Introduction
Apoptosis and NETosis, two important pathways of programed cell death, differ in their morphologic features and their effects on the immune system. In apoptosis, nuclear chromatin compacts as it is packaged into nuclear fragments and apoptotic blebs (1), and uptake of apoptotic cells by phagocytes generally suppresses the immune response (2). In NETosis, named after neutrophil extracellular traps (NETs), nuclear chromatin relaxes and forms a fibrous meshwork upon release from the cell (3). In general, NETosis is induced by infection, inflammation, or trauma and represents a mechanism of innate immune activation (4). Neutrophils, the most abundant type of white blood cells, migrate toward a stimulus in coordinated fashion, and NETs may synchronize such neutrophil swarms (5). Despite the structural and functional differences between apoptosis and NETosis, significant aspects of their clearance pathways likely overlap, as specific serum proteins participate in the recognition and uptake of remnants from either cell death pathway. In vivo, it is likely that both cell death pathways are concurrently present and that apoptotic bodies and NETs entangle (6). Yet, a third type of DNA may intertwine with DNA from apoptotic and NETotic cells, as certain bacteria and fungi release extracellular DNA that is used to construct biofilms (7). How apoptotic bodies, NETs, and biofilm DNA (Figure 1) are safely cleared is of great interest, because incomplete clearance leads to systemic inflammation and autoantibody production.
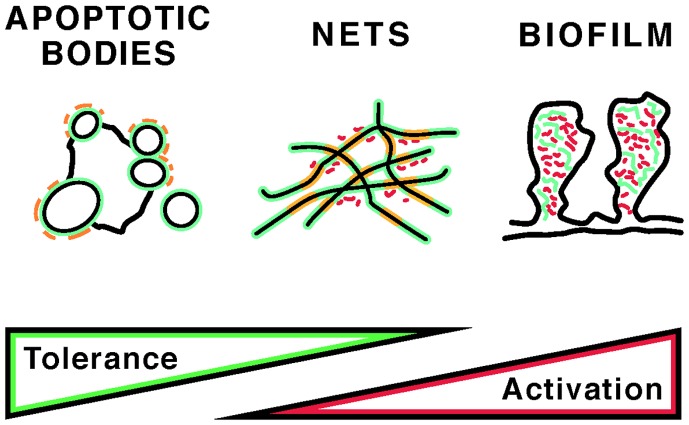
Figure 1.
Self and foreign antigens that may induce autoantibodies in autoimmunity. The potential contribution of apoptotic bodies, NETs, and bacterial biofilms to immune tolerance versus stimulation is indicated. The distribution and content of self (green) and foreign (red) antigens is diagrammed. In apoptotic bodies, “foreign” structures may include post-translational modifications that are present only during late stages of apoptosis (orange). NETs, in addition to modified chromatin (orange), may also contain bacterial adjuvants, whereas biofilms may incorporate host DNA. Short red rods indicate bacteria in NETs and biofilms. For details, see text.
Systemic Autoimmune Diseases and Autoantibodies to Nuclear Antigens
Molecular structures associated with dying cells are targets of autoantibodies in autoimmune diseases such as systemic lupus (SLE) (8), antiphospholipid syndrome (APS) (9), as well as other musculoskeletal/rheumatoid disorders (10). The resulting autoreactivities are idiosyncratic for each condition and thus are useful for clinical diagnosis. However, the antigens recognized by the autoantibodies are also involved in pathogenesis, as they accumulate at the sites of tissue damage and contribute to immune complex deposition (11). Tissue damage may worsen in the absence of serum nucleases such as DNAse I (12). Furthermore, the interactions between dying cells and the adaptive immune system strengthen over time, as somatic mutations and antigen selection optimize antibodies for improved binding (13). In SLE, antibodies to nuclear or plasma membrane antigens arise in the course of disease (14, 15). These antibodies avidly bind to apoptotic cells (16). Classical studies recognized that apoptotic cells are far better substrates for autoantibody binding than viable cells (17). However, monoclonal antibodies from mouse lupus models that bind to apoptotic blebs (16) also tightly bind to NETs released in response to bacterial pathogens (18). Our laboratory showed that NETotic cells provide suitable targets for autoantibodies from diverse human autoimmune disorders (19). Whether apoptotic or NETotic cell death, or both, provide antigens that induce autoantibody production is essential information for understanding the etiopathogenesis of autoimmune diseases (20).
Apoptotic and NETotic Cell Death
Apoptosis is characterized by dramatic morphologic changes that are orchestrated by a family of specific proteases called caspases (21). The chromatin in the nucleus condenses tightly despite the fact that caspase-activated DNAse cleaves certain regions of genomic DNA to produce an oligonucleosome “ladder” (22). Curiously, the diameter (and thus the permeability) of nuclear pores transiently increases during this stage of apoptosis (23), and oligonucleosomes pass through the pores into the cytoplasm (16). The chromatin fragments associate with the outer nuclear envelope, the nucleus breaks up, and nuclear fragments migrate toward the cellular plasma membrane. These nuclear fragments form “blebs” at the cell surface, which are characteristic protrusions that give apoptotic cells their typical “grape cluster” appearance. Blebs display DNA, chromatin, and ribonucleoproteins at the cell surface (16, 24) such that these autoantigens become accessible to antibodies and pattern recognition receptors.
An alternative form of cell death was discovered by Brinkmann et al. (18). These authors reported that, upon exposure to bacteria, LPS, or PMA, neutrophils dissolve nuclear and cytoplasmic granule membranes, relax nuclear chromatin, associate the chromatin with granule components such as myeloperoxidase or elastase, and release the relaxed chromatin across the plasma membrane (4). The chromatin appears as disorganized fibers that spread widely to form an extracellular network. The authors named the fibers “NETs” because this chromatin could immobilize or “trap” bacteria. Mouse anti-chromatin antibodies were used to demonstrate that the NETs consisted of DNA and histones. These results immediately suggested that a tangle of bacteria and nuclear chromatin should be viewed as a “dangerous liaison” between lupus autoantigens and bacterial adjuvants that, by acting as a molecular complex, could trigger an adaptive immune response (25).
Follow-up studies revealed that NETs are not always an impediment to microbes. Proliferation assays identified certain species of bacteria that are resistant to any bactericidal effects of the released neutrophil chromatin (26), even though NETs organize bactericidal granule contents such as peroxidase and serine proteases (27), and even though histones also exhibit bactericidal activity (28). In fact, NET chromatin has found a novel use for certain bacteria that can incorporate NET chromatin into their extracellular matrix (29, 30). Such biofilms protect the microbes from physiological and pharmaceutical antibiotics and help to colonize various host tissues (7). DNA gives biofilms their structural integrity because nuclease treatment efficiently dissolves biofilms (31). The biofilms can also incorporate microbial DNA, as particular bacteria and fungi have mechanisms to release sections of genomic DNA for use in forming biofilms. Such DNA could be of particular significance in inducing anti-DNA responses because bacterial DNA has hypomethylated CpG motifs that directly stimulate toll-like receptors (32) and other DNA receptors (33) in B cells and other antigen- presenting cells.
Evidence for Apoptosis and NETosis in the Induction of Autoimmunity
Evidence supporting apoptotic cells as the source of autoantigens that induce and promote the development of autoimmunity derives from a close inspection of autoantibody specificities. The observation that lupus serum IgG bind to apoptotic cells (17) initiated an active area of research. Because apoptotic cells externalize phosphatidylserine at the cell surface, binding of serum factors or lupus antibodies to phosphatidylserine could interfere with clearance in a way that would alter recognition of apoptotic cells and potentially induce disease. This view is consistent with genetic defects in cell clearance that in many instances recreate the full set of lupus manifestations (8).
Completion of the apoptotic program without adequate clearance may lead to the exposure of highly modified autoantigens (34). Autoantibodies to apoptotic cells may be induced by unique antigenic structures that are produced by enzymatic reactions in apoptotic cells. Granzyme B activation in apoptosis was identified as one possible mechanism whereby apoptosis generates novel self antigens that stimulate autoantibody binding (35). Importantly, characteristic post-translational modifications (PTM) of histones are induced during apoptosis. These include the acetylation of lysine 12 in the H2B core histone, a PTM that was shown to enhance the binding of lupus autoantibodies (36). However, lysine 12 acetylation also occurs in NETosis, and tri-acetylated histone H4, a specific target of the KM-2 murine lupus autoantibody, is more abundant in NETs from SLE patients than in controls (37). Therefore, antibody reactivity against any single histone PTM may not unambiguously establish which biological process supplies nuclear antigens in autoimmunity (38).
The generation of apoptotic cells during development and under conditions of rapid cell turnover, such as exist physiologically in primary lymphoid organs, suggests that apoptotic lymphocytes provide a steady supply of tolerogenic autoantigens (39). The idea that apoptosis provides self antigens that maintain tolerance is supported by immune suppression following injection of apoptotic cells (40). Immune suppression by apoptotic cells can also be recreated in vitro (41) and can be converted to immune activation by opsonization of apoptotic cells with antibodies (42). On balance, NETosis is a more likely alternative source of autoantigens that stimulate autoreactive B cells. This follows directly from the observation that, in autoimmunity, autoantibodies arise to various known NET components (43, 44). These include the proteases cathepsin G, proteinase 3, and elastase, as well as granule peptides, including LL37 and other defensins that have bactericidal properties.
Detailed analysis revealed that neutrophils from autoimmune patients are more prone to NETosis than controls and that NETosis is associated with particular autoantigen modifications (45, 46). Such autoantigen PTM may arise through reactive oxygen species liberated in NETosis or through enzymes that are activated during the progression of NETosis. Amino acids such as tryptophan and tyrosine are modified by oxidation or reactions with hypochlorous acid and peroxynitrite (47). NETosis also activates peptidylarginine deiminases (PADs), enzymes that convert arginine residues in proteins to citrulline residues. Our laboratory was first to link deimination (also known as “citrullination”) of nucleohistones to steps that are set in motion during NETosis (25). Importantly, we also showed that histone deimination is independent of caspase activity and that induction of apoptosis prevents PAD activation. Thus, deimination of histones clearly distinguishes NETosis from apoptosis.
In subsequent studies, we showed that citrullinated histones, including core and linker histones, are recognized in preference over non-modified histones by antibodies from patients with various autoimmune diseases, including SLE and Felty’s syndrome, a more severe form of rheumatoid arthritis (10). In confirming our results, others have shown that autoantibodies to deiminated histones are remarkably useful in the diagnosis of rheumatoid arthritis (48). In earlier studies, it was reported that citrullinated proteins are frequently targets of IgG antibodies from patients with arthritis (49), and antibodies to citrullinated antigens have been a focus of a growing number of research studies (50, 51). These observations represent a solid link between NETosis and the induction of disease-specific autoantibodies.
Clearance Mechanisms
Clearance of apoptotic cells has been a focus of research for more than two decades (52), and a bewildering complexity of pathways has emerged (53). Different cell types participate in the uptake of apoptotic cells, the cells employ different combinations of receptors, and clearance may be enhanced or suppressed by various plasma proteins. Soluble plasma proteins that participate in apoptotic cell clearance include members of the pentraxin (54) and collectin families (55), the complement protein C1q (56), and milk fat globule epidermal growth factor 8 (MFG-E8) (57). An important “eat-me” signal is generated by the endoplasmic reticulum chaperone calreticulin. Apoptotic cells release calreticulin from the endoplasmic reticulum into the cytoplasm (58). The cytoplasmic calreticulin binds to phosphatidylserine in the inner leaflet of the plasma membrane from where it is externalized as the plasma membrane loses its asymmetry. At the cell surface, calreticulin combines with C1q and binds CD91 on the surface of the macrophage, leading to the phagocytosis of the apoptotic cell (59). Other receptors for uptake of apoptotic cells include SCARF1, a highly conserved receptor for C1q (60), and the integrin βVα5, a receptor for MFG-E8 (61). The importance of C1q, MFG-E8, and SCARF1 for tissue homeostasis is emphasized by the fact that mice deficient for any of these molecules show a reduced capacity for apoptotic cell clearance and exhibit a concomitant induction of autoantibodies (60, 62, 63). In SLE, altered levels of MFG-E8 in the serum and impaired C1q recognition of apoptotic cells correlate with the severity of disease manifestations (64, 65).
Additional receptors for the recognition and clearance of apoptotic cells are the Mer, Axl, and Tyro3 receptor tyrosine kinases (66). Mice deficient in any of these receptors manifest symptoms of autoimmune disease (67), and patients show altered serum levels of Mer family ligands GAS6 and protein S (68). Whereas Axl determines apoptotic cell clearance by dendritic cells (69), Mer is induced by C1q and serves to enhance apoptotic cell uptake by macrophage (70). It is important to note that several of these receptor–ligand systems are not specific for apoptotic cells but instead participate in the clearance of infectious microbes such as bacteria, fungi, and viruses (53). Possibly, some of these clearance pathways also serve to eliminate other cellular remnants.
Little is known about the clearance of NETotic cells, although a systematic analysis of the relevant mechanisms for NET clearance is urgently needed. Good starting points would be proteins and receptors that bind DNA or chromatin and that participate in the clearance of apoptotic cells. For example, several pentraxins (71) and collectins (55) bind to nucleic acids and chromatin, and calreticulin exhibits high affinity for chromatin and nucleosomes (72). It is likely that these proteins and receptors also bind NETs, although NETs are not efficiently recognized by the pentraxin C-reactive protein, or the complement protein C3b (73). In contrast, C1q binds NETs and activates the complement cascade (74, 75). The search for additional factors that regulate NET clearance is timely because NETosis has been linked to atherosclerosis (76), small vessel vasculitis (77), deep vein thrombosis (78), and various autoimmune conditions (79). Conversely, autoimmune diseases show an aberrant persistence of NETs, and NET clearance is impaired in APS (80), SLE (81), and gout (82). A better knowledge of NET clearance is expected to lead to new treatments for autoimmune diseases, as inhibitors of PAD4 show promise in various animal models of autoimmune disorders (83–86).
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Thanks to Tim Higgins, Senior Scientific Illustrator for the design of (Figure 1). Research in the Radic lab is supported by the ORR Fund of Memphis, TN, USA.
References
- 1.Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer (1994) 73:2013–26 [DOI] [PubMed] [Google Scholar]
- 2.Ravishankar B, Shinde R, Liu H, Chaudhary K, Bradley J, Lemos HP, et al. Marginal zone CD169+ macrophages coordinate apoptotic cell-driven cellular recruitment and tolerance. Proc Natl Acad Sci U S A (2014) 111:4215–20 10.1073/pnas.1320924111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol (2007) 176:231–41 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V, Zychlinsky A. Neutrophil extracellular traps: is immunity the second function of chromatin? J Cell Biol (2012) 198:773–83 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature (2013) 498:371–5 10.1038/nature12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manfredi AA, Covino C, Rovere-Querini P, Maugeri N. Instructive influences of phagocytic clearance of dying cells on neutrophil extracellular trap generation. Clin Exp Immunol (2014). 10.1111/cei.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol (2013). 10.3109/1040841X.2013.841639 [DOI] [PubMed] [Google Scholar]
- 8.Munoz LE, Lauber K, Schiller M, Manfredi AA, Herrmann M. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat Rev Rheumatol (2010) 6:280–9 10.1038/nrrheum.2010.46 [DOI] [PubMed] [Google Scholar]
- 9.Andreoli L, Fredi M, Nalli C, Franceschini F, Meroni PL, Tincani A. Antiphospholipid antibodies mediate autoimmunity against dying cells. Autoimmunity (2013) 46:302–6 10.3109/08916934.2013.783025 [DOI] [PubMed] [Google Scholar]
- 10.Dwivedi N, Radic M. Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Ann Rheum Dis (2014) 73:483–91 10.1136/annrheumdis-2013-203844 [DOI] [PubMed] [Google Scholar]
- 11.van der Vlag J, Berden JH. Lupus nephritis: role of antinucleosome autoantibodies. Semin Nephrol (2011) 31:376–89 10.1016/j.semnephrol.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 12.Rekvig OP, Mortensen ES. Immunity and autoimmunity to dsDNA and chromatin – the role of immunogenic DNA-binding proteins and nuclease deficiencies. Autoimmunity (2012) 45:588–92 10.3109/08916934.2012.719954 [DOI] [PubMed] [Google Scholar]
- 13.Shlomchik MJ, Aucoin AH, Pisetsky DS, Weigert MG. Structure and function of anti-DNA autoantibodies derived from a single autoimmune mouse. Proc Natl Acad Sci U S A (1987) 84: 9150–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cocca BA, Cline AM, Radic MZ. Blebs and apoptotic bodies are B cell autoantigens. J Immunol (2002) 169:159–66 10.4049/jimmunol.169.1.159 [DOI] [PubMed] [Google Scholar]
- 15.Cocca BA, Seal SN, D’Agnillo P, Mueller YM, Katsikis PD, Rauch J, et al. Structural basis for autoantibody recognition of phosphatidylserine-beta 2 glycoprotein I and apoptotic cells. Proc Natl Acad Sci U S A (2001) 98:13826–31 10.1073/pnas.241510698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol (2004) 172:6692–700 10.4049/jimmunol.172.11.6692 [DOI] [PubMed] [Google Scholar]
- 17.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med (1994) 179:1317–30 10.1084/jem.179.4.1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science (2004) 303:1532–5 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 19.Dwivedi N, Upadhyay J, Neeli I, Khan S, Pattanaik D, Myers L, et al. Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis Rheum (2012) 64:982–92 10.1002/art.33432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bouts YM, Wolthuis DF, Dirkx MF, Pieterse E, Simons EM, van Boekel AM, et al. Apoptosis and NET formation in the pathogenesis of SLE. Autoimmunity (2012) 45:597–601 10.3109/08916934.2012.719953 [DOI] [PubMed] [Google Scholar]
- 21.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol (2014) 15:81–94 10.1038/nrm3735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iglesias-Guimarais V, Gil-Guinon E, Sanchez-Osuna M, Casanelles E, Garcia-Belinchon M, Comella JX, et al. Chromatin collapse during caspase-dependent apoptotic cell death requires DNA fragmentation factor, 40-kDa subunit-/caspase-activated deoxyribonuclease-mediated 3’-OH single-strand DNA breaks. J Biol Chem (2013) 288:9200–15 10.1074/jbc.M112.411371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strasser C, Grote P, Schauble K, Ganz M, Ferrando-May E. Regulation of nuclear envelope permeability in cell death and survival. Nucleus (2012) 3:540–51 10.4161/nucl.21982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radic MZ, Shah K, Zhang W, Lu Q, Lemke G, Hilliard GM. Heterogeneous nuclear ribonucleoprotein P2 is an autoantibody target in mice deficient for Mer, Axl, and Tyro3 receptor tyrosine kinases. J Immunol (2006) 176:68–74 10.4049/jimmunol.176.1.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neeli I, Khan SN, Radic M. Histone deimination as a response to inflammatory stimuli in neutrophils. J Immunol (2008) 180:1895–902 10.4049/jimmunol.180.3.1895 [DOI] [PubMed] [Google Scholar]
- 26.Juneau RA, Pang B, Weimer KE, Armbruster CE, Swords WE. Nontypeable Haemophilus influenzae initiates formation of neutrophil extracellular traps. Infect Immun (2011) 79:431–8 10.1128/IAI.00660-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoo DG, Winn M, Pang L, Moskowitz SM, Malech HL, Leto TL, et al. Release of cystic fibrosis airway inflammatory markers from Pseudomonas aeruginosa-stimulated human neutrophils involves NADPH oxidase-dependent extracellular DNA trap formation. J Immunol (2014) 192:4728–38 10.4049/jimmunol.1301589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirsch JG. Bactericidal action of histone. J Exp Med (1958) 108:925–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malcolm KC, Nichols EM, Caceres SM, Kret JE, Martiniano SL, Sagel SD, et al. Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival. PLoS One (2013) 8:e57402. 10.1371/journal.pone.0057402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Gennip M, Christensen LD, Alhede M, Qvortrup K, Jensen PO, Hoiby N, et al. Interactions between polymorphonuclear leukocytes and Pseudomonas aeruginosa biofilms on silicone implants in vivo. Infect Immun (2012) 80:2601–7 10.1128/IAI.06215-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science (2002) 295:1487. 10.1126/science.295.5559.1487 [DOI] [PubMed] [Google Scholar]
- 32.Green NM, Marshak-Rothstein A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin Immunol (2011) 23:106–12 10.1016/j.smim.2011.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paludan SR, Bowie AG. Immune sensing of DNA. Immunity (2013) 38:870–80 10.1016/j.immuni.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cline AM, Radic MZ. Apoptosis, subcellular particles, and autoimmunity. Clin Immunol (2004) 112:175–82 10.1016/j.clim.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 35.Casciola-Rosen L, Andrade F, Ulanet D, Wong WB, Rosen A. Cleavage by Granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J Exp Med (1999) 190:815–26 10.1084/jem.190.6.815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Bavel CC, Dieker J, Muller S, Briand JP, Monestier M, Berden JH, et al. Apoptosis-associated acetylation on histone H2B is an epitope for lupus autoantibodies. Mol Immunol (2009) 47:511–6 10.1016/j.molimm.2009.08.009 [DOI] [PubMed] [Google Scholar]
- 37.Pieterse E, Hofstra J, Berden J, Herrmann M, Dieker J, van der Vlag J. Acetylated histones contribute to the immunostimulatory potential of neutrophil extracellular traps in systemic lupus erythematosus. Clin Exp Immunol (2014). 10.1111/cei.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieterse E, van der Vlag J. Breaking immunological tolerance in systemic lupus erythematosus. Front Immunol (2014) 5:164. 10.3389/fimmu.2014.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature (2000) 407:784–8 10.1038/35037722 [DOI] [PubMed] [Google Scholar]
- 40.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity (2011) 35:445–55 10.1016/j.immuni.2011.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature (1997) 390:350–1 [DOI] [PubMed] [Google Scholar]
- 42.Janko C, Franz S, Munoz LE, Siebig S, Winkler S, Schett G, et al. CRP/anti-CRP antibodies assembly on the surfaces of cell remnants switches their phagocytic clearance toward inflammation. Front Immunol (2011) 2:70. 10.3389/fimmu.2011.00070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol (2012) 189:2689–95 10.4049/jimmunol.1201719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radic M, Marion TN. Neutrophil extracellular chromatin traps connect innate immune response to autoimmunity. Semin Immunopathol (2013) 35:465–80 10.1007/s00281-013-0376-6 [DOI] [PubMed] [Google Scholar]
- 45.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med (2013) 5:178ra140. 10.1126/scitranslmed.3005580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, Lin AM, et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol (2011) 187:538–52 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heinecke JW. Mass spectrometric quantification of amino acid oxidation products in proteins: insights into pathways that promote LDL oxidation in the human artery wall. FASEB J (1999) 13:1113–20 [DOI] [PubMed] [Google Scholar]
- 48.Pratesi F, Dioni I, Tommasi C, Alcaro MC, Paolini I, Barbetti F, et al. Antibodies from patients with rheumatoid arthritis target citrullinated histone 4 contained in neutrophils extracellular traps. Ann Rheum Dis (2014) 73:1414–22 10.1136/annrheumdis-2012-202765 [DOI] [PubMed] [Google Scholar]
- 49.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest (1998) 101:273–81 10.1172/JCI1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amara K, Steen J, Murray F, Morbach H, Fernandez-Rodriguez BM, Joshua V, et al. Monoclonal IgG antibodies generated from joint-derived B cells of RA patients have a strong bias toward citrullinated autoantigen recognition. J Exp Med (2013) 210:445–55 10.1084/jem.20121486 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Haag S, Schneider N, Mason DE, Tuncel J, Andersson IE, Peters EC, et al. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type ii collagen. Arthritis Rheumatol (2014) 66:1440–9 10.1002/art.38383 [DOI] [PubMed] [Google Scholar]
- 52.Platt N, da Silva RP, Gordon S. Recognizing death: the phagocytosis of apoptotic cells. Trends Cell Biol (1998) 8:365–72 10.1016/S0962-8924(98)01329-4 [DOI] [PubMed] [Google Scholar]
- 53.Poon IK, Hulett MD, Parish CR. Molecular mechanisms of late apoptotic/necrotic cell clearance. Cell Death Differ (2010) 17:381–97 10.1038/cdd.2009.195 [DOI] [PubMed] [Google Scholar]
- 54.Deban L, Bottazzi B, Garlanda C, de la Torre YM, Mantovani A. Pentraxins: multifunctional proteins at the interface of innate immunity and inflammation. Biofactors (2009) 35:138–45 10.1002/biof.21 [DOI] [PubMed] [Google Scholar]
- 55.Palaniyar N, Nadesalingam J, Clark H, Shih MJ, Dodds AW, Reid KB. Nucleic acid is a novel ligand for innate, immune pattern recognition collectins surfactant proteins A and D and mannose-binding lectin. J Biol Chem (2004) 279:32728–36 10.1074/jbc.M403763200 [DOI] [PubMed] [Google Scholar]
- 56.Liang YY, Arnold T, Michlmayr A, Rainprecht D, Perticevic B, Spittler A, et al. Serum-dependent processing of late apoptotic cells for enhanced efferocytosis. Cell Death Dis (2014) 5:e1264. 10.1038/cddis.2014.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauber K, Keppeler H, Munoz LE, Koppe U, Schroder K, Yamaguchi H, et al. Milk fat globule-EGF factor 8 mediates the enhancement of apoptotic cell clearance by glucocorticoids. Cell Death Differ (2013) 20:1230–40 10.1038/cdd.2013.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tarr JM, Young PJ, Morse R, Shaw DJ, Haigh R, Petrov PG, et al. A mechanism of release of calreticulin from cells during apoptosis. J Mol Biol (2010) 401:799–812 10.1016/j.jmb.2010.06.064 [DOI] [PubMed] [Google Scholar]
- 59.Ogden CA, deCathelineau A, Hoffmann PR, Bratton D, Ghebrehiwet B, Fadok VA, et al. C1q and mannose binding lectin engagement of cell surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J Exp Med (2001) 194:781–95 10.1084/jem.194.6.781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramirez-Ortiz ZG, Pendergraft WF, III, Prasad A, Byrne MH, Iram T, Blanchette CJ, et al. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat Immunol (2013) 14:917–26 10.1038/ni.2670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Toda S, Hanayama R, Nagata S. Two-step engulfment of apoptotic cells. Mol Cell Biol (2012) 32:118–25 10.1128/MCB.05993-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, et al. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science (2004) 304:1147–50 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]
- 63.Mitchell DA, Pickering MC, Warren J, Fossati-Jimack L, Cortes-Hernandez J, Cook HT, et al. C1q deficiency and autoimmunity: the effects of genetic background on disease expression. J Immunol (2002) 168:2538–43 10.4049/jimmunol.168.5.2538 [DOI] [PubMed] [Google Scholar]
- 64.Donnelly S, Roake W, Brown S, Young P, Naik H, Wordsworth P, et al. Impaired recognition of apoptotic neutrophils by the C1q/calreticulin and CD91 pathway in systemic lupus erythematosus. Arthritis Rheum (2006) 54:1543–56 10.1002/art.21783 [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto N, Yamaguchi H, Ohmura K, Yokoyama T, Yoshifuji H, Yukawa N, et al. Serum milk fat globule epidermal growth factor 8 elevation may subdivide systemic lupus erythematosus into two pathophysiologically distinct subsets. Lupus (2014) 23:386–94 10.1177/0961203314523870 [DOI] [PubMed] [Google Scholar]
- 66.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science (2001) 293:306–11 10.1126/science.1061663 [DOI] [PubMed] [Google Scholar]
- 67.Scott RS, McMahon EJ, Pop SM, Reap EA, Caricchio R, Cohen PL, et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature (2001) 411:207–11 10.1038/35079659 [DOI] [PubMed] [Google Scholar]
- 68.Recarte-Pelz P, Tassies D, Espinosa G, Hurtado B, Sala N, Cervera R, et al. Vitamin K-dependent proteins GAS6 and protein S and TAM receptors in patients of systemic lupus erythematosus: correlation with common genetic variants and disease activity. Arthritis Res Ther (2013) 15:R41. 10.1186/ar4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Subramanian M, Hayes CD, Thome JJ, Thorp E, Matsushima GK, Herz J, et al. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J Clin Invest (2014) 124:1296–308 10.1172/JCI72051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galvan MD, Foreman DB, Zeng E, Tan JC, Bohlson SS. Complement component C1q regulates macrophage expression of Mer tyrosine kinase to promote clearance of apoptotic cells. J Immunol (2012) 188:3716–23 10.4049/jimmunol.1102920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du Clos TW. Pentraxins: structure, function, and role in inflammation. ISRN Inflamm (2013) 2013:379040. 10.1155/2013/379040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kobayashi S, Uchiyama S, Sone T, Noda M, Lin L, Mizuno H, et al. Calreticulin as a new histone binding protein in mitotic chromosomes. Cytogenet Genome Res (2006) 115:10–5 10.1159/000094795 [DOI] [PubMed] [Google Scholar]
- 73.Schorn C, Janko C, Latzko M, Chaurio R, Schett G, Herrmann M. Monosodium urate crystals induce extracellular DNA traps in neutrophils, eosinophils, and basophils but not in mononuclear cells. Front Immunol (2012) 3:277. 10.3389/fimmu.2012.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Farrera C, Fadeel B. Macrophage clearance of neutrophil extracellular traps is a silent process. J Immunol (2013) 191:2647–56 10.4049/jimmunol.1300436 [DOI] [PubMed] [Google Scholar]
- 75.Leffler J, Martin M, Gullstrand B, Tyden H, Lood C, Truedsson L, et al. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J Immunol (2012) 188:3522–31 10.4049/jimmunol.1102404 [DOI] [PubMed] [Google Scholar]
- 76.Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, Soehnlein O, et al. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation (2012) 125:1673–83 10.1161/CIRCULATIONAHA.111.046755 [DOI] [PubMed] [Google Scholar]
- 77.Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, Werb Z, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med (2009) 15:623–5 10.1038/nm.1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A (2013) 110:8674–9 10.1073/pnas.1301059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knight JS, Carmona-Rivera C, Kaplan MJ. Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol (2012) 3:380. 10.3389/fimmu.2012.00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leffler J, Stojanovich L, Shoenfeld Y, Bogdanovic G, Hesselstrand R, Blom AM. Degradation of neutrophil extracellular traps is decreased in patients with antiphospholipid syndrome. Clin Exp Rheumatol (2014) 32:66–70 [PubMed] [Google Scholar]
- 81.Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, et al. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A (2010) 107:9813–8 10.1073/pnas.0909927107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B, et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med (2014) 20:511–7 10.1038/nm.3547 [DOI] [PubMed] [Google Scholar]
- 83.Chumanevich AA, Causey CP, Knuckley BA, Jones JE, Poudyal D, Chumanevich AP, et al. Suppression of colitis in mice by Cl-amidine: a novel peptidylarginine deiminase inhibitor. Am J Physiol Gastrointest Liver Physiol (2011) 300:G929–38 10.1152/ajpgi.00435.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Knight JS, Zhao W, Luo W, Subramanian V, O’Dell AA, Yalavarthi S, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest (2013) 123:2981–93 10.1172/JCI67390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wei L, Wasilewski E, Chakka SK, Bello AM, Moscarello MA, Kotra LP. Novel inhibitors of protein arginine deiminase with potential activity in multiple sclerosis animal model. J Med Chem (2013) 56:1715–22 10.1021/jm301755q [DOI] [PubMed] [Google Scholar]
- 86.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-alpha-benzoyl-N5-(2-chloro-1-iminoethyl)-L-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol (2011) 186:4396–404 10.4049/jimmunol.1001620 [DOI] [PMC free article] [PubMed] [Google Scholar]