Abstract
Objective:
To determine whether allopregnanolone (AP) may mediate seizure reduction in progesterone-treated women with epilepsy.
Methods:
The NIH Progesterone Trial compared the efficacy of adjunctive cyclic natural progesterone therapy vs placebo treatment of intractable seizures in 294 subjects, randomized 2:1 to progesterone or placebo, stratified by catamenial vs noncatamenial designation. Treatments were compared on proportions of 50% responders, and changes in seizure frequency from 3 baseline to 3 treatment cycles. Serum AP levels were measured by radioimmunoassay from 155 women with intractable focal-onset seizures who had baseline and treatment-phase midluteal serum samples drawn each cycle for hormone measurements.
Results:
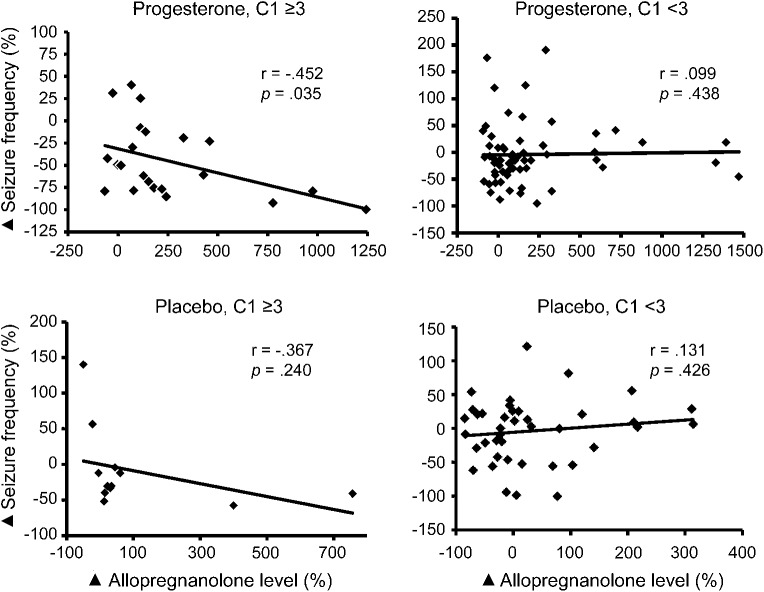
There was no significant correlation between percentage changes in AP levels and seizure frequencies from baseline to treatment for either the catamenial or noncatamenial stratum. There was a significant correlation for the subset of subjects who showed a significantly greater responder rate in the post hoc analysis of the trial, i.e., subjects who had a 3-fold or greater increase in average daily seizure frequency perimenstrually compared with the midfollicular and midluteal phases (C1 ≥3: r = −0.442, p = 0.013, and specifically for C1 ≥3 progesterone-treated subjects [r = −0.452, p = 0.035], but not other groups [C1 ≥3 placebo: r = −0.367; C1 <3 progesterone: r = 0.099; C1 <3 placebo: r = 0.131; p = not significant]).
Conclusions:
The findings support AP as a mediator of seizure reduction in progesterone-treated women who have a substantial level of perimenstrually exacerbated seizures.
Preclinical studies suggest that the reduced progesterone metabolite, allopregnanolone (AP), a positive allosteric modulator of γ-aminobutyric acid type A (GABAA) receptor–mediated conduction, can reduce neuronal excitability and increase seizure thresholds.1,2 The attenuation of AP production using an inhibitor of 5-α-reductase (finasteride) or actions of AP with a GABAA antagonist (bicuculline) increases seizure activity in a rat pentylenetetrazole-induced seizure model.1,2 Clinically, the case report of a woman with catamenial epilepsy who had seizure control on cyclic progesterone supplement abrogated when finasteride was added for the treatment of male pattern baldness also suggests a role for AP as an intermediary in clinical seizure management.3
The NIH Progesterone Trial showed no significant reduction in seizure frequency with adjunctive cyclic progesterone supplement, as compared with placebo, for women with intractable focal-onset seizures, regardless of whether they were designated at the end of the baseline phase to the catamenial or noncatamenial stratum for randomization.4 A prespecified post hoc analysis did find that the level of perimenstrual seizure exacerbation was the most significant predictor of ≥50% progesterone responders.4 A 3-fold increase in seizure frequency was associated with a significantly greater, and clinically important, level of responder rate with adjunctive cyclic progesterone supplement (37.8%) compared with placebo (11.1%).4 The purpose of this analysis is to address a secondary outcome of the trial, which is to determine whether AP may mediate the seizure reduction obtained with progesterone treatment.
METHODS
The AP data come from 155 women with intractable focal-onset seizures who had sufficient baseline and treatment-phase midluteal serum samples drawn each cycle for AP measurements in the randomized, double-blind, placebo-controlled, phase III, multicenter NIH Progesterone Trial. The trial compared the efficacy and safety of adjunctive cyclic natural progesterone therapy vs placebo treatment of intractable seizures in 294 subjects randomized 2:1 to progesterone or placebo, stratified by catamenial vs noncatamenial status using previously reported, mathematically based cutoffs.4,5 The women took study progesterone 200 mg or placebo lozenges 3 times daily from day 14 to 25 of each cycle followed by a 3-day gradual taper.4 The trial compared treatments on proportions of 50% responders and changes in seizure frequency from 3 baseline to 3 treated cycles.4 Because there was no significant difference between progesterone and placebo efficacy using the original stratification into catamenial and noncatamenial strata that considered all 3 patterns of catamenial seizure exacerbation and used previously established cutoffs for designation,5 we assigned the data to groups using the post hoc analysis finding, which showed a significantly superior response to progesterone treatment in women who had a 3-fold or greater level of perimenstrual (days −3 to +3) seizure exacerbation (C1 level ≥3), expressed as multiples of the combined midfollicular (days 4–9) and midluteal (days −12 to −4) baseline average daily seizure frequency. We tested whether changes in seizure frequency were related significantly to changes in serum levels of AP using correlational analyses in each of 4 groups: progesterone-treated, C1 ≥3 level; progesterone-treated, C1 <3 level; placebo-treated, C1 ≥3 level; and placebo-treated, C1 <3 level.
Women used printed calendars to chart seizures and menses during 3 baseline and 3 treatment cycles. They had blood samples drawn during the midluteal phase between days 20 and 24 of each cycle, 4 hours after taking a study lozenge for measurement of serum AP levels using standard methods for radioimmunoassay, described briefly as follows. Serum was thawed on ice. AP was extracted from serum with ether after incubation with dH20 and 100 μL of tritiated AP (800 counts per minute, specific activity 65.0 Ci/mmol; PerkinElmer, Boston, MA). After snap freezing twice, samples were evaporated to dryness in a heated vacuum centrifuge to remove ether. Dried samples were reconstituted with phosphate assay buffer to the original sample volume. Samples were centrifuged at 3,000g at 4°C, and supernatant was used for AP measurement with standard methods2 including addition of tritiated AP and AP antibody (no. 921412-5; Dr. Robert Purdy, Veterans Medical Affairs, La Jolla, CA) to tubes, their incubation, and termination of binding by addition of ice-cold charcoal buffer to each assay tube. Binding was determined by standard liquid scintillation spectroscopy, with correction for recovery and efficiency of the scintillation counter. AP concentrations were determined in relation to standard curves run concurrently with the unknown samples. Intra- and interassay variability were 2.74% and 6.80%, respectively.
We performed statistical analysis using SPSS version 21 software (IBM Corp., Armonk, NY). Descriptive results included mean and SD values for average midluteal baseline and treatment-phase AP levels for each group. We compared baseline and treatment mean AP values for each group using paired t tests. We used Pearson correlational analyses to test the relationships between changes in seizure frequencies and changes in AP levels for each treatment by C1 level group.
Standard protocol approvals, registrations, and patient consents.
Written consent was obtained from all participants. The study was approved by the institutional review boards of the Beth Israel Deaconess Medical Center and each of the participating study centers. The study is registered as NCT00029536 at clinicaltrials.gov.
RESULTS
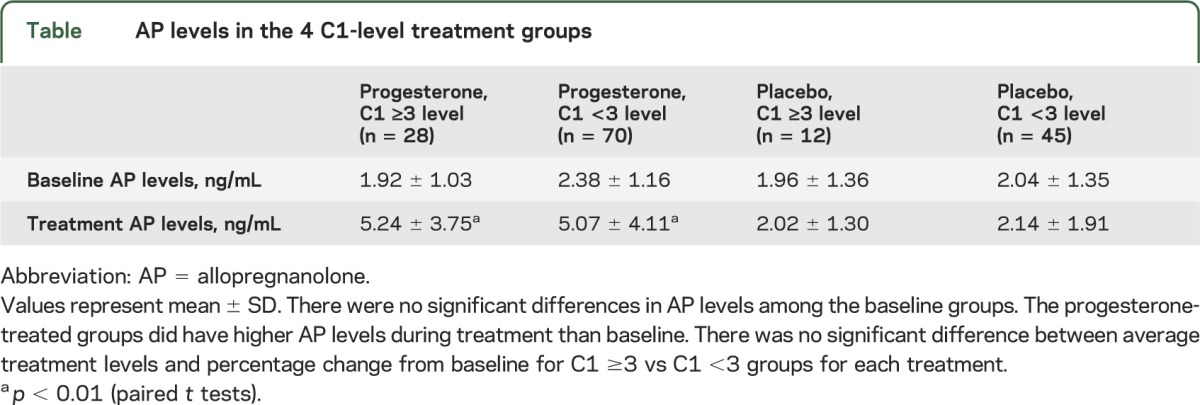
There was no significant difference in age, seizure frequency, antiepileptic drug use for the top 5 drugs, or responder rate for the 155 women under consideration in this analysis as compared with the 294 who were randomized. Baseline and treatment-phase AP serum levels for each of the 4 groups are presented in the table. There was no significant difference in AP levels among the baseline groups. AP levels were significantly greater in treated than in baseline cycles for the progesterone groups, but not for the placebo groups, regardless of catamenial designation. There was no significant difference between average treatment levels and percentage change from baseline for C1 ≥3 vs C1 <3 groups for each treatment.
Table.
AP levels in the 4 C1-level treatment groups
There was a significant correlation between changes in seizure frequency and changes in AP levels from baseline to treatment for subjects who had a 3-fold or greater increase in average daily seizure frequency perimenstrually as compared with the midfollicular and midluteal phases (C1 ≥3: r = −0.449, p = 0.013, and specifically for C1 ≥3 progesterone-treated subjects [r = −0.452, p = 0.035], but not other groups [C1 ≥3 placebo: r = −0.367; C1 <3 progesterone: r = 0.099; C1 <3 placebo: r = 0.131; p = not significant]) (figure). There was no significant correlation between changes in progesterone level and seizure frequency for any group.
Figure. Correlational relationships between changes in serum allopregnanolone levels and changes in seizure frequency for the 4 catamenial-level treatment groups.
There was a significant inverse correlation between percentage change (black diamond) in serum allopregnanolone levels and percentage change (black diamond) in seizure frequencies for the progesterone-treated women with 3-fold or greater perimenstrual increase in seizure frequency (C1 ≥3 group: r = −0.452, p = 0.035), but not for the 3 other catamenial-level treatment groups.
DISCUSSION
The finding of a significant correlation between changes in serum AP levels and seizure frequencies lends support to the hypothesis that AP may mediate the seizure reduction obtained with progesterone treatment in a subset of women with higher (C1 ≥3) levels of perimenstrual seizure exacerbation. The premenstrual withdrawal of progesterone, and hence AP, has been implicated in perimenstrual seizure exacerbation.1,2,6–9 The regimen of progesterone treatment on days 14 to 28 of each cycle did not provide significant benefit for women who had low progesterone levels during the entire luteal phase, i.e., inadequate luteal-phase cycles as documented by low midluteal progesterone levels, and entire luteal phase (C3 pattern) seizure exacerbation.4 Thus, the study finding is consistent with the notion that progesterone withdrawal, rather than just low progesterone, may be an important factor for this progesterone response.1,2,6–9
Animal experimental models suggest that the GABAA receptor has an important role in the occurrence and treatment of seizures.1,2,6–9 The specific subunit assembly of the pentameric receptor determines its function.1,6–9 Whereas benzodiazepines and barbiturates act at a synaptic GABAA receptor that is generally composed of α1–α3, β, and γ2 subunits to modulate phasic inhibition, neuroactive steroids act at an extrasynaptic GABAA receptor site to modulate GABA-mediated neurotransmission.1,6–9 The neuroactive steroids preferentially bind receptors that contain the δ subunit.1,6–9 Progesterone and AP withdrawal, by most accounts, increases the α4-subunit content of the synaptic GABAA receptor.1 The incorporation of this subunit results in a loss of sensitivity to benzodiazepines and GABA-mediated neurotransmission.1,6,7,9 Response to neuroactive steroids, however, may be retained as suggested by the finding that gaboxadol, a synthetic analog of AP, has 6-fold–greater antiseizure efficacy in late diestrus than estrus.8 These findings in animal experimental studies may support a role for both progesterone withdrawal in the occurrence of seizures and the efficacy of progesterone in perimenstrually exacerbated seizures.
The finding that significant efficacy is limited to a particular subset of women with epilepsy focuses emphasis on the importance of the brain substrate in addition to progesterone. An animal model of temporal lobe epilepsy does suggest that there is an increased insertion of α4γ2-subunit–containing receptors that have diminished sensitivity to both benzodiazepines and neuroactive steroids in the dentate gyrus.1 Clinically, the frequency of catamenial epilepsy varies with the laterality and focality of the epilepsy focus with preference for left-sided laterality and temporal lobe focus.10
The principal finding of this study, along with the results of experimental animal studies1,2,6–9 and the clinical case report cited above,3 provides support for the notion that AP may be an important mediator in the seizure reduction obtained with progesterone treatment.
Supplementary Material
ACKNOWLEDGMENT
The authors wish to recognize the technical contributions of Jason Paris, Jennifer Torgersen, Carolyn Koonce, and Dr. Alicia Walf to the analysis of allopregnanolone levels.
GLOSSARY
- AP
allopregnanolone
- GABAA
γ-aminobutyric acid type A
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Herzog and Dr. Frye: study concept and design, acquisition of data, analysis and interpretation of data, drafting/revising the manuscript for intellectual content.
STUDY FUNDING
Supported by a grant from the National Institute of Neurological Disorders and Stroke (NIH R01 NS39466).
DISCLOSURE
A. Herzog was the principal investigator on this research that was supported by NIH National Institute of Neurological Disorders and Stroke grant R01 39466. He also received support for investigator-initiated research projects funded by GlaxoSmithKline and Abbott as well as the Epilepsy Foundation. He serves on the editorial board of Epilepsy & Behavior. C. Frye received support for the allopregnanolone assays from the NIH National Institute of Neurological Disorders and Stroke grant RO1 39466. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Joshi S, Rajasekaran K, Kapur J. GABAergic transmission in temporal lobe epilepsy: the role of neurosteroids. Exp Neurol 2013;244:36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhodes ME, Harney JP, Frye CA. Gonadal, adrenal, and neuroactive steroids' role in ictal activity. Brain Res 2004;1000:8–18 [DOI] [PubMed] [Google Scholar]
- 3.Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol 2003;53:390–391 [DOI] [PubMed] [Google Scholar]
- 4.Herzog AG, Fowler KM, Smithson SD, et al. ; Progesterone Trial Study Group. Progesterone versus placebo therapy for women with epilepsy: a randomized clinical trial. Neurology 2012;78:1959–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia 1997;38:1082–1088 [DOI] [PubMed] [Google Scholar]
- 6.Smith SS, Gong QH, Hsu FC, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor alpha4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature 1998;392:926–929 [DOI] [PubMed] [Google Scholar]
- 7.Frye CA, Bayon LE. Cyclic withdrawal from endogenous and exogenous progesterone increases kainic acid and perforant pathway induced seizures. Pharmacol Biochem Behav 1999;62:315–321 [DOI] [PubMed] [Google Scholar]
- 8.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci 2005;8:797–804 [DOI] [PubMed] [Google Scholar]
- 9.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res 2010;186:113–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quigg M, Smithson SD, Fowler KM, Sursal T, Herzog AG; Progesterone Trial Study Group. Laterality and location influence catamenial seizure expression in women with partial epilepsy. Neurology 2009;73:223–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.