Abstract
Objective:
To provide guidance on standards for reporting studies of diagnostic test accuracy for dementia disorders.
Methods:
An international consensus process on reporting standards in dementia and cognitive impairment (STARDdem) was established, focusing on studies presenting data from which sensitivity and specificity were reported or could be derived. A working group led the initiative through 4 rounds of consensus work, using a modified Delphi process and culminating in a face-to-face consensus meeting in October 2012. The aim of this process was to agree on how best to supplement the generic standards of the STARD statement to enhance their utility and encourage their use in dementia research.
Results:
More than 200 comments were received during the wider consultation rounds. The areas at most risk of inadequate reporting were identified and a set of dementia-specific recommendations to supplement the STARD guidance were developed, including better reporting of patient selection, the reference standard used, avoidance of circularity, and reporting of test-retest reliability.
Conclusion:
STARDdem is an implementation of the STARD statement in which the original checklist is elaborated and supplemented with guidance pertinent to studies of cognitive disorders. Its adoption is expected to increase transparency, enable more effective evaluation of diagnostic tests in Alzheimer disease and dementia, contribute to greater adherence to methodologic standards, and advance the development of Alzheimer biomarkers.
Over the past decade, there has been an impressive increase in the number of reports published on Alzheimer disease (AD) and dementia biomarkers, describing both proof-of-concept and diagnostic test accuracy (DTA) studies. New diagnostic criteria proposed in the United States and in Europe place greater emphasis on the use of biomarkers and imaging techniques in the diagnosis of AD (in both symptomatic and asymptomatic subjects).1–4 An amyloid PET ligand has been licensed on the basis of its utility in excluding a diagnosis of AD.5,6 “Appropriate Use” criteria have since been proposed that suggest routine use of florbetapir PET scanning in mild cognitive impairment (MCI), but the lack of evidence for enhancing diagnostic certainty or for predicting progression has been acknowledged.7 The European Medicines Agency supports the use of CSF biomarkers (β-amyloid 42 and tau) to enrich clinical populations with prodromal AD.8 Numerous other potential biomarkers are in development.
Diagnostic tests for diseases that may cause cognitive problems are not restricted to biochemical and neuroimaging biomarkers. There are a variety of clinical assessment scales, both for “screening” and “diagnosis,” such as the Alzheimer's Disease Assessment Scale,9 Montreal Cognitive Assessment,10 and the Addenbrooke's Cognitive Examination–Revised.11 At present, there is little guidance on the optimal assessment scale for a particular purpose or setting; this has resulted in considerable variation in approaches to cognitive testing. As many countries move toward large-scale cognitive screening of older adults,12 there is considerable need for studies of DTA.13,14
In dementia, diagnostic studies can be divided into proof-of-concept studies (whether a test result is different in healthy controls vs dementia patients) and studies investigating the clinical applicability of a new diagnostic test. For the latter, there are 3 main questions:
Are those with certain test results more likely to have a particular form of dementia, for example, Lewy body dementia, than persons with other test results (differential diagnosis)?
Are those with certain test results more likely to progress to, for example, AD dementia, than persons with other test results (delayed determination or prediction)?
Does the test provide incremental benefit in the diagnostic workup, considering ease of administration of the test, costs, and burden for the patient?
In this context, the quality of reporting of DTA studies is particularly important. Poor or inconsistent reporting can hamper effective evaluation of study methods, assessment of potential for bias, and interpretation of the results.15–18 It also limits the ability to synthesize data across studies, precluding methodologically sound meta-analyses. Guidelines for reporting standards in other contexts,19 such as the Consolidated Standards of Reporting Trials (CONSORT) statement, are effective in raising reporting standards and, indeed, can also drive improvements in standards of trial design.20–22
In 2003, Bossuyt et al.23 published the STARD statement: Standards for Reporting of Diagnostic Accuracy studies, aiming to “improve the accuracy and completeness of reporting of studies of diagnostic accuracy, to allow readers to assess the potential for bias in the study (internal validity) and to evaluate its generalizability (external validity).”24 To date, more than 200 scientific journals have become STARD “adopters.” Although the impact of STARD may not yet be comparable to CONSORT, STARD has raised standards of reporting in diagnostic accuracy studies.25
Despite this, a recent systematic review found that, within the field of dementia, the majority of reports of diagnostic biomarker studies were missing important information, particularly for blinding of results of either the biomarker or reference standard, handling of missing data, sample selection methods, and test reproducibility.26 Although DTA studies in dementia are similar to those in any field, some features specific to dementia research are not fully addressed in the STARD criteria. Therefore, we aimed to identify aspects of reporting that are particularly important in the context of AD and dementia, produce dementia-specific supplementary guidance to STARD, and thereby enhance use (and utility) of STARD for dementia studies.
METHODS
The STARDdem Initiative sought to establish an international consensus on reporting standards for DTA studies in dementia, highlighting the most important issues and identifying any areas in which additional reporting recommendations might enhance the usefulness of the STARD guideline. The method used was derived from guidance on effective implementation of reporting standards published by the EQUATOR (Enhancing the Quality and Transparency of Health Research) Network,27 which proposes a step-wise development approach and deems a consensus process to be “a crucial characteristic” of developing reporting guidelines.22 We adapted the guidance leading to a development process comprising 3 broad phases: (1) evaluation, (2) drafting with widespread discussion and feedback using a modified Delphi technique,28 and (3) delivery. This report describes the first 2 phases and itself constitutes part of the delivery phase.
Phase 1: Evaluation.
We conducted a comprehensive literature review on biomarker DTA studies in dementia, focusing on studies that included patients with cognitive impairment (but no dementia) at baseline and used progression to dementia of the AD type as a reference standard (for methods, see Noel-Storr et al.26). Applying STARD, we assessed the quality of reporting in all identified DTA studies by calculating the percentage of studies complying with each of the STARD items.23
Phase 2: Drafting and discussion.
An international and multidisciplinary working group of dementia experts and methodologists was established, organized by the Cochrane Dementia and Cognitive Improvement Group.29 The objectives of this group were to (1) define the scope of STARD for dementia, (2) assess the applicability of existing reporting guidelines (STARD) to dementia, (3) draft dementia-specific supplementary recommendations, and (4) seek feedback and consensus from the dementia research community.
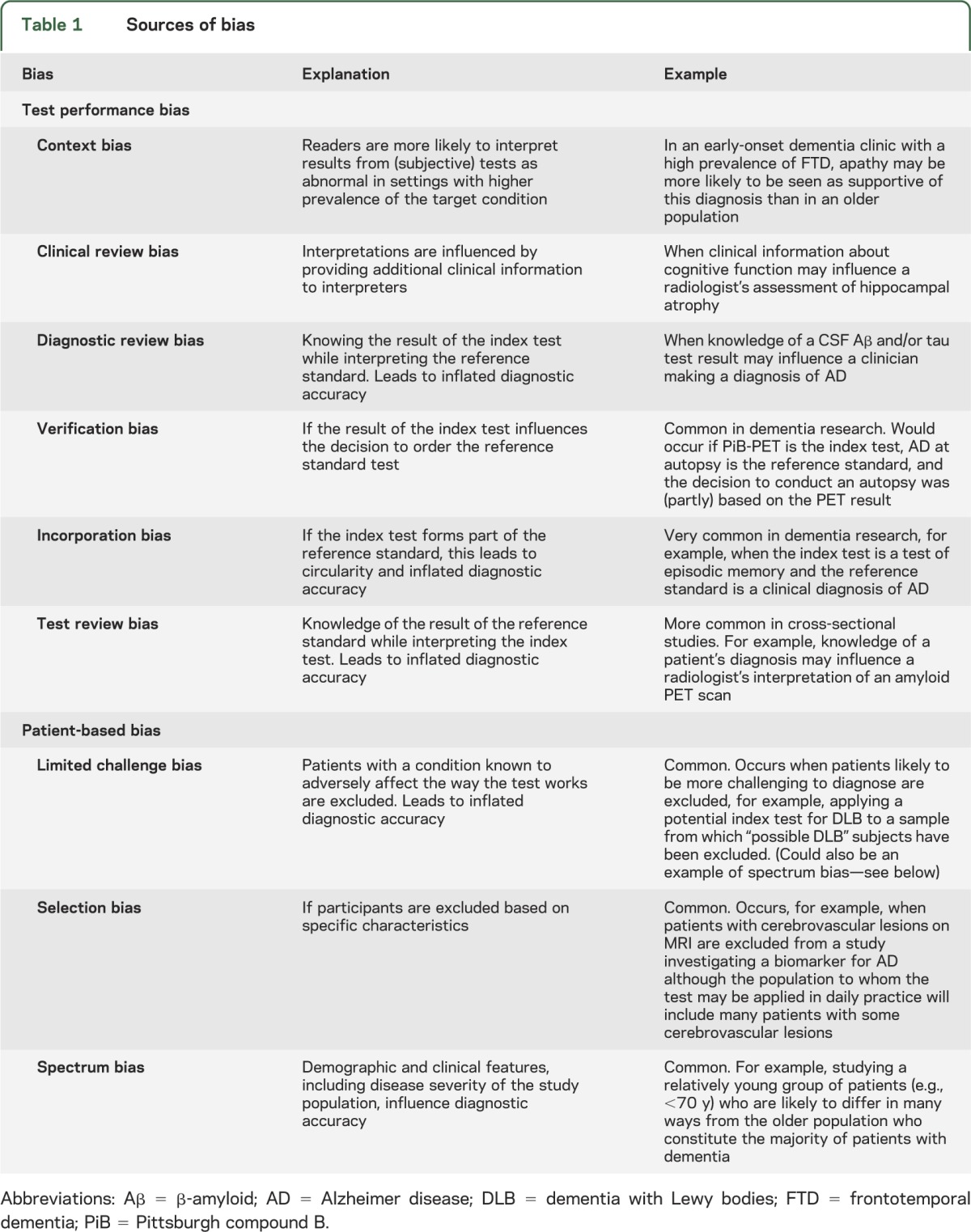
In round 1 of phase 2 (see the figure), the working group held a series of 4 meetings (one face-to-face, 3 teleconferences). Before each, members independently assessed 3 papers using the STARD tool, rating whether each STARD criterion was met and with the option to record free text comments. The 12 papers were randomly selected from studies identified by searches for DTA systematic reviews in progress by the Cochrane Dementia and Cognitive Improvement Group.30 Omissions, agreements, and disagreements were identified and discussed at these meetings. This stage of the process highlighted areas in which there was lack of clarity about what constituted “clear” or “poor” reporting within a dementia context, and hence identifying a number of focus areas for STARDdem. Supplementary guidance in these areas was drafted, and examples of adequate/clear reporting were identified from dementia diagnostic research for each relevant item. Round 1 also helped to highlight the different types of bias that can arise in dementia studies (see table 1). Bias, in this context, is defined as a systematic error, often unintentional and sometimes unavoidable, in an observed measurement from the true value. If bias existed, a study would consistently over- or underestimate the true accuracy parameters (such as test sensitivity or specificity) were the study to be replicated and repeated.
Figure. Phases and rounds of the STARDdem Consensus Initiative.
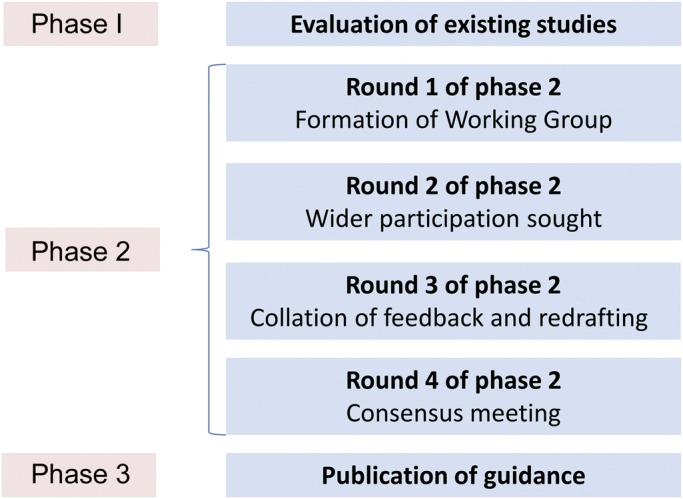
Table 1.
Sources of bias
In round 2 (figure), draft additional guidance and examples, together with the generic STARD guidance, were uploaded to the STARDdem Web site.31 More than 350 individuals were invited to comment via the Web site. These individuals had been identified as the following: corresponding authors of diagnostic studies using imaging, biochemical biomarkers, or neuropsychiatric tests; presenting authors of relevant oral presentations and posters identified from the abstract books of the Alzheimer's Association International Conference 2012 and Clinical Trials in Alzheimer's Disease conference 2012; editors of journals who publish significant numbers of diagnostic studies in dementia; and DTA methodologists. The site was open access with an encouraged branching dissemination strategy whereby participants shared the site address with other interested parties. Participants could post general feedback and/or comments on specific items, anonymously if they wished. This period of feedback was open for 2 months. The comments obtained were all discussed in the working group during a further 3 teleconferences, and revised dementia-specific additions to STARD were drafted (round 3, figure).
Round 4 (figure) consisted of a half-day consensus meeting, held in October 2012. The meeting had 2 main aims: (1) to reach consensus on the reporting of items of key significance in dementia DTA studies and on the choice of examples to illustrate good reporting, and (2) to decide on the best method for disseminating the outcome of the process and ensuring its adoption by the research community. The meeting ran as both a face-to-face meeting and Web conference to try to maximize attendance and participation. Participants comprised 40 individuals, with key groups represented by researchers/authors in this field and journal editors. After this meeting, the working group then produced a final version of the STARDdem supplementary recommendations.
RESULTS
See appendix (table e-2) on the Neurology® Web site at Neurology.org for key definitions pertinent to studies of DTA.
Phase 1.
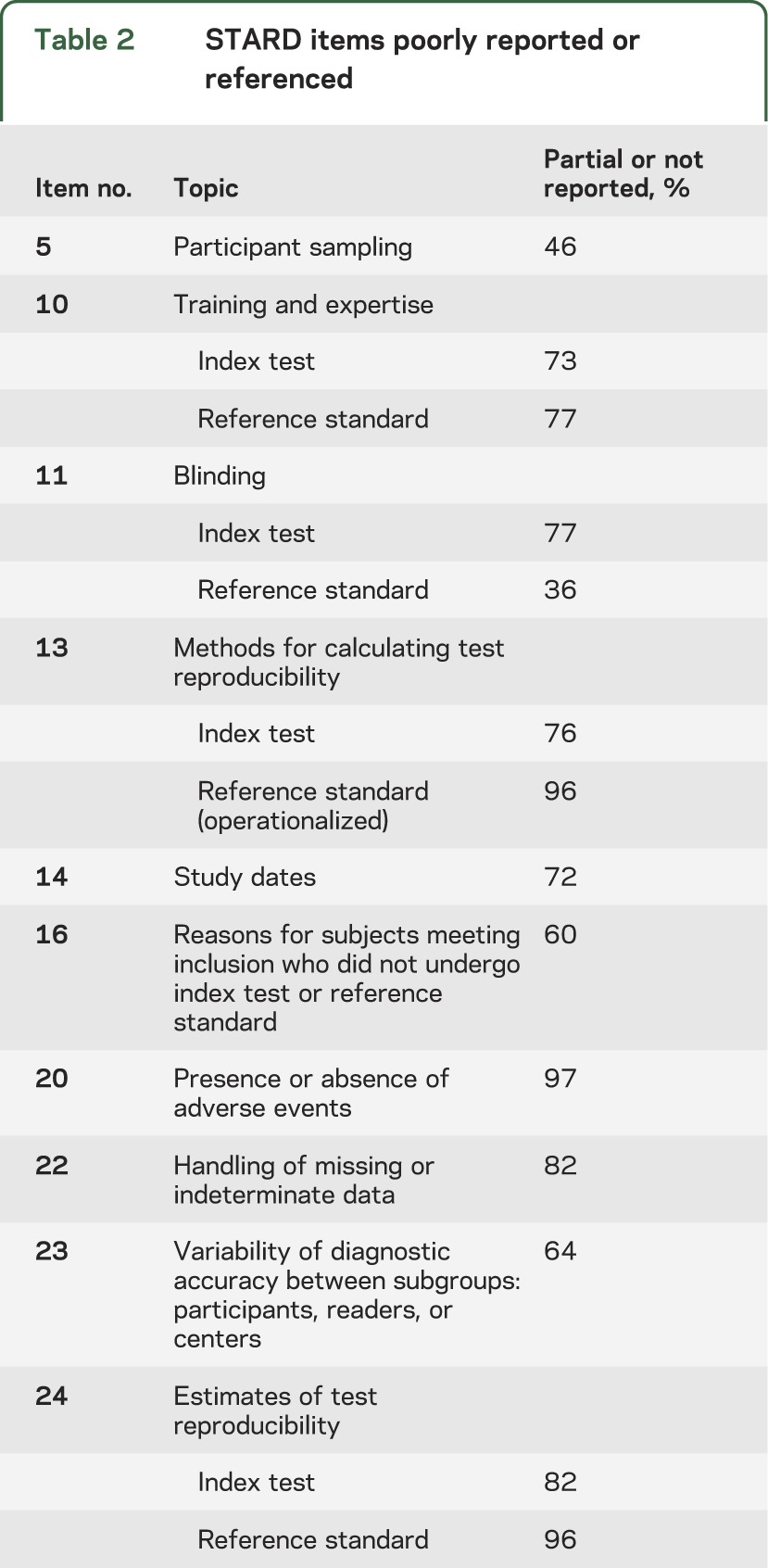
The results of the STARD assessment for studies included in our systematic review have been reported in detail elsewhere.26 In brief, this review found that of the 142 studies identified, there was marked variation in the quality of reporting between several STARD items. Items particularly poorly reported or referenced are listed in table 2.
Table 2.
STARD items poorly reported or referenced
Phase 2.
STARD is applicable to studies in which the results of one or more (index) tests are compared with the results of a reference standard applied to the same subjects, allowing production of a 2 × 2 table from which estimates of test accuracy—usually sensitivity and specificity—may be obtained. Binary diagnostic categories retain core utility as the basis for prognosis, treatment, management, and legal decision-making: the fact that the pathology and symptoms of dementias occur on a spectrum does not negate the need for the clear delineation of thresholds in index tests and for categorical reference standards. Correlations between continuous variables (e.g., biomarker level and cognitive decline) are of value for establishing etiology and point to potential as a diagnostic test, but do not guide clinicians or reimbursers about when the benefits of starting treatment outweigh the risks and costs.
Although most diagnostic test studies are cross-sectional by design, in dementia studies, the reference standard of “progression from MCI to dementia” is frequently used. These “delayed verification” studies (entailing some additional complexities of design) were included if 2 × 2 data were presented or could be derived. Studies were included regardless of the phase of development of the test.
The initial draft of reporting items, produced by the working group for consultation, may be viewed on the STARDdem Web site.31 During the open comment period of 2 months, more than 200 comments were posted by clinicians, statisticians, methodologists, neuropsychologists, molecular biologists, clinical chemists, and radiologists. Based on the comments of the working group, the most important items to address were (1) the description of the population under study, (2) reporting of the operationalization and application of the reference standard, (3) identification of potential incorporation bias or “circularity” when the index test forms a part of the reference standard (e.g., a neuropsychological test that also contributes to the diagnosis of dementia), and (4) reporting of test-retest reliability.
The second draft, which was circulated to consensus meeting participants, may also be viewed on the STARDdem Web site.31 After the discussion at the consensus meeting, a final version was produced in the form of brief additions to the concise tabular format of STARD (see table 3; print version accessible from table e-1). For 18 of the 25 items, dementia-specific additions were deemed necessary. Also, dementia-specific examples of adequate reporting were derived from the literature and are available in table e-2.
Table 3.
STARDdem checklist for the reporting of diagnostic accuracy studies in dementia
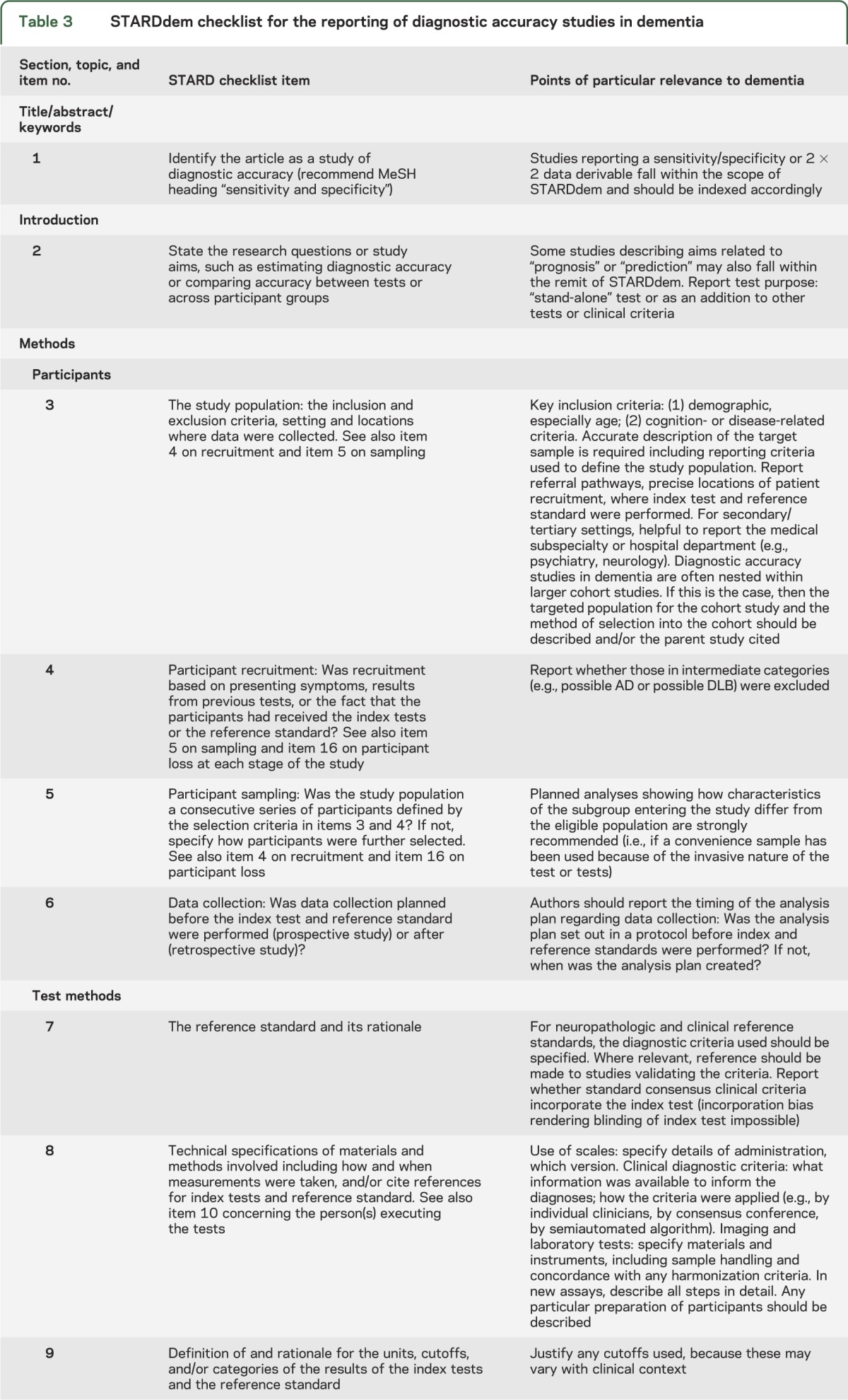
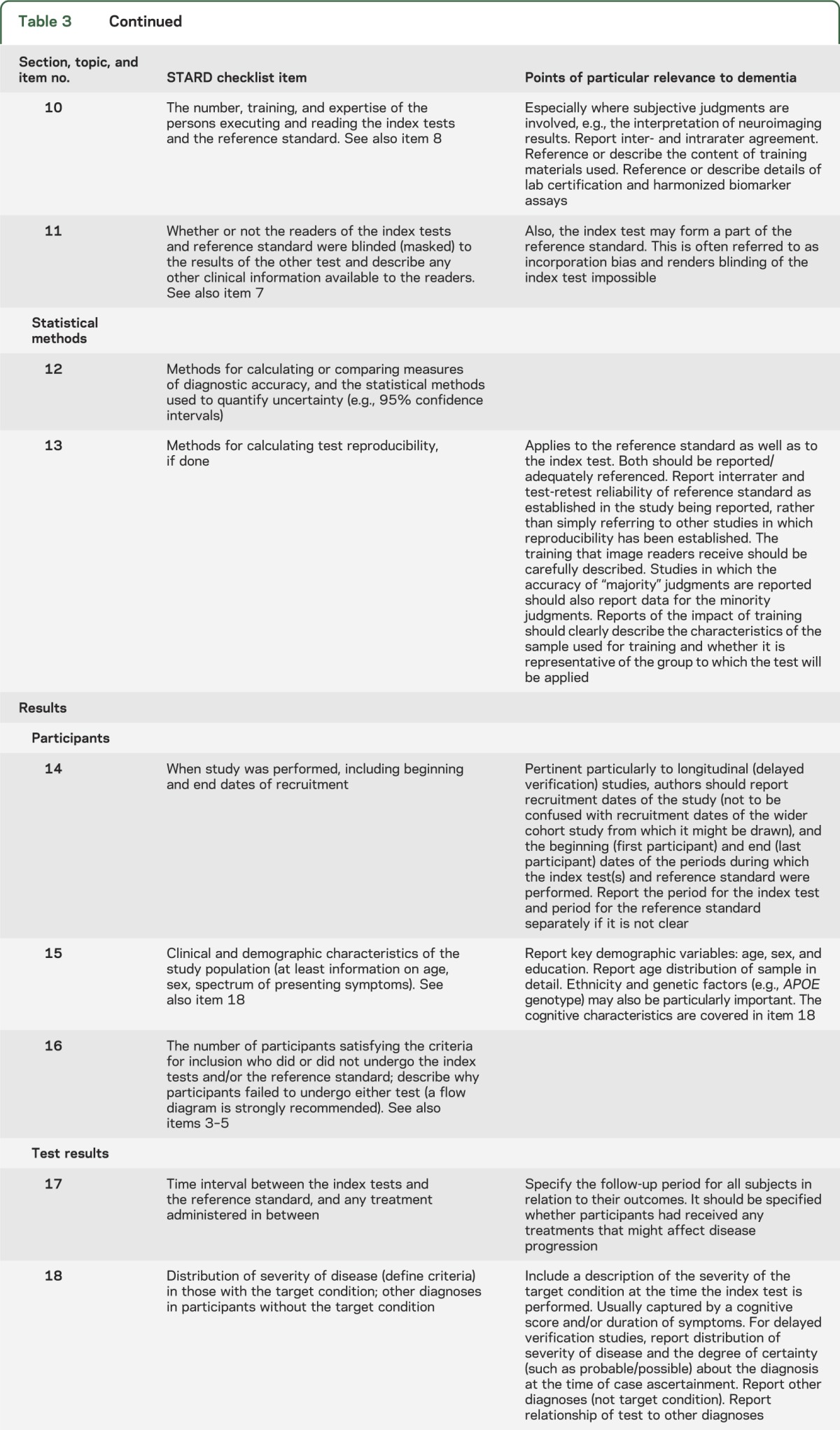
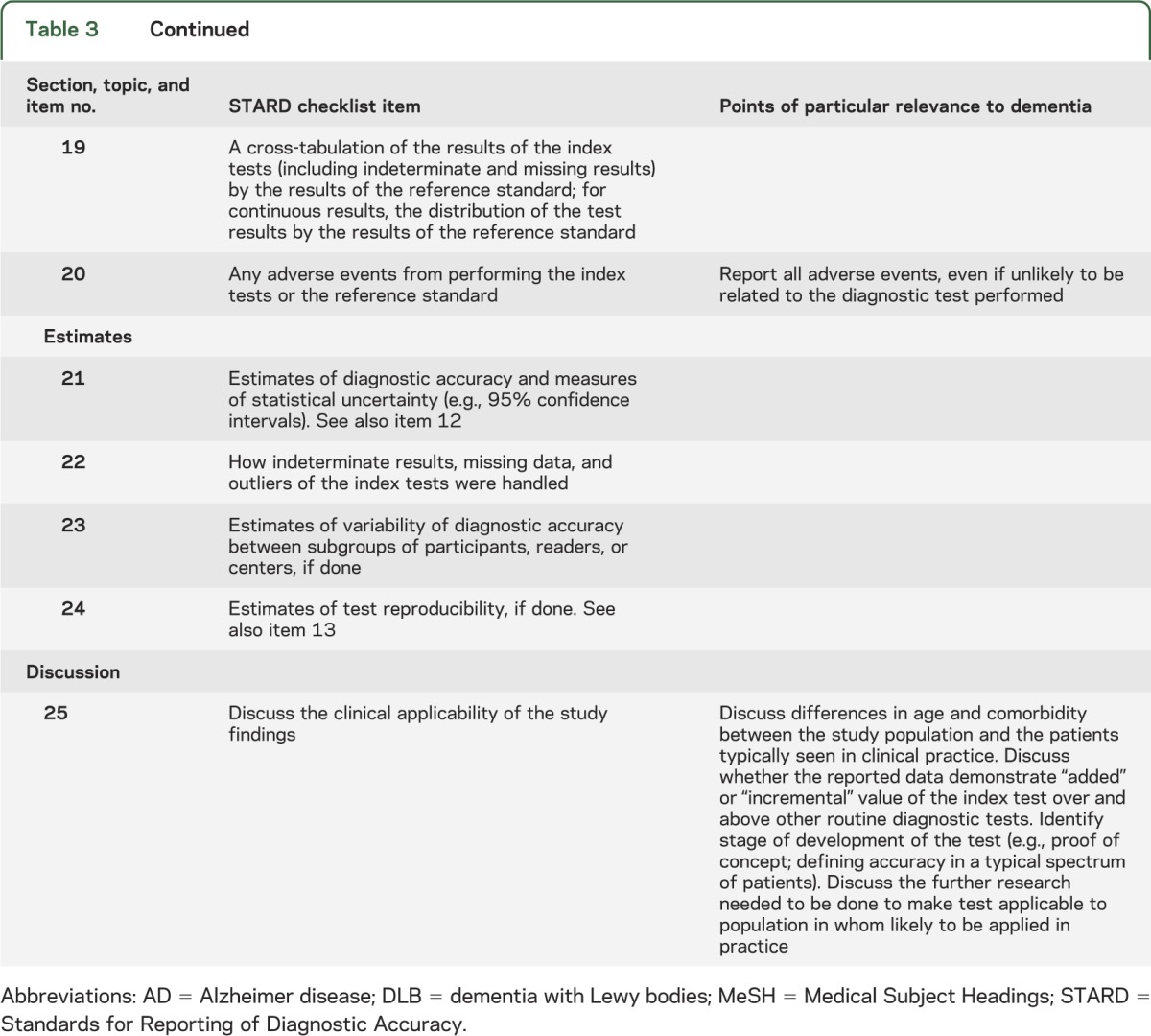
There are 4 key areas central to effective evaluation of studies of diagnostic tests in dementia and cognitive impairment to which special attention should be given when reporting results of DTA studies in dementia and cognitive impairment:
Study population: Many DTA studies report on a highly selected population, e.g., from a tertiary memory clinic or a sample of convenience. Generalizability to the population with cognitive impairment at large, or even to other speciality clinics, may be questionable. The report should address whether that sample was representative in terms of spectrum of disorders, proportion of cases with the disease for which the index test is intended, and severity of cognitive impairment of the population in whom the test would be applied in practice. If not, then test accuracy may be over- or underestimated.
Reference standard: The current limitations in our reference standards in dementia are responsible for some of the inaccuracy and bias that bedevil studies of test accuracy. The 2 major classes of reference standard are (1) postmortem verification, and (2) progression from MCI to dementia due to AD or other conditions according to clinical criteria. Both fall short of the ideal and carry risks of disease misclassifications (bias by the reference standard). An inconsistently applied reference standard creates obvious difficulties in effectively evaluating the performance of a test across studies. Careful specification of the reference standard(s), its operationalization, and application are essential. If more than one reference standard is applied, the index test results should also be displayed by the different reference standards.
Circularity: “Incorporation bias,” whereby the index test forms a part of the reference standard, is common in dementia diagnostic studies. This is inevitable given the composite nature of the reference standards and is a particular problem in the evaluation of neurocognitive tests. Incorporation bias is associated with a tendency to overestimate the value of a specific diagnostic test. The risk of such bias should be acknowledged and reported.
Reliability: Reporting on the test-retest reliability is important. Intra- and interobserver variability may have important effects on neurocognitive scales. For many of the CSF biomarkers, significant intraindividual variation can be found on repeated testing. In addition, there may be substantial interlaboratory variability.32,33 Initiatives are underway to pinpoint and minimize causes of test variation, particularly for protein biomarkers.34,35 Clear reporting of test-retest reliability should help to complement these efforts.
DISCUSSION
It is striking that many of the causes of bias in studies of DTA are similar to those in clinical trials: population selection, blinding, missing data and dropouts, and unreliable outcomes. Other common causes of bias more specific to DTA studies are use of healthy controls and mixed reference standards.15,36 Without full and transparent reporting, readers are unable to assess the validity of individual studies, and thus the overall body of evidence available for a particular test or biomarker. This increases the risk of misinterpretation and misuse of the test data.37 High-quality reporting is of particular importance as patients increasingly present early with equivocal symptoms.12 A diagnosis (or indeed, misdiagnosis) of such a disease has profound implications for patients and their families. Although no disease-modifying treatments or treatments for early-stage illness have reached clinical practice, it is imperative that, when they do, confidence in the diagnostic process is high. At present, the patchy quality of reporting damages the confidence with which findings from studies of DTA can be translated and applied to clinical practice.
The STARD guidelines are important in raising awareness of the key reporting issues in DTA studies.25 However, despite widespread adoption by journals, our earlier work shows that standards of reporting in the dementia field are not uniformly high.26 The STARDdem Consensus Initiative serves to raise awareness of the issues in test accuracy, and emphasizes those that are particularly important to dementia and cognitive impairment.
Supplementary Material
ACKNOWLEDGMENT
STARDdem Initiative contributors (not including authors or members of the STARDdem Working Group) during the consensus process are listed on the Neurology® Web site at Neurology.org.
GLOSSARY
- AD
Alzheimer disease
- CONSORT
Consolidated Standards of Reporting Trials
- DTA
diagnostic test accuracy
- MCI
mild cognitive impairment
- STARD
Standards for Reporting of Diagnostic Accuracy
Footnotes
Supplemental data at Neurology.org
AUTHOR AFFILIATIONS
From the Cochrane Dementia and Cognitive Improvement Group (A.H.N.-S., J.M.M., N.S., S.M.), Department of Geratology, Nuffield Department of Clinical Neurosciences (G.W.), and Department of Psychiatry (R.M.), University of Oxford, UK; Department of Neurology, Academic Medical Centre (E.R.), and Department of Clinical Epidemiology and Biostatistics (P.M.M.B.), University of Amsterdam, the Netherlands; Centre for Mental Health (C.W.R.), Imperial College London, UK; Western Australian Centre for Health & Ageing–WACHA (L.F.), Western Australian Institute for Medical Research, University of Western Australia, Perth; Centre for Academic Mental Health (S.J.C.), School of Social and Community Medicine, University of Bristol; Institute of Public Health (D.D., C.B.), University of Cambridge; Institute of Cardiovascular and Medical Sciences (T.J.Q.), University of Glasgow, The New Lister Building, Glasgow; Peninsula Technology Assessment Group (PenTAG) (C.H.), University of Exeter, UK; Institute of Social and Preventive Medicine (A.W.S.R.), University of Bern, Switzerland; Sunnybrook Research Institute (S.B.), Sunnybrook Health Sciences Centre, Toronto, Canada; Institute of Neuroscience and Physiology (K.B., N.M., H.Z.), Department of Psychiatry and Neurochemistry, the Sahlgrenska Academy at the University of Gothenburg, Mölndal, Sweden; Department of Neurology and Psychiatry (M.F.), University of Rome–Sapienza, Rome, Italy; Institute for Health & Aging, Department of Social and Behavioral Sciences (J.K.J.), and Departments of Neurology (J.K.J.) and Radiology and Biomedical Imaging (N.M.), University of California, San Francisco; Nursing Research Group (S.K.), Institute for Social Medicine and Epidemiology, University of Lübeck, Germany; Department of Psychiatry, Neurology, and Gerontology (L.S.S.), University of Southern California Keck School of Medicine; and King's College London (A.S.), Institute of Psychiatry, Department of Neuroimaging, London, UK.
AUTHOR CONTRIBUTIONS
Anna H. Noel-Storr: substantial contribution to conception and design of initiative and its methods; primarily responsible for formation and coordination of STARDdem Working Group; coordinated Web site design and content management during consensus period; collated consensus round comments; codrafted initial draft guidance; drafted first draft of manuscript; corevised subsequent drafts. Jenny M. McCleery: member of the STARDdem Working Group, contributed to conception and design of initiative and its methods; substantial input into codrafting of initial draft of guidance; substantial input into subsequent drafts based on consensus round feedback; substantial input into manuscript development. Edo Richard: member of the STARDdem Working Group; made substantial contributions during the redrafting of the guidance based on consensus round feedback; substantial input into the manuscript development. Craig W. Ritchie and Leon Flicker: member of the STARDdem Working Group; substantial role in conception of initiative; input into manuscript development. Sarah J. Cullum, Daniel Davis, Terence J. Quinn, Chris Hyde, Anne W.S. Rutjes, Nadja Smailagic, and Sue Marcus: member of the STARDdem Working Group; input into manuscript development. Sandra Black, Kaj Blennow, Carol Brayne, Mario Fiorivanti, Julene K. Johnson, Sascha Köpke, and Lon S. Schneider: contributed to the consensus round; contributed to the drafting of the manuscript. Andrew Simmons, Niklas Mattsson, Henrik Zetterberg, and Patrick M.M. Bossuyt: contributed during the consensus round; contributed to the drafting of the manuscript. Gordon Wilcock: Member of the STARDdem Working Group; chaired the STARDdem consensus meeting; contributed to the consensus round; contributed to the drafting of the manuscript. Rupert McShane: Coconvenor of the STARDdem Working Group; substantial contribution to conception and design of initiative and its methods; substantial input into codrafting of initial draft of guidance; substantial input into subsequent drafts based on consensus round feedback; substantial input into manuscript development.
STUDY FUNDING
This project was funded by the National Institute for Health Research Cochrane Collaboration programme grant scheme: Programme Grant of Diagnostic Test Accuracy Reviews (project 10/4001/05). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS, or the Department of Health.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging and Alzheimer's Association workgroup. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol 2010;9:1118–1127 [DOI] [PubMed] [Google Scholar]
- 5.FDA approves imaging drug Amyvid [online]. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm299678.htm. Accessed February 10, 2014
- 6.European Medicines Agency. Summary of opinion: Amyvid, October 2012 [online]. Available at: http://www.ema.europa.eu/ema. Accessed February 10, 2014
- 7.Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. Alzheimers Dement 2013;9:e-1–e-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaac M, Vamvakas S, Abadie E, Jonsson B, Gispen C, Pani L. Qualification opinion of novel methodologies in the predementia stage of Alzheimer's disease: cerebro-spinal-fluid related biomarkers for drugs affecting amyloid burden—regulatory considerations by European Medicines Agency focusing in improving benefit/risk in regulatory trials. Eur Neuropsychopharmacol 2011;21:781–788 [DOI] [PubMed] [Google Scholar]
- 9.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry 1984;141:1356–1364 [DOI] [PubMed] [Google Scholar]
- 10.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699 [DOI] [PubMed] [Google Scholar]
- 11.Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry 2006;21:1078–1085 [DOI] [PubMed] [Google Scholar]
- 12.Goodyear-Smith F. Government's plans for universal health checks for people aged 40–75. BMJ 2013;347:f4788. [DOI] [PubMed] [Google Scholar]
- 13.Cordell CB, Borson S, Boustani M, et al. Alzheimer's Association recommendations for operationalizing the detection of cognitive impairment during the Medicare Annual Wellness Visit in a primary care setting. Alzheimers Dement 2013;9:141–150 [DOI] [PubMed] [Google Scholar]
- 14.Le Couteur DG, Doust J, Creasey H, Brayne C. Political drive to screen for pre-dementia: not evidence based and ignores the harms of diagnosis. BMJ 2013;347:f5125. [DOI] [PubMed] [Google Scholar]
- 15.Rutjes AWS, Reitsma JB, Di Nisio M, Smidt N, van Rijn JC, Bossuyt PMM. Evidence of bias and variation in diagnostic accuracy studies. CMAJ 2006;174:469–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smidt N, Rutjes AW, van der Windt DA, et al. Quality of reporting of diagnostic accuracy studies. Radiology 2005;235:347–353 [DOI] [PubMed] [Google Scholar]
- 17.Smidt N, Rutjes AW, van der Windt DA, Ostelo RW, Bossuyt PM. The quality of diagnostic accuracy studies since the STARD statement: has it improved? Neurology 2006;67:792–797 [DOI] [PubMed] [Google Scholar]
- 18.Young NS, Ioannidis JP, Al-Ubaydli O. Why current publication practices may distort science. PLoS Med 2008;5:e201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simera I, Moher D, Hoey J, Schulz KF, Altman DG. A catalogue of reporting guidelines for health research. Eur J Clin Invest 2010;40:35–53 [DOI] [PubMed] [Google Scholar]
- 20.Simera I, Altman DG, Moher D, Schulz KF, Hoey J. Guidelines for reporting health research: the EQUATOR Network's survey of guideline authors. PLoS Med 2008;5:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman DG, Schulz KF, Moher D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663–694 [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz F, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossuyt PM, Reitsma JB, Bruns DE, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD Initiative. Clin Chem 2003;49:1–6 [DOI] [PubMed] [Google Scholar]
- 24.STARD. STARD statement [online]. Available at: www.stard-statement.org. Accessed February 10, 2014
- 25.Ochodo EA, Bossuyt PM. Reporting the accuracy of diagnostic tests: the STARD Initiative 10 years on. Clin Chem 2013;59:917–919 [DOI] [PubMed] [Google Scholar]
- 26.Noel-Storr AH, Flicker L, Ritchie CW, et al. Systematic review of the body of evidence for use of biomarkers in the diagnosis of dementia. Alzheimers Dement 2013;9:e96–e105 [DOI] [PubMed] [Google Scholar]
- 27.EQUATOR Network. Enhancing the QUAlity and Transparency of Health Research [online]. Available at: www.equator-network.org. Accessed February 10, 2014
- 28.Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs 2000;32:1008–1015 [PubMed] [Google Scholar]
- 29.National Institute for Health Research. Cochrane Dementia and Cognitive Improvement Group (CDCIG) [online]. Available at: http://dementia.cochrane.org. Accessed February 10, 2014
- 30.Davis DHJ, Creavin ST, Noel-Storr AH, et al. Neuropsychological tests for the diagnosis of Alzheimer's disease dementia and other dementias: a generic protocol for cross-sectional and delayed-verification studies. Cochrane Database Syst Rev 2013;(3):CD010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.STARDdem. Reporting standards for studies of diagnostic test accuracy in Alzheimer's disease and dementia: the STARDdem (STAndards for the Reporting of Diagnostic accuracy studies–dementia) Initiative [online]. Available at: http://www.starddem.org. Accessed February 10, 2014 [DOI] [PMC free article] [PubMed]
- 32.Verwey NA, van der Flier WM, Blennow K, et al. A worldwide multicentre comparison of assays for cerebrospinal fluid biomarkers in Alzheimer's disease. Ann Clin Biochem 2009;46:235–240 [DOI] [PubMed] [Google Scholar]
- 33.Mattsson N, Andreasson U, Persson S, et al. CSF biomarker variability in the Alzheimer's Association Quality Control Program. Alzheimers Dement 2013;9:251–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanderstichele H, Bibl M, Engelborghs S, et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's Biomarkers Standardization Initiative. Alzheimers Dement 2012;8:65–73 [DOI] [PubMed] [Google Scholar]
- 35.Carrillo MC, Blennow K, Soares H, et al. Global standardization measurement of cerebral spinal fluid for Alzheimer's disease: an update from the Alzheimer's Association Global Biomarkers Consortium. Alzheimers Dement 2013;9:137–140 [DOI] [PubMed] [Google Scholar]
- 36.Lijmer JG, Mol BW, Heisterkamp S, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061–1066 [DOI] [PubMed] [Google Scholar]
- 37.Chalmers I, Glasziou P. Avoidable waste in the production and reporting of research evidence. Lancet 2009;374:86–89 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


