire cohort and a population based cohort were evaluated.
Results
807 biopsies from 666 patients were re-examined; 198 patients had ≥ 15 eos/hpf. Among a population-based cohort of patients with ≥ 15 eos/hpf, there was a modest increase in incidence (p < 0.001; IRR 1.18; CI 1.09–1.28). After correcting for a 40-fold increase in the number of endoscopies during this time period, the proportion of biopsies with ≥ 15 eos/hpf did not change (0.08 in 1982 vs. 0.08 in 1996 (peak), (p = 0.9; IRR 1.02; CI 0.73 –1.44). Patients who had as few as 5 eos/hpf were more likely to have persistent esophageal eosinophilia on repeat EGD, evidence of basal layer hyperplasia and lamina propria fibrosis compared to patients with < 5 eos/hpf (p <0.001).
Conclusions
Esophageal Eosinophilia, at levels consistent with EE, was present among 30% of patients diagnosed with reflux esophagitis and the incidence of esophageal eosinophilia did not change over time. Patients with ≥ 5 eos/hpf had evidence of other histologic abnormalities and were likely to have persistent esophageal eosinophilia.
Keywords: eosinophilic esophagitis, incidence, diagnosis, chronic esophagitis, eosinophil, esophagitis, epidemiology, retrospective
Introduction
Eosinophilic esophagitis (EE) has garnered great interest as a newly appreciated disorder with a clinical presentation that can mimic gastroesophageal reflux disease (GERD).1–4 In an effort to standardize the diagnostic approach to EE, consensus guidelines were published in 2007 that define EE as a clinicohistopathologic disorder requiring the presence of ≥ 15 eosinophils per high power field (eos/hpf) on esophageal biopsy, and the exclusion of GERD based on a trial of high dose proton pump inhibitor (PPI) therapy or a negative pH probe.5, 6 However, the clinical and histopathologic distinctions between EE and GERD are based on a paucity of data and remain controversial.7, 8 In particular, studies evaluating the number of esophageal eos/hpf that distinguish EE from GERD are limited, and the minimum number of eosinophils used to define EE has varied widely in the medical literature.9–11 Further investigation is needed to identify the esophageal eosinophil count at which pathological features and disease morbidity begin to emerge.
In addition to the uncertainty surrounding the diagnostic criteria, the reason for the sudden rise in EE cases is also unclear. In 2004, we reported that the first case series of recognized EE at our institution was in 1999, that the subsequent incidence of EE was approximately 1/10,000 children and this incidence remained constant between 2000 to 2004.12 Subsequent epidemiologic studies have attempted to address whether the sudden burst of new patients with EE reflects a true rise in the number of new cases or increasing disease recognition.13–15 The data from these studies are conflicting. These studies were not performed among population based cohorts and do not account for dramatic changes in the practice of pediatric esophagogastroduodenoscopy (EGD) over the past two decades.15, 16 Finally, there have been no pediatric studies to evaluate fluctuations in eosinophil counts over time among patients with esophageal eosinophilia who were not treated with currently accepted therapies for EE.
We aimed to determine if there was a significant cohort of pediatric patients who were previously diagnosed with esophagitis prior to the late 1990s who had currently accepted histopathologic features of EE upon histologic re-evaluation. Additionally, we aimed to determine the eosinophil level at which other pathologic abnormalities begin to arise. We also aimed to assess whether esophageal eosinophilia (≥ 15 eos/hpf) was a persistent histologic finding. Finally, we aimed to determine the epidemiology of esophageal eosinophilia from 1982 to 1999 utilizing a population based pediatric cohort.
Methods
Study Setting
This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center (CCHMC).
Data Sources
The CCHMC histopathology database includes all specimens obtained at our institution from 1971 through present day. To identify esophageal biopsies that may contain eosinophils, we searched the database using the terms “reflux esophagitis,” “chronic esophagitis,” or “eosinophilic esophagitis.” Once this cohort was identified, a subsample query was performed to investigate for Barrett’s esophagus using the terms “Barrett’s,” “metaplasia,” “goblet,” “columnar,” “dysplasia,” and “cancer.”
The onset of our study period was defined by the first year in which a substantial number of esophageal biopsies with a diagnosis of “chronic esophagitis” or “reflux esophagitis” were present (1982). The end of our study period was designated as the year in which the first diagnosis of EE at our institution was made (1999). The total number of esophageal biopsies obtained during this study period was also identified.
Patient Population
Patient demographics and indication for initial endoscopy were obtained from chart review. There were 32 (6.8%) missing indications for endoscopy among patients with < 15 eos/hpf. Patient race was determined by self report. Patients were assigned to one of two groups based on histopathologic re-evaluation (≥ 15 eosinophils in at least one high power field (eos/hpf) on any biopsy, < 15 eos/hpf). As trends began to emerge in our analysis, the data were also analyzed utilizing a lower eosinophil threshold count (≥ 5 eos/hpf, < 5 eos/hpf).
Histopathologic Analysis
Slides of all biopsies identified by our search criteria were reviewed by a single blinded reviewer (CWD). A second blinded reviewer (MHC) also generated peak eosinophil counts for all biopsies with ≥ 15 eos/hpf early in the study. All slides for which the peak counts generated by the two reviewers differed by more than 10% were re-reviewed simultaneously by both reviewers and the discrepancies were resolved. Subsequently, second reviews were obtained at the request of the primary reviewer.
The peak eosinophil count was determined for each biopsy, and was defined as the greatest number of intraepithelial eosinophils visualized at 400× magnification (area= 0.23mm2). If more than one level was biopsied, then all levels were reviewed and the highest count was reported. The majority of biopsies were obtained from distal esophagus and the number of biopsies taken at each level was not reported at our institution during the study period. Each biopsy was also assessed for other histologic abnormalities. The percentage of biopsies with evidence basal layer hyperplasia, lamina propria fibrosis, microabscesses and surface layering was calculated. To avoid including a patient more than once, only the incident biopsy was used for these calculations. Biopsies were excluded from analysis if the amount of tissue present was less than 1 hpf. Biopsies were also excluded if the hematoxylin and eosin staining was too faint to allow for accurate identification of epithelial cells, basal cells or eosinophils. Eosinophil surface layering was defined as 4 contiguous eosinophils along the luminal surface of the epithelium and an eosinophil microabscess was defined as an intraepithelial space occupying collection of ≥ 10 eosinophils. Basal layer hyperplasia was defined as expansion of the basal layer to ≥ 25% of the total epithelial thickness in a well-oriented section.17 Lamina propria fibrosis was defined as an increase in the deposition of thickened collagen fibers. The lamina propria was present in biopsy specimens in approximately 38% (75/198) of patients with ≥ 15 eos/hpf and 20% (93/468) patients with < 15 eos/hpf, and a similar proportion of specimens evaluated at lower threshold counts (34% ≥ 5 eos/hpf, 19% < 5 eos/hpf). A Receiver Operator Characteristic (ROC) curve comparing peak esophageal eosinophil level with the occurrence of basal layer hyperplasia and also for the occurrence of either hyperplasia or lamina propria fibrosis was used to identify the optimal cut-off value.
Assessment of Disease Chronicity and Natural History
All patients who had 2 or more EGDs with biopsies were assigned to one of two groups (≥ 15 eos/hpf, < 15 eos/hpf) based on the peak intraepithelial eosinophil count observed on their initial study biopsy. The percentage of patients in each group who demonstrated ≥ 15 eos/hpf and either complete (0 eos/hpf) or partial (< 15 eos/hpf) histologic resolution/remission of esophageal eosinophilia on subsequent biopsies was determined. As trends in the data began to emerge at peak eosinophil counts of ≥ 5, data were also compared at a lower threshold (≥ 5 eos/hpf, < 5 eos/hpf). Among subjects with multiple study biopsies, assessments of disease chronicity included: scatter plots comparing the peak eosinophil counts on incident EGD and subsequent EGDs, and the odds of repeat EGD at defined eosinophil counts ranging from 5–40 eos/hpf.
Epidemiology
We conducted our study at CCHMC which is the sole pediatric gastroenterology and pathology provider for Hamilton County, Ohio and the greater Cincinnati area. Census data from Hamilton County was obtained for our entire study period. Subjects 0–19 years of age with ≥ 15 eos/hpf on their initial study biopsy who resided in Hamilton County within a given year were compared to Hamilton County population estimates for individuals 0–19 years of age as defined by U.S. Census data. Subsequently, pediatric population based incidence rate ratios (IRR) for patients with ≥ 15 eos/hpf and ≥ 5 eos/hpf residing in Hamilton County, Ohio were calculated.
Statistical Analysis
Data were analyzed using JMP version 7.0 (SAS, Cary, NC), SPSS® version 16.0 (SPSS, Chicago, IL) and STATA® version 10.0 (Stata Corporation, College Station, TX). Data are reported as raw numbers, as proportions and as population based incidence rates. Descriptive statistics for basic subject demographics and histopathologic findings are reported as mean (± SD) and medians (IQR) depending on the variable distributions. To compare groups for continuous variables, the ANOVA or Kruskal-Wallis test was used depending on the distribution of the data. Categorical data were compared using Chi-Square tests. All tests were considered significant at the p < 0.05 level and 95% confidence intervals (CI) were calculated.
Receiver operating characteristic (ROC) curves were developed for each of the following diagnostic variables: basilar hyperplasia present (Yes/No) and fibrosis present (Yes/No). The optimal cut-off value for each variable was determined by the minimum Euclidean distance approach.. The sensitivity, specificity, and area under the ROC curve are reported for the cut-point of 15 (currently accepted value) and the newly identified cut-point.
Results
Patient Population
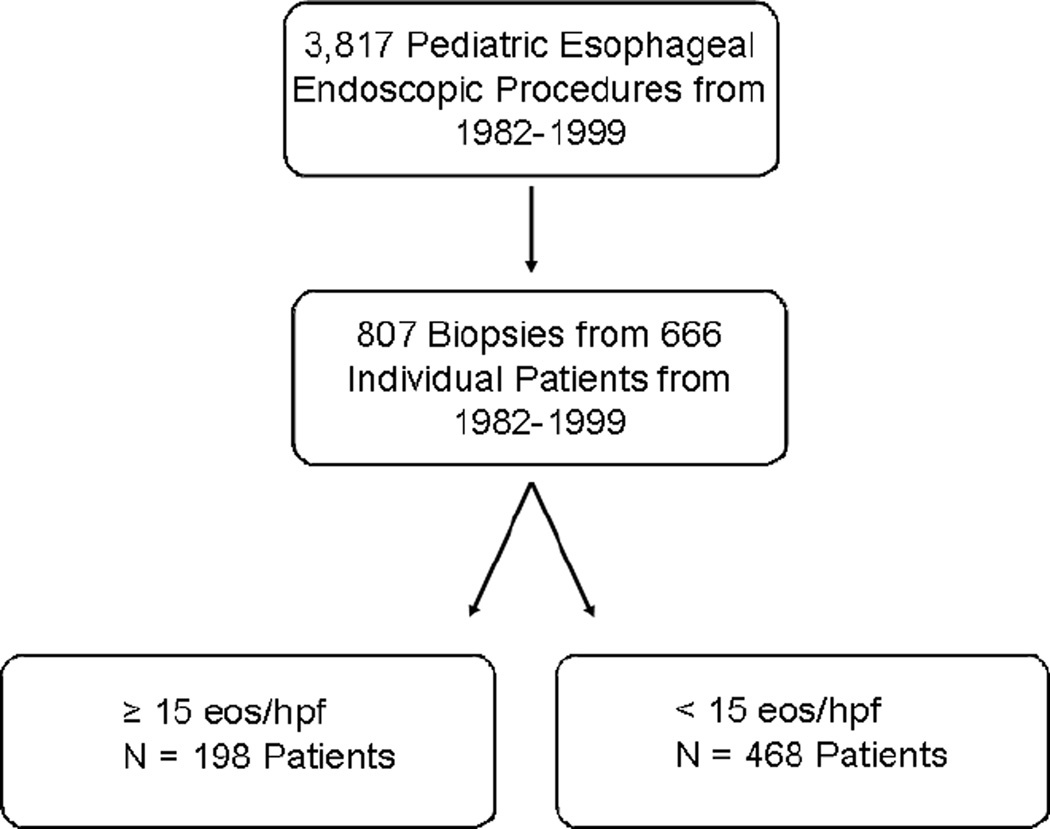
The search of the CCHMC pathology database yielded 3,817 esophageal biopsies obtained between 1982 and 1999. 868 biopsies contained the terms “chronic esophagitis,” “reflux esophagitis,” or “eosinophilic esophagitis” within the pathologic diagnosis. Of those 868 biopsies, 807 biopsies from 666 patients were sufficient for analysis. The remaining 61 (7.0%) biopsies were either inadequately stained or did not contain enough tissue to be analyzed adequately. Among our cohort of 666 patients, there were 430 (64.6%) males. The average age was 8.7 ± 5.9 years. Subject race was 535 (80.5%) Caucasian, 50 (7.5%) African-American, 1 (0.15%) Asian and 80 (12.6%) unknown (not available). Subject ethnicity data were not available. Histologic evaluation of these biopsies demonstrated that 198 (30%) of the 666 patients had evidence of ≥ 15 eos/hpf (Figure 1). Seventy two percent of patients with ≥ 15 eos/hpf were male compared to just 62% of patients with < 15 eos/hpf (p < 0.05; OR 1.56; CI 1.08 – 2.24). Mean age for patients with ≥ 15 eos/hpf was 8.1 years compared to 8.9 years for patients with < 15 eos/hpf (p =0.38). The percentage of Caucasian subjects was 81% and 76% among patients with ≥ 15 eos/hpf and < 15 eos/hpf, respectively (p = 0.47). Approximately 12% (24/198) of patients with ≥ 15 eos/hpf had a chief complaint of dysphagia at the time of endoscopy compared to just 2.4% (11/436) of patients with < 15 eos/hpf (p < .0001). The chief complaint of vomiting, esophagitis or abdominal pain was present in similar rates in both groups (Table I). The indication for the second biopsy was also obtained and suggested that 87% of patients were actively symptomatic on repeat biopsy. A subsample of medical records were reviewed for additional clinical and endoscopic data (history of atopic disease, esophageal rings, etc), however, these data were not consistently documented.
Figure 1. Initial Study Cohort identification.
Between 1982 and 1999, a total of 3,817 esophageal biopsies were obtained and 666 patients met study criteria. Upon reevaluation, 198 of these patients had ≥ 15 eos/hpf on esophageal biopsy.
Table I. Indications for Initial Endoscopy.
The 5 most common indications for endoscopy for patients with ≥ 15 eos/hpf and for patients with < 5 eos/hpf are shown. The rates of esophageal strictures and the percentage of patients with other indications for endoscopy (GI bleeding, Inflammatory Bowel Disease, airway abnormalities, etc.) are shown.
| ≥ 15 eos/hpf (N=198) | < 15 eos/hpf (N=436) | |
|---|---|---|
| Abdominal Pain (%) | 17.68 | 20.41 |
| Reflux (%) | 14.14 | 10.78 |
| Esophagitis (%) | 14.65 | 16.74 |
| Vomiting (%) | 12.63 | 8.26 |
| Dysphagia (%) | 12.12** | 2.52 |
| Esophageal Stricture or Foreign Body(%) | 2.00 | 1.15 |
| Other (%) | 26.78 | 40.14** |
p < 0.001
Histopathologic Analysis
We evaluated all 807 biopsies for evidence of other histologic abnormalities. The median eosinophil number for the entire study population was 1 eos/hpf and the median eosinophil number for patients with ≥ 15 eos/hpf was 35 eos/hpf. Peak eosinophil counts ranged from 15 eos/hpf to 187 eos/hpf in patients with ≥ 15 eos/hpf. Interestingly, the median eosinophil number among patients with ≥ 15 eos/hpf was inversely associated with patient age (p < 0.02). Among patients with ≥ 15 eos/hpf, the median peak eosinophil number was 50 eos/hpf for children ≤ 3 years of age (N= 61), 35 eos/hpf for children 4–12 years of age (N= 88), and 30 eos/hpf for children 13–18 years of age (N= 49).
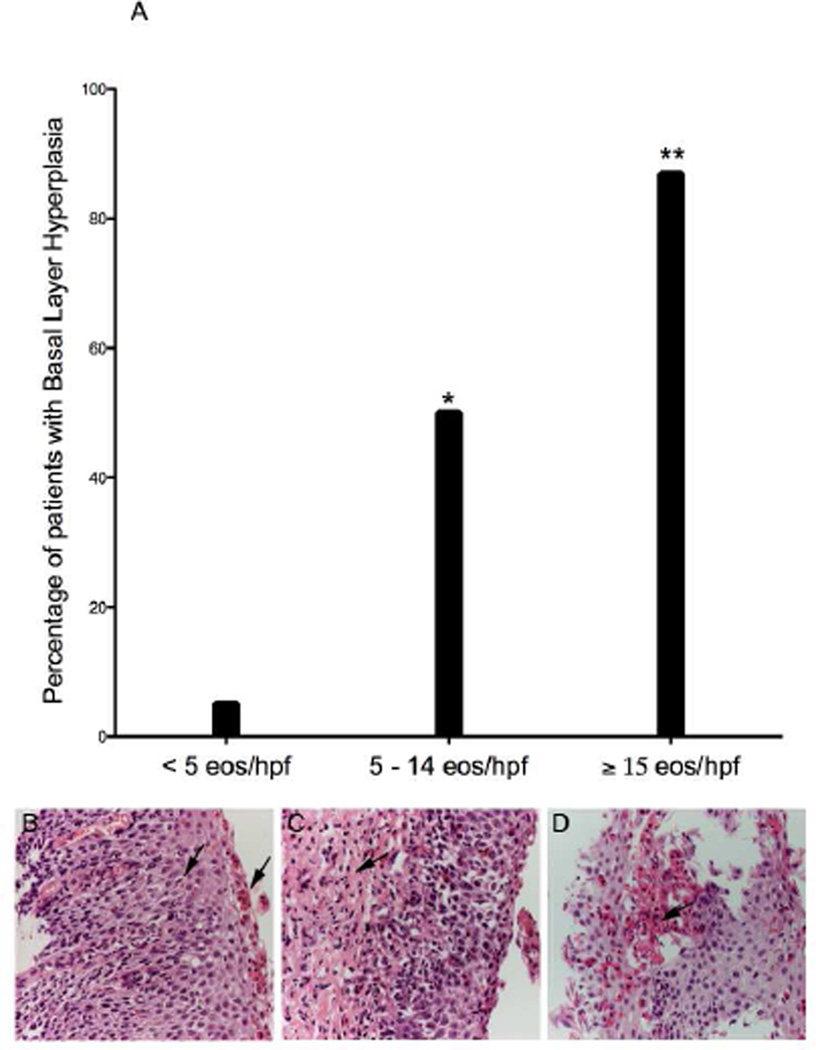
Basal cell hyperplasia was much more common among biopsies with ≥ 15 eos/hpf (87%, N= 170) than those with < 15 eos/hpf (13%, N= 61; p < 0.001; OR 38.09; CI 23.55 – 61.60, Figure 2a). Among 75 biopsies where the lamina propria was present and there was a peak count of ≥ 15 eos/hpf, 85% had evidence of lamina propria fibrosis. Only 36% (34/93) of biopsies with < 15 eos/hpf had lamina propria fibrosis (p < 0.001; OR 10.40; CI 4.86 – 22.22).
Figure 2. Histologic Re-evaluation.
Patients with 5 – 14 eos/hpf and ≥ 15 eos/hpf were more likely to have basal layer hyperplasia than patients with < 5 eos/hpf (*p < .001) and < 15 eos/hpf (**p < .001), respectively. Examples of basal layer hyperplasia and surface layering (b), lamina propria fibrosis (c) and microabscesses (d) are shown.
Histologic findings of surface layering and eosinophilic microabscesses were exclusively present among biopsies with ≥ 15 eos/hpf. The median eosinophil count among biopsies with microabscesses was 85 eos/hpf and the median eosinophil count among biopsies with evidence of surface layering was 65 eos/hpf. The histologic data are summarized in Table II. Among our cohort of 807 biopsies, 11 patients from our study demonstrated histopathologic changes consistent with Barrett’s esophagus: 6 patients with < 5 eos/hpf, 2 patients with 5 –14 eos/hpf and 3 patients with ≥ 15 eos/hpf. Compared to patients with < 5 eos/hpf. patients with ≥ 15 eos/hpf (OR 0.89; CI 0.25 – 3.11, p > 0.05) and those with 5 –14 (OR 1.16; CI 0.37 – 3.64, p > 0.05) were not more likely to have histologic findings of Barrett’s. The data are included in Table II.
Table II.
Demographic and Histologic Features of Study Population
| < 5 eos/hpf | 5 – 14 eos/hpf | ≥ 15 eos/hpf | |
|---|---|---|---|
| N (patient number) | 388 | 80 | 198 |
| Mean age (years) | 8.9 | 8.9 | 8.1 |
| Male (%) | 60 | 71 | 72** |
| Median Eos Count (eos/hpf) | 0 | 10* | 35** |
| Basal Cell Hyperplasia (%) | 5 | 50* | 87** |
| Lamina Propria Fibrosis (%) | 27 | 70* | 85** |
| Barrett's Esopahgus (OR; 95% CI) | N/A | 1.16; 0.37 – 3.64 | 0.89; 0.25 – 3.11 |
= p < 0.001 compared to < 5 eos/hpf
= p < 0.001 compared to < 15 eos/hpf
= p < 0.05 compared to < 15 eos/hpf
Receiver Operating Characteristic Curves
ROC curves identified an optimal cut-point of 6 eos/hpf based on the presence or absence of basal cell hyperplasia. This cut-point resulted in a sensitivity of 89% and a specificity of 87% with an area under the ROC curve of 0.92. In contrast, the sensitivity and specificity values based on basal cell hyperplasia were 72% and 94% for the currently accepted cut-point of 15 eos/hpf. Cut-points were also investigated based on presence or absence of either lamina propria fibrosis or basal cell hyperplasia (optimal cut-point = 4 eos/hpf, area under ROC = 0.925).
Assessment of Histopathologic Disease Chronicity and Natural History
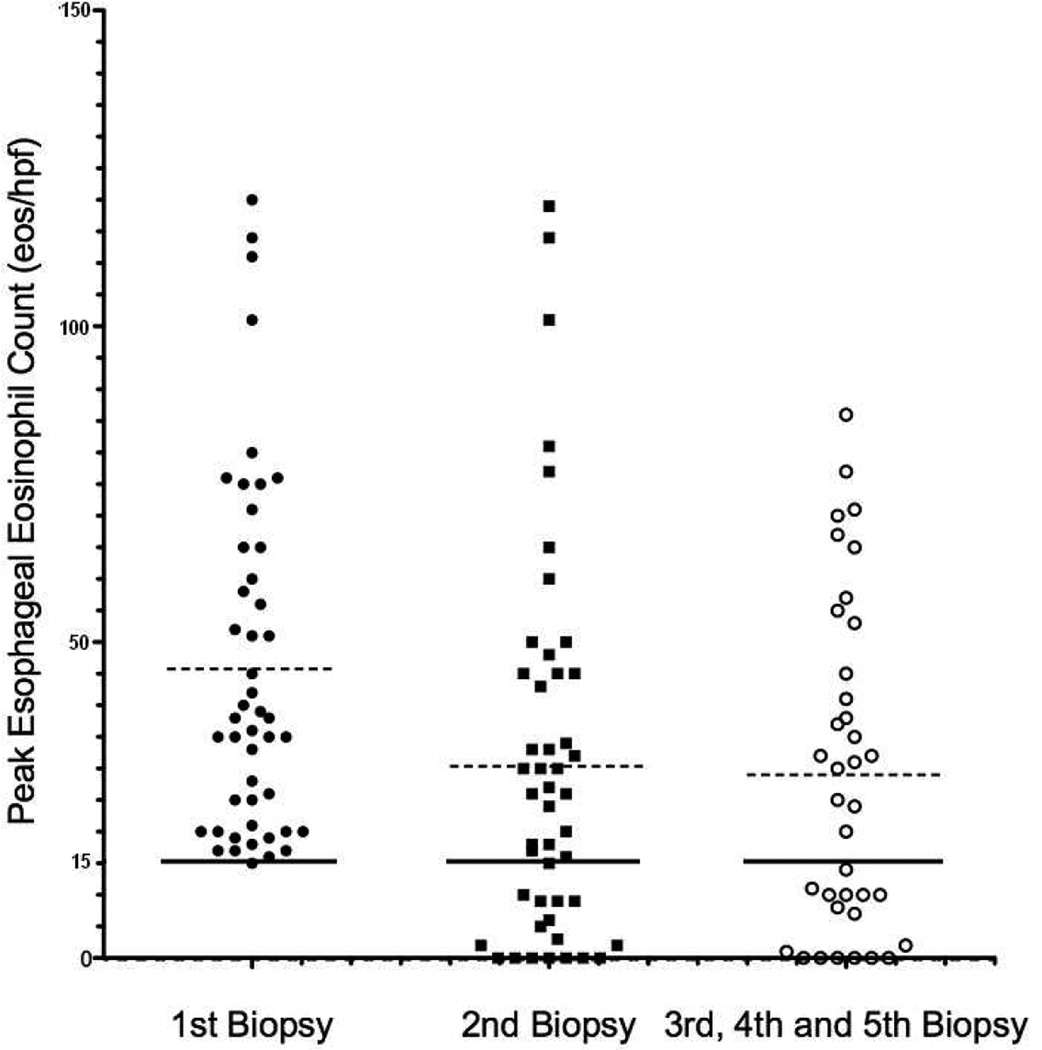
For subjects who had ≥ 15 eos/hpf on initial biopsy and at least one subsequent esophageal biopsy (N = 47), the peak eosinophil count at each biopsy is shown in a scatter plot (Figure 3). Among patients with ≥ 15 eos/hpf on initial biopsy, 66% had ≥ 15 eos/hpf on the subsequent biopsy, and 75% had evidence of esophageal eosinophilia when all subsequent endoscopies were evaluated. A smaller fraction of patients (25%, N =13/49) with < 15 eos/hpf had ≥ 15 eos/hpf on their second biopsy (p < 0.001; OR 6.54; CI 2.70 – 15.81).
Figure 3. Chronicity of Initial Esophageal Eosinophilia (≥ 15 eos/hpf).
Among the 47 patients with ≥ 15 eos/hpf and at least 2 esophageal biopsies, the peak eosinophil count for each biopsy is shown. The solid line represents 15 eos/hpf and the dashed line represents the median eosinophil level for each group.
Only 13% of patients with ≥ 15 eos/hpf demonstrated evidence of histologic resolution/remission over time (p < 0.0001; OR 7.72; CI 2.77–21.50). In contrast, 55% patients with < 15 eos/hpf on incident biopsy had histologic resolution/remission (0 eos/hpf) on a subsequent biopsy.
We noted that the majority of patients with ≥ 5 eos/hpf in the initial EGD with biopsy were more likely to have persistent esophageal eosinophilia on repeat EGD with biopsy when compared to those with < 5 eos/hpf on initial EGD with biopsy (83.6% vs. 34.3%; p < 0.0001; OR 7.78; CI 3.69 –25.86). Conversely, patients with < 5 eos/hpf on incident EGD with biopsy were far more likely have a peak count of 0 eos/hpf on subsequent biopsies than patients with initial eosinophilia (≥ 5 eos/HPF) (54% vs. 20%; p < 0.001; OR 4.85; CI 1.94–12.13).
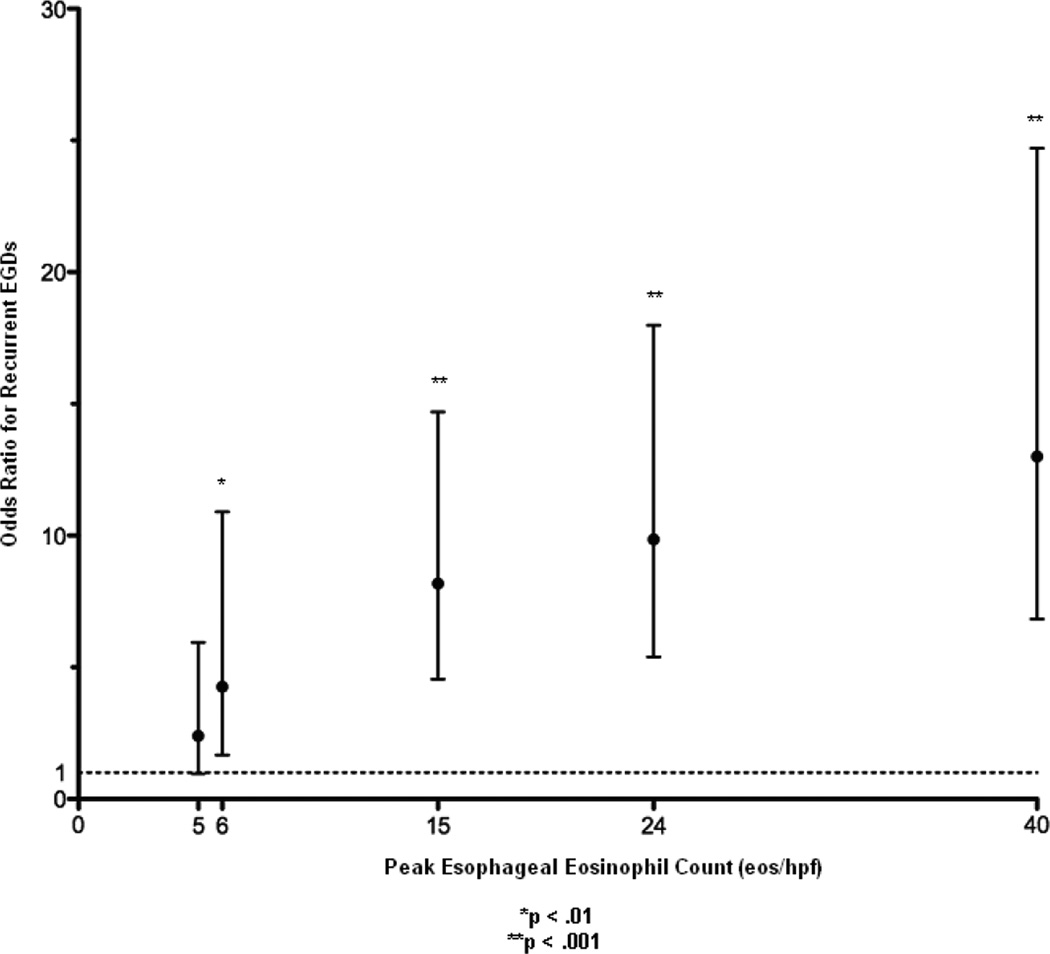
The odds of requiring repeat endoscopy increased as peak eosinophil count increased (Figure 4). Patients with > 5 eos/hpf were significantly more likely to undergo repeat endoscopy (p < 0.01; OR 4.26; CI 1.67–10.90) compared to patients without esophageal eosinophilia (0–1 eos/hpf). Overall, there was a correlation between peak esophageal eosinophil counts on initial EGD and the number of endoscopies (r2 = 0.32, p < 0.001).
Figure 4. Odds Ratio of Repeat EGD as a Function of Initial Peak Eosinophil Count.
The odds ratio and 95% CI of repeat EGDs with biopsy as a function of the initial eosinophil count is shown. An odds ratio of 1 is represented by the dotted line. (*p < .01, **p < .001)
Epidemiology
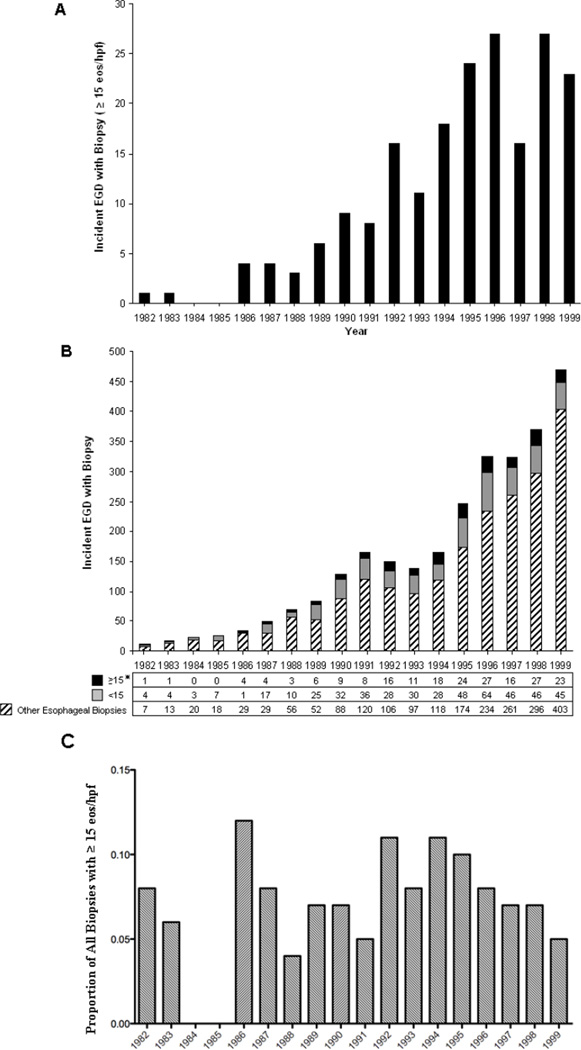
The number of incident biopsies with ≥ 15 eos/hpf ranged from 0 to 27 cases per year, with the number of incident cases steadily increasing over time (p < 0.001) (Figure 5a). In parallel with this change, the number of esophageal biopsies obtained during each year of our study period increased substantially from 12 esophageal biopsies in 1982 to 471 biopsies in 1999, a 40-fold increase (p < 0.001) (Figure 5b). Among all biopsies that met our study criteria, the proportion of biopsies with ≥ 15 eos/hpf was 0.20 in 1982 and was not increasing from 1982–1999 (p = 0.68; IRR 1.04; CI 0.87–1.23) (Table IIIA). The rate of biopsies with ≥ 15 eos/hpf per total number of esophageal biopsies at our institution was 0.08 in 1982 compared to 0.05 in 1999, and was not increasing from 1982 to 1999 (p = 0.9; IRR 1.02; CI 0.73 –1.44). When subjects were restricted to a population based cohort, the incidence rate was 0/100,000 children in 1982 vs. 4.9/100,000 children in 1996 (peak); (p < 0.001; IRR 1.18; CI 1.09–1.28) (Table IIIB). The number of esophageal biopsies with ≥ 5 eos/hpf among patients in Hamilton County rose steadily throughout the study (p < .001). Of note, the proportion of biopsies with ≥ 5 eos/hpf remained the same (p = 0.93; IRR 1.01; CI 0.76–1.35).
Figure 5. The Incidence of Esophageal Eosinophilia Over Time.
The number of incident biopsies with ≥ 15 eos/hpf occurring each year is shown* (a). The number of incident biopsies with ≥ 15 eos/hpf, < 15 eos/hpf and all other esophageal biopsies is displayed (b). The proportion of biopsies with ≥15 eos/hpf per year is shown. (*p < .001).
Table III.
| The Incidence of biopsies with ≥ 15 eos/hpf in Hamilton County A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1982 | 1983 | 1984 | 1985 | 1986 | 1987 | 1988 | 1989 | 1990 | |
| ≥ 15 eos/hpf (N) | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 3 | 4 |
| Incidence Rate* | 0.00 | 0.38 | 0.00 | 0.00 | 0.39 | 0.78 | 0.00 | 1.19 | 1.61 |
| 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | |
| ≥ 15 eos/hpf (N) | 4 | 6 | 5 | 12 | 11 | 12 | 8 | 11 | 5 |
| Incidence Rate* | 1.61 | 2.42 | 2.02 | 4.86 | 4.49 | 4.93 | 3.29 | 4.56 | 2.09 |
| The Proportion of Biopsies with ≥ 15 eos/hpf over time B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1982 | 1983 | 1984 | 1985 | 1986 | 1987 | 1988 | 1989 | 1990 | |
| ≥ 15 eos/hpf / Study Biopsies + | 0.20 | 0.20 | 0.00 | 0.00 | 0.80 | 0.19 | 0.23 | 0.19 | 0.22 |
| ≥ 15 eos/hpf / All Biopsies ++ | 0.08 | 0.06 | 0.00 | 0.00 | 0.12 | 0.08 | 0.04 | 0.07 | 0.07 |
| 1991 | 1992 | 1993 | 1994 | 1995 | 1996 | 1997 | 1998 | 1999 | |
| ≥ 15 eos/hpf / Study Biopsies + | 0.18 | 0.36 | 0.27 | 0.39 | 0.33 | 0.30 | 0.26 | 0.37 | 0.34 |
| ≥ 15 eos/hpf / All Biopsies ++ | 0.05 | 0.11 | 0.08 | 0.11 | 0.10 | 0.08 | 0.05 | 0.07 | 0.05 |
= p < 0.001; IRR 1.18, CI 1.09–1.28. Incidence of biopsies with ≥ 15 eos/hpf per 100,000 children.
= p= 0.7; IRR 1.04, Ci 0.87–1.23. Incidence of biopsies with ≥ 15 eos/hpf per total number of study biopsies.
= p=0.9; IRR 1.02, CI 0.73 – 1.44. Incidence of biopsies with ≥ 15 eos/hpf per total number of esophageal biopsies.
Discussion
Herein we report that a substantial number (n = 198) of patients (30%) previously diagnosed with reflux esophagitis between 1982–1999 had histologic evidence of EE. These 198 patients were predominantly male and distinguished from patients with chronic esophagitis by a chief complaint of dysphagia. The incidence of new cases of esophageal eosinophilia dramatically rose during the study interval, but when corrected for the large increase in the number of EGDs performed, there was a stable proportion of esophageal eosinophilia per EGD. Furthermore, we have identified that threshold levels of eosinophils associated with disease persistence emerge at ≥ 5 eos/hpf and that the presence of other histologic abnormalities begins to arise at a threshold of 6 eos/hpf. Taken together, our data strongly suggest that EE is not a new disease but, it is a new classification of a persistent esophageal disorder.
Of the retrospective studies currently published, our cohort of 198 patients represents the largest group of pediatric patients with unrecognized histologic esophageal eosinophilia. We find the similarities between our cohort of patients with esophageal eosinophilia and cohorts of EE patients to be striking. Our retrospective cohort of patients with ≥ 15 eos/hpf includes a predominance of males, and Caucasians, a mean age of 8.7 years at endoscopic presentation, and dysphagia as a common indication for endoscopy.18, 19 The identification of histologic features of EE in nearly 30% of patients previously diagnosed with reflux esophagitis suggests that EE may have been under-diagnosed in the 1980s and 1990s. We report a novel inverse correlation between peak eosinophil counts and age at histologic presentation in children, consistent with Straumman et al who demonstrated decreasing esophageal eosinophil counts with age in adult EE patients treated only with esophageal dilation.20
While the incidence of patients with ≥ 15 eos/hpf increased during our study, increasing disease recognition and re-classification are likely responsible, at least in large part. Notably, the proportion of biopsies with ≥ 15 eos/hpf was stable despite a 40-fold increase in the number of biopsies obtained. This data suggests that the “epidemic” of EE may be the result of increased recognition of EE rather than a genuine increase in disease incidence. Yet, a small rise in the true incidence of EE may still be occurring in conjunction with recognition and ascertainment biases.
It is interesting that the majority of patients with ≥ 15 eos/hpf were more likely to undergo multiple endoscopic procedures and had greater evidence of persistent disease on repeat biopsies compared with patients that presented with < 15 eos/hpf. This data suggests that patients with ≥ 15 eos/hpf had more persistent and severe symptomatology. Given our lack of clinical data, we cannot rule out that increased surveillance of patients with esophageal eosinophilia did not contribute to this finding. It is noteworthy that 13% of patients with ≥ 15 eos/hpf experienced complete histologic resolution/remission. This finding is supported by two prior studies that have demonstrated histologic resolution/remission of esophageal eosinophilia in only a small sub-group of EE patients.18, 21
We were struck by our finding that 25% of patients with < 15 eos/hpf on initial biopsy went on to develop peak eosinophil counts ≥ 15 eos/hpf. Peak eosinophil counts of ≥ 15 eos/hpf are currently used to define EE, and have been associated with basal cell hyperplasia and esophageal fibrosis.22 Interestingly, the proportion of patients with basal layer hyperplasia and fibrosis were also increased among patients with ≥ 5 eos/hpf on initial EGD with biopsy. Patients who had > 5 eos/hpf were also likely to undergo multiple endoscopic procedures and had evidence of persistent esophageal eosinophilia on repeat biopsy. Our findings are supported by clinical data published by Ruchelli et al., who reported that patients with a mean eosinophil level > 7 eos/hpf were less likely to respond to PPI therapy, and Orenstein et al., who identified classic clinical features in a series of 30 children while defining EE at levels as low as 5 eos/hpf.23, 24 These data suggest that the histologic features associated with the clinicopathologic diagnosis of EE begin to emerge with relatively low numbers of eosinophils in the epithelium.
By only examining esophageal eosinophilia without clinical data and addressing the contribution of acid-induced esophagitis, our results have limitations. Without definitive proof that our patients failed to respond to acid blockade, we cannot definitively attribute our findings to EE. However, it remains notable that patients with ≥ 5 eos/hpf had persistent esophageal eosinophilia, basal layer hyperplasia, lamina propria fibrosis and required multiple endoscopic procedures suggesting that disease persistence and severity emerge at low threshold levels.
Clinical Implications.
Esophageal Eosinophilia is a common histologic finding that was under-recognized during the 1980s and 1990s. The identification of esophageal eosinophilia, even at low thresholds (~5/hpf), raises concerns for persistent disease.
Acknowledgments
The Authors would like to thank Simon Hogan for review of this manuscript and Andrea Lippelman for editorial assistance.
Support: This work has been supported by the Campaign Urging Research for Eosinophilic Disease (CURED), the Food Allergy Project, National Institute of Allergy and Infectious Diseases T32 AI060515 (Charles W. DeBrosse), PHS Grant P30 DK078392, the Buckeye Foundation and The International Group of Eosinophilic Researchers (TIGER).
Abbreviations
- EE
Eosinophilic Esophagitis
- EGD
Esophagogastroduodenoscopy
- eos
Eosinophils
- GERD
Gastroesophageal reflux disease
- GI
Gastrointestinal
- hpf
High power field
- IRR
Incidence Rate Ratio
- PPI
Proton Pump Inhibitor
- ROC
Receiver Operator Characteristic
- CCHMC
Cincinnati Children’s Hospital Medical Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rothenberg ME. Biology and Treatment of Eosinophilic Esophagitis. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBrosse CW, Rothenberg ME. Allergy and eosinophil-associated gastrointestinal disorders (EGID) Curr Opin Immunol. 2008;20:703–708. doi: 10.1016/j.coi.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkins D, Kramer R, Capocelli K, Lovell M, Furuta GT. Eosinophilic esophagitis: the newest esophageal inflammatory disease. Nat Rev Gastroenterol Hepatol. 2009;6:267–278. doi: 10.1038/nrgastro.2009.45. [DOI] [PubMed] [Google Scholar]
- 4.Franciosi JP, Liacouras CA. Eosinophilic esophagitis. Immunol Allergy Clin North Am. 2009;29:19–27. doi: 10.1016/j.iac.2008.09.001. viii. [DOI] [PubMed] [Google Scholar]
- 5.Liacouras CA, Bonis P, Putnam PE, Straumann A, Ruchelli E, Gupta SK, et al. Summary of the First International Gastrointestinal Eosinophil Research Symposium. J Pediatr Gastroenterol Nutr. 2007;45:370–391. doi: 10.1097/MPG.0b013e318142b4f8. [DOI] [PubMed] [Google Scholar]
- 6.Furuta GT, Liacouras CA, Collins MH, Gupta SK, Justinich C, Putnam PE, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133:1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 7.Protheroe C, Woodruff SA, de Petris G, Mukkada V, Ochkur SI, Janarthanan S, et al. A novel histologic scoring system to evaluate mucosal biopsies from patients with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2009;7:749–55. doi: 10.1016/j.cgh.2009.03.022. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ngo P, Furuta GT, Antonioli DA, Fox VL. Eosinophils in the esophagus--peptic or allergic eosinophilic esophagitis? Case series of three patients with esophageal eosinophilia. Am J Gastroenterol. 2006;101:1666–1670. doi: 10.1111/j.1572-0241.2006.00562.x. [DOI] [PubMed] [Google Scholar]
- 9.Noel RJ, Putnam PE, Collins MH, Assa'ad AH, Guajardo JR, Jameson SC, et al. Clinical and immunopathologic effects of swallowed fluticasone for eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2004;2:568–575. doi: 10.1016/s1542-3565(04)00240-x. [DOI] [PubMed] [Google Scholar]
- 10.Walsh SV, Antonioli DA, Goldman H, Fox VL, Bousvaros A, Leichtner AM, et al. Allergic esophagitis in children: a clinicopathological entity. Am J Surg Pathol. 1999;23:390–396. doi: 10.1097/00000478-199904000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Dellon ES, Aderoju A, Woosley JT, Sandler RS, Shaheen NJ. Variability in diagnostic criteria for eosinophilic esophagitis: a systematic review. Am J Gastroenterol. 2007;102:2300–2313. doi: 10.1111/j.1572-0241.2007.01396.x. [DOI] [PubMed] [Google Scholar]
- 12.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351:940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 13.Straumann A, Simon HU. Eosinophilic esophagitis. escalating epidemiology? J Allergy Clin Immunol. 2005;115:418–419. doi: 10.1016/j.jaci.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Vanderheyden AD, Petras RE, DeYoung BR, Mitros FA. Emerging eosinophilic (allergic) esophagitis: increased incidence or increased recognition? Arch Pathol Lab Med. 2007;131:777–779. doi: 10.5858/2007-131-777-EEAEII. [DOI] [PubMed] [Google Scholar]
- 15.Whitney-Miller CL, Katzka D, Furth EE. Eosinophilic esophagitis. A retrospective review of esophageal biopsy specimens from 1992 to 2004 at an adult academic medical center. Am J Clin Pathol. 2009;131:788–792. doi: 10.1309/AJCPOMPXJFP7EB4P. [DOI] [PubMed] [Google Scholar]
- 16.Gill R, Durst P, Rewalt M, Elitsur Y. Eosinophilic esophagitis disease in children from West Virginia: a review of the last decade (1995–2004) Am J Gastroenterol. 2007;102:2281–2285. doi: 10.1111/j.1572-0241.2007.01352.x. [DOI] [PubMed] [Google Scholar]
- 17.Steiner SJ, Kernek KM, Fitzgerald JF. Severity of basal cell hyperplasia differs in reflux versus eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2006;42:506–509. doi: 10.1097/01.mpg.0000221906.06899.1b. [DOI] [PubMed] [Google Scholar]
- 18.Spergel JM, Brown-Whitehorn TF, Beausoleil JL, Franciosi J, Shuker M, Verma R, et al. 14 years of eosinophilic esophagitis: clinical features and prognosis. J Pediatr Gastroenterol Nutr. 2009;48:30–36. doi: 10.1097/MPG.0b013e3181788282. [DOI] [PubMed] [Google Scholar]
- 19.Assa'ad AH, Putnam PE, Collins MH, Akers RM, Jameson SC, Kirby CL, et al. Pediatric patients with eosinophilic esophagitis: an 8-year follow-up. J Allergy Clin Immunol. 2007;119:731–738. doi: 10.1016/j.jaci.2006.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Straumann A, Spichtin HP, Grize L, Bucher KA, Beglinger C, Simon HU. Natural history of primary eosinophilic esophagitis: a follow-up of 30 adult patients for up to 11.5 years. Gastroenterology. 2003;125:1660–1669. doi: 10.1053/j.gastro.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 21.Konikoff MR, Noel RJ, Blanchard C, Kirby C, Jameson SC, Buckmeier BK, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131:1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 22.Ruchelli E, Wenner W, Voytek T, Brown K, Liacouras C. Severity of esophageal eosinophilia predicts response to conventional gastroesophageal reflux therapy. Pediatr Dev Pathol. 1999;2:15–18. doi: 10.1007/s100249900084. [DOI] [PubMed] [Google Scholar]
- 23.Orenstein SR, Shalaby TM, Di Lorenzo C, Putnam PE, Sigurdsson L, Mousa H, et al. The spectrum of pediatric eosinophilic esophagitis beyond infancy: a clinical series of 30 children. Am J Gastroenterol. 2000;95:1422–1430. doi: 10.1111/j.1572-0241.2000.02073.x. [DOI] [PubMed] [Google Scholar]