Abstract
Objectives:
Traumatic brain injury (TBI) is common in military personnel, and there is growing concern about the long-term effects of TBI on the brain; however, few studies have examined the association between TBI and risk of dementia in veterans.
Methods:
We performed a retrospective cohort study of 188,764 US veterans aged 55 years or older who had at least one inpatient or outpatient visit during both the baseline (2000–2003) and follow-up (2003–2012) periods and did not have a dementia diagnosis at baseline. TBI and dementia diagnoses were determined using ICD-9 codes in electronic medical records. Fine-Gray proportional hazards models were used to determine whether TBI was associated with greater risk of incident dementia, accounting for the competing risk of death and adjusting for demographics, medical comorbidities, and psychiatric disorders.
Results:
Veterans were a mean age of 68 years at baseline. During the 9-year follow-up period, 16% of those with TBI developed dementia compared with 10% of those without TBI (adjusted hazard ratio, 1.57; 95% confidence interval: 1.35–1.83). There was evidence of an additive association between TBI and other conditions on risk of dementia.
Conclusions:
TBI in older veterans was associated with a 60% increase in the risk of developing dementia over 9 years after accounting for competing risks and potential confounders. Our results suggest that TBI in older veterans may predispose toward development of symptomatic dementia and raise concern about the potential long-term consequences of TBI in younger veterans and civilians.
There is growing evidence that traumatic brain injury (TBI) is associated with a variety of short- and long-term adverse health outcomes. A 2008 Institute of Medicine report concluded that TBIs are consistently associated with an increased risk of unprovoked seizures, premature mortality, and neurocognitive deficits in the affected region, with evidence strongest for penetrating wounds and severe or moderate TBIs.1 However, prior research on the relationship between TBI and risk of Alzheimer disease (AD) and all-cause dementia has been mixed.2–14 Most prior studies have not adequately controlled for potential confounders, such as medical and psychiatric comorbidities, and none have considered death as a competing risk.
Furthermore, to our knowledge, only one prior study has specifically focused on examining the relationship between TBI and risk of dementia in veterans.11 Many veterans have other combat-related risk factors such as posttraumatic stress disorder (PTSD) and depression, which have been associated with an increased risk of dementia in veterans in prior studies,15,16 and could act as either confounders or effect modifiers of the association between TBI and dementia in veterans.
The objective of our study was to determine whether TBI is independently associated with the risk of incident dementia in older veterans after accounting for the competing risk of mortality and adjusting for potential confounding. We also examined PTSD, depression, and cerebrovascular disease as potential effect modifiers.
METHODS
Subject population.
Data were extracted from the Veterans Health Administration (VHA) National Patient Care Database, an electronic database that captures information on all inpatient and outpatient encounters that occur at VHA health care facilities nationwide. Subjects were a random sample of 200,000 veterans aged 55 years or older who had at least one inpatient or outpatient visit during both the baseline (October 1, 2000 to September 30, 2003) and follow-up (October 1, 2003 to September 30, 2012) periods. A total of 11,236 subjects (5.6%) had dementia at baseline and were excluded for a final sample size of 188,764.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Committee on Human Research at the University of California, San Francisco; the Research & Development Committee at the San Francisco VA Medical Center; and the Human Research Protection Office of the US Army Medical Research and Materiel Command. Informed consent was waived because this was a low-risk, retrospective analysis of existing medical record data, and it would not have been practicable to obtain consent because many study participants had died. We did not have access to identifying information such as names or contact information, and scrambled social security numbers were used to track participants over time.
Measures.
Information related to all inpatient and outpatient medical encounters that occur within the VHA system is entered by the clinician into an electronic medical record system. Required data include diagnostic codes related to the reason(s) for the visit and procedure codes related to any procedures performed. Diagnoses and inpatient procedures are coded using the ICD-9-CM; outpatient procedures are coded using Current Procedural Terminology codes.
Traumatic brain injury.
Veterans who had a current head injury diagnosis during the baseline period were identified through ICD-9-CM codes; however, the TBI date was not recorded, and TBIs could have occurred recently or in the past. Specific codes included skull fracture (800–804); intracranial injury without skull fracture (850–854); late effects of skull fracture (905.0) or late effects of intracranial injury without skull fracture (907.0); postconcussion syndrome (310.2); and unspecified head injury (873.8, 873.9, 959.01). Extension codes were used when available to examine level of severity (open vs closed wounds). This approach has been validated in civilian populations.17
Dementia.
Dementia at baseline was defined using a broad range of diagnostic codes that were designed to maximize sensitivity and identify as many prevalent cases as possible.18,19 Dementia at follow-up was defined using previously defined criteria based on a restricted set of codes to maximize specificity.15,16 Codes included those for AD (331.0), vascular dementia (VaD) (290.4), senile dementia (290.0, 290.2, 290.3, 331.2), frontotemporal dementia (FTD) (331.1), Lewy body dementia (331.82), and dementia not otherwise specified (294.8).
Mortality.
Date of death was determined using the VHA Vital Status File, which combines information from the VHA, the Center for Medicare & Medicaid Services, and the Social Security Administration to determine date of death.20 Prior studies have found that the VHA Vital Status File is comparable to the National Death Index in accuracy and completeness.21
Other measures.
Demographic variables included age and sex. Socioeconomic status was determined based on neighborhood education and income data from the 2000 US Census. Education was dichotomized based on whether veterans were living in a zip code tabulation area where ≤25% or >25% of the adult population had completed a college degree (bachelor's degree or higher). Income was defined using tertiles of median income in the zip code tabulation area. ICD-9 codes were used to identify veterans with specific medical diagnoses (diabetes, hypertension, myocardial infarction, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, renal disease, obesity) and psychiatric diagnoses (major depressive disorder, PTSD, alcohol abuse, drug abuse, tobacco use) at baseline.
Statistical analyses.
Prevalence of TBI at baseline (2000–2003) was determined, and characteristics of veterans with and without TBI at baseline were compared using t tests for continuous variables and χ2 for categorical variables. The proportion of veterans who developed dementia was determined for veterans with vs without TBI.
Cumulative incidence of dementia accounting for the competing risk of death was plotted by age at diagnosis for veterans with and without TBI. Time to event was calculated from the date of first visit or first recorded TBI diagnosis during baseline (2000–2003) until the date of dementia diagnosis or death (whichever occurred first) during follow-up (2003–2012). Veterans who did not die or develop dementia were censored at the end of follow-up (September 30, 2012).
Fine-Gray proportional hazards regression was used to examine time to dementia onset with age as the time scale while accounting for the competing risk of death.22 Traditional Cox proportional hazards regression treats subjects who die as censored, which assumes that they would still be “at risk” if additional follow-up data had been available.23 In contrast, Fine-Gray regression models mortality as an alternate “competing risk,” thereby providing a more conservative estimate of the association.
Models were unadjusted and adjusted for groups of potential confounders that were significantly associated with TBI or dementia in bivariate analyses. Model 1 included demographic factors (education, income). Model 2 included demographic factors plus medical conditions (hypertension, diabetes mellitus, myocardial infarction, cerebrovascular disease, peripheral vascular disease, chronic pulmonary disease, renal disease). Model 3 included demographic and medical factors plus psychiatric conditions (PTSD, major depressive disorder, alcohol abuse, drug abuse, and tobacco use). Analyses were performed examining the primary outcome of all-cause dementia as well as specific dementia subtypes (AD, VaD, Lewy body dementia, FTD). Analyses of a specific dementia subtype excluded veterans with other subtypes.
Additional analyses examined different TBI diagnoses and severity levels. In addition, the potential interactive effects of TBI with PTSD, depression, and cerebrovascular disease were examined by including interaction terms in the models to test for greater-than-additive effects; thus, nonsignificant p values are consistent with additive associations. Sensitivity analyses also were performed excluding subjects with cerebrovascular disease at baseline. Data were missing only for census data variables (education, income). Because the amount of missing data was minimal (approximately 3%), these data points were excluded from multivariable models. Standard statistical and graphical techniques were used to assess proportional hazards assumptions.
RESULTS
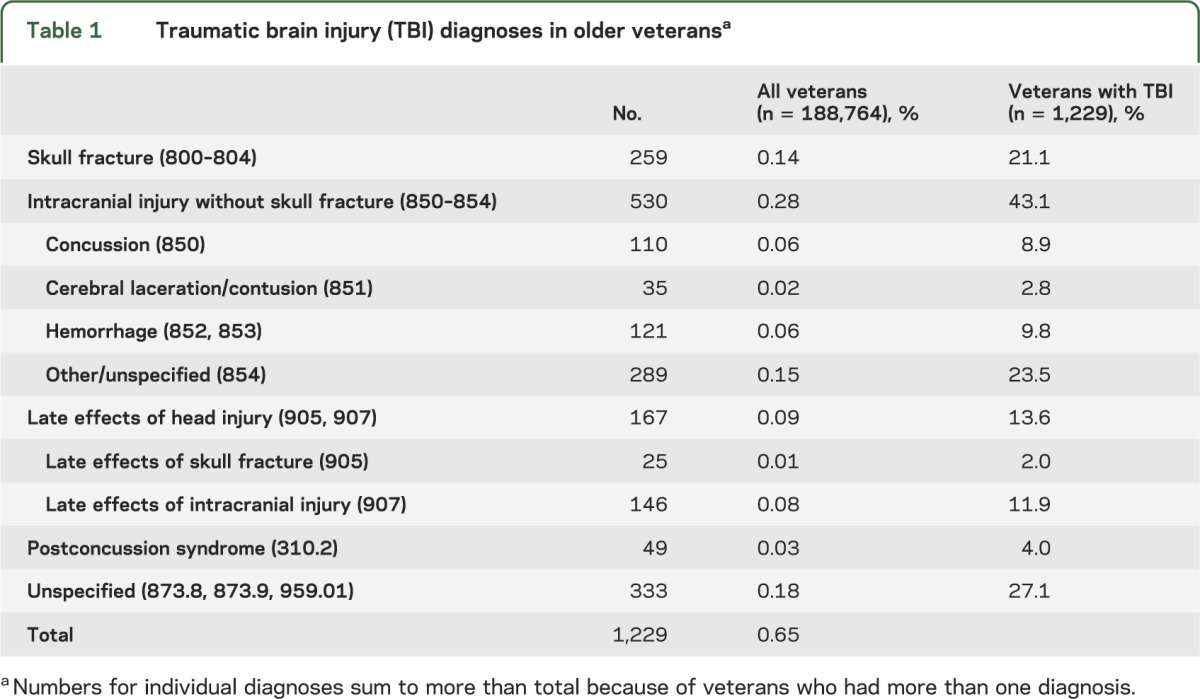
A total of 1,229 veterans (0.65%) had a current TBI diagnosis during the baseline assessment period (table 1). The most common types of TBI were intracranial injury without skull fracture (43%), skull fracture (21%), late effects of TBI (14%), and postconcussion syndrome (4%), while 27% of TBIs were of an unspecified nature; 57% were classified as open wounds while 4% were closed and 39% had unspecified severity; 9% of veterans with TBI had more than one diagnosis.
Table 1.
Traumatic brain injury (TBI) diagnoses in older veteransa
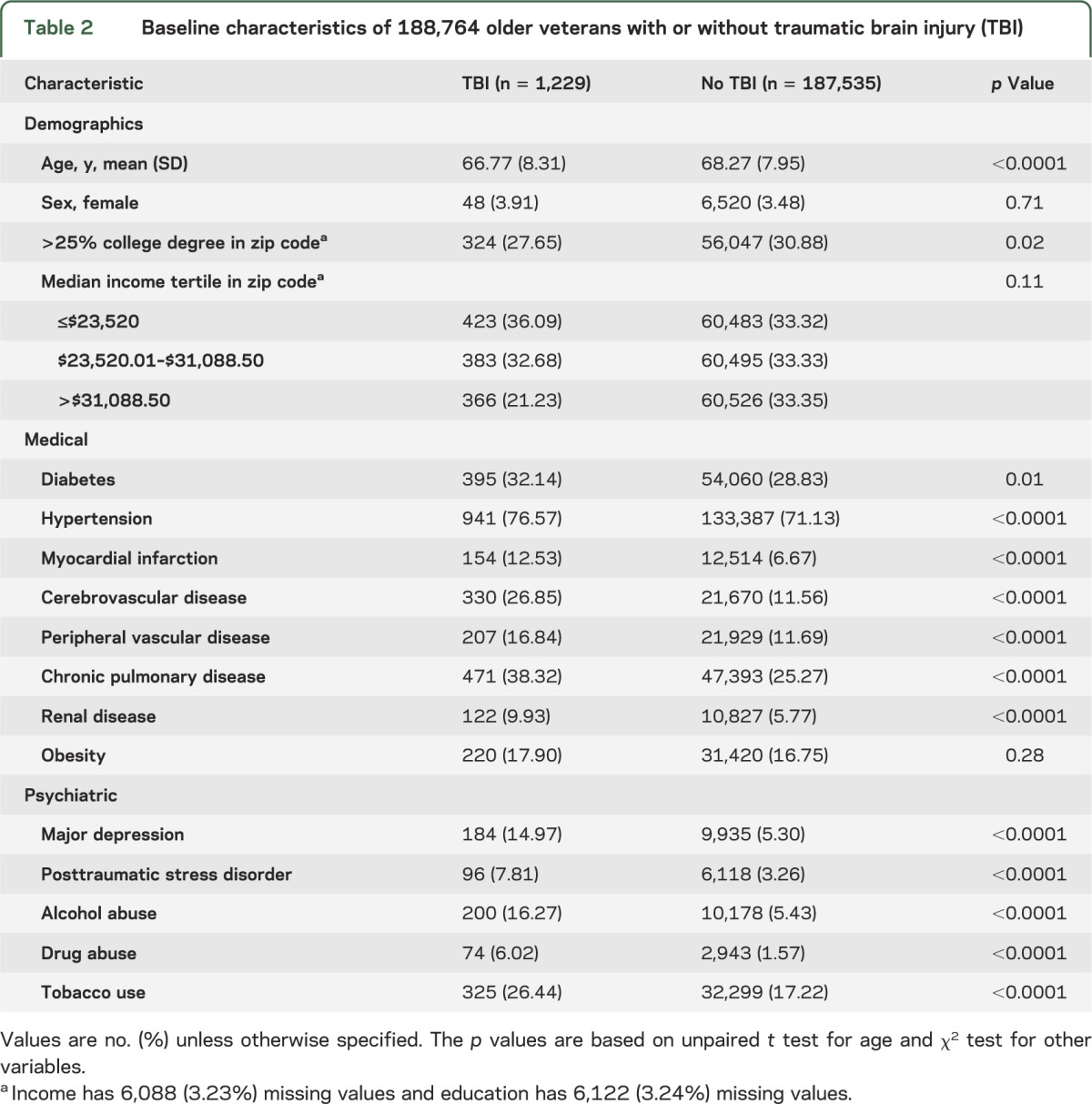
Veterans with TBI were slightly younger than those without TBI (67 vs 68 years, p < 0.001; table 2) but the groups were similar in sex and income distributions. Despite being younger, veterans with TBI had significantly higher prevalence of most comorbid medical conditions including diabetes (32% vs 29%, p = 0.01), hypertension (77% vs 71%, p < 0.001), myocardial infarction (13% vs 7%, p < 0.001), and cerebrovascular disease (27% vs 12%, p < 0.001) as well as most comorbid mental health conditions including depression (15% vs 5%, p < 0.001) and PTSD (8% vs 3%, p < 0.001).
Table 2.
Baseline characteristics of 188,764 older veterans with or without traumatic brain injury (TBI)
The mean length of follow-up was 7.44 years (maximum: 9.67 years; total: 1,404,729 years). During the follow-up period, 196 (16%) veterans with TBI developed dementia compared with 18,255 (10%) of those without TBI (p < 0.001). Figure 1 shows time to dementia onset with age as the time scale and accounting for the competing risk of death in veterans with and without TBI. On average, veterans with TBI developed dementia 2.1 years earlier than those without TBI (78.5 vs 80.7 years, p < 0.001). In addition, those who did not develop dementia died 2.3 years earlier if they had a TBI vs no TBI (77.0 vs 79.3 years, p < 0.001).
Figure 1. Age at dementia diagnosis in veterans with and without TBI, accounting for mortality.
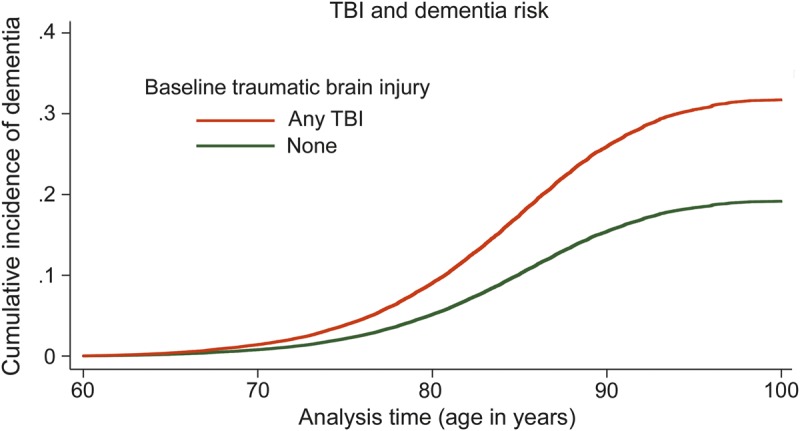
The cumulative incidence of dementia is shown for veterans with traumatic brain injury (TBI) at baseline (orange) and without TBI at baseline (green), accounting for the competing risk of mortality. Age is used as the time scale to indicate age at dementia diagnosis.
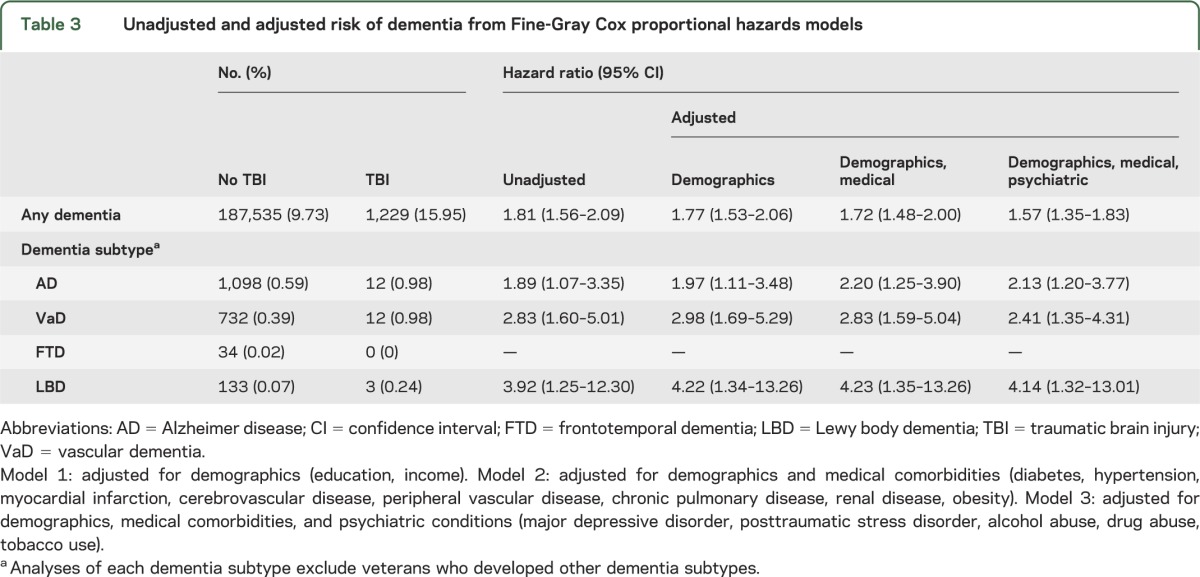
The unadjusted hazard of dementia accounting for the competing risk of death was 81% higher in veterans with TBI than in those without TBI (hazard ratio [95% confidence interval]: 1.81 [1.56–2.09]; table 3). The magnitude of increase was generally similar for all TBI diagnoses and severity levels (intracranial injury: 1.79 [1.41–2.27]; skull fracture: 1.41 [0.92–2.15]; postconcussion: 4.33 [1.91–9.78]; late effects: 1.74 [1.08–2.82]; open: 1.70 [1.38–2.08]; unspecified: 1.49 [1.03–2.17]). Furthermore, risk was significantly elevated for all dementia subtypes, although we did not have enough cases to examine the association between TBI and FTD (table 3). The association between TBI and dementia risk was attenuated slightly by adjustment for demographic, medical, and psychiatric factors but remained statistically significant (1.57 [1.35–1.83]). Results were similar using traditional Cox proportional hazards regression (unadjusted, 2.05 [1.78–2.35]; adjusted, 1.56 [1.35–1.80]).
Table 3.
Unadjusted and adjusted risk of dementia from Fine-Gray Cox proportional hazards models
There was no evidence of multiplicative interaction between TBI and depression (p = 0.14), PTSD (p = 0.18), or cerebrovascular disease (p = 0.41) on dementia risk. Instead, there was evidence of an independent, additive association, such that the risk of dementia was higher in veterans with TBI plus depression, PTSD, or cerebrovascular disease than in those with either TBI or these other conditions alone (figure 2). In addition, the association between TBI and dementia remained significant in sensitivity analyses that excluded veterans with cerebrovascular disease (1.34 [1.08–1.65]).
Figure 2. Additive effects of TBI and other risk factors on dementia incidence.
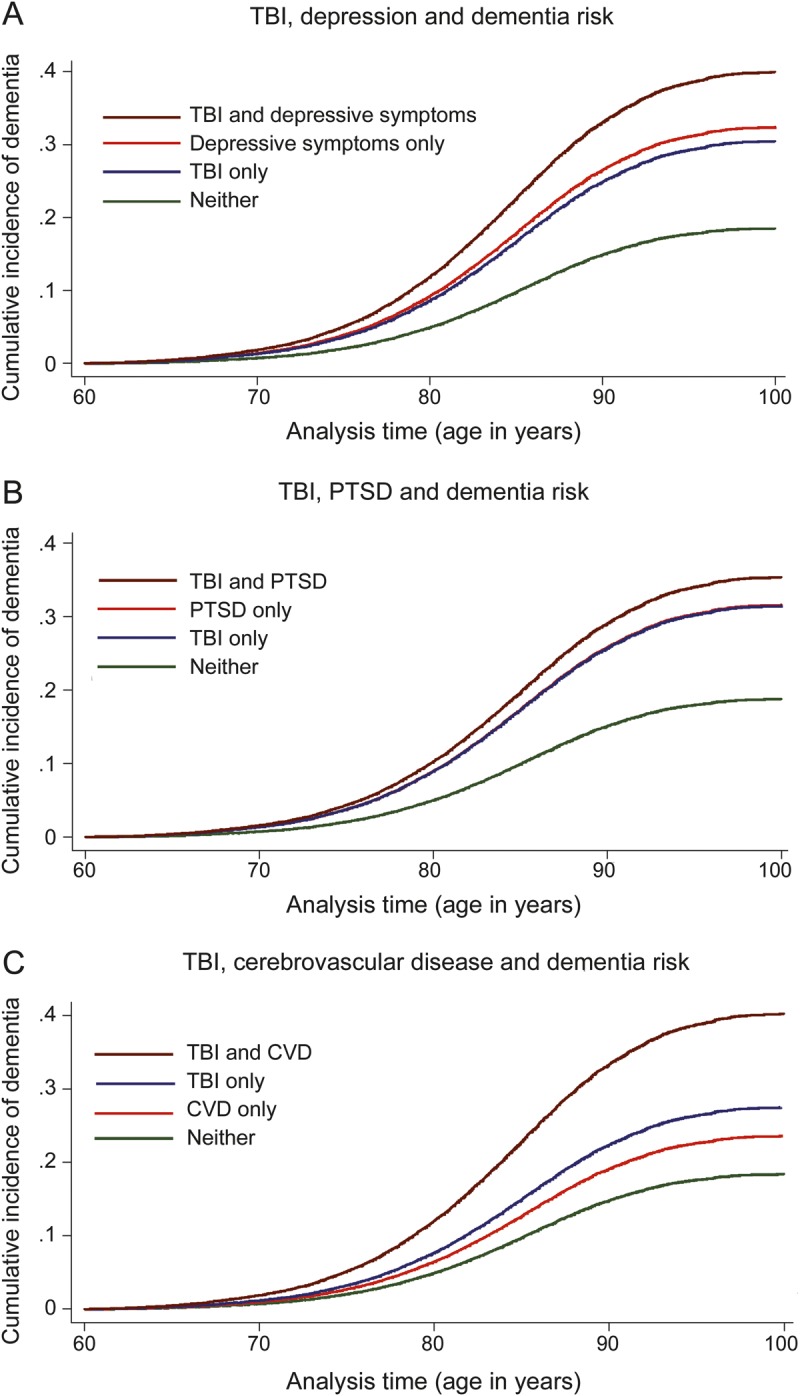
The additive association between traumatic brain injury (TBI) and other dementia risk factors is illustrated by showing the cumulative incidence of dementia with age as the time scale for veterans with TBI only (blue), other risk factor only (orange), both risk factors (brown), or neither risk factor (green). Other risk factors examined include depression (A), posttraumatic stress disorder (PTSD) (B), and cerebrovascular disease (CVD) (C).
DISCUSSION
In this study of more than 188,000 older veterans, those who had a TBI diagnosis at baseline were 60% more likely to develop dementia over 9 years of follow-up, and the age of dementia onset was approximately 2 years earlier, compared with those without TBI. Furthermore, these associations remained strong after accounting for the competing risk of death, and there was evidence of an additive association between TBI and other dementia risk factors.
Prior studies on the association between head injury and risk of dementia in the general population have been mixed, with some studies suggesting an increased risk6,12,14 or earlier onset5,10 and others suggesting no association.2,3,8 Still others have found that the association between TBI and dementia is restricted to those positive for the APOE ε4 allele7,13 or is stronger in men.4,9
Several recent studies have examined the association between concussions in athletes and adverse neurocognitive outcomes. A study of retired football players found that those who had experienced 3 or more concussions were more likely to develop mild cognitive impairment; in addition, onset of AD was earlier than in the general population.5 Another study of former university hockey or football players found that those who had experienced one or more sports-related concussions had worse cognitive performance on several measures.24 In addition, a study examining causes of death in retired football players found that, although their overall mortality rates were lower than the general population, their risk of dying of a neurodegenerative disease (AD, Parkinson disease, or amyotrophic lateral sclerosis) was 3 to 4 times higher.25
Relatively few prior studies have examined the association between TBI and dementia in veterans. One study found that World War II navy and marine veterans who had experienced moderate or severe service-related head injuries, but not mild head injuries, had an increased risk of dementia.11 Another study found that Vietnam veterans who had penetrating head injuries experienced an accelerated rate of cognitive decline over 30 years compared with noninjured controls; however, this decline was unrelated to dementia, and preinjury intelligence was the most consistent predictor of cognitive function over time.26 Finally, a study of veterans seen at VA Cognitive Disorders Clinics found that TBIs were more than 4 times more likely to be present in FTD than other forms of dementia such as AD, VaD, and dementia with Lewy bodies.27
A limitation of prior studies in both veteran and nonveteran populations is that they did not account for the competing risk of death. Our study adds to the existing literature by finding that there is a consistent association between TBI in older veterans and the subsequent risk of developing dementia, even after accounting for mortality as a competing risk. In addition, we found that the risk of dementia increased in an additive manner when TBI was combined with other medical and psychiatric comorbidities, including PTSD, depression, and cerebrovascular disease.
There are several potential mechanisms by which TBIs may increase dementia risk, because TBI can involve a variety of neural changes, depending on the nature and severity of the injury as well as the cumulative number of TBIs an individual has experienced.28–30 In closed head injury, the brain is subjected to forces of acceleration and deceleration, which can stretch and tear axons and blood vessels, resulting in diffuse axonal and vascular injury.28 TBI can also lead to gross alterations in brain structure. For example, in a recent study of former athletes, remote sports-related concussion was associated with ventricular enlargement and cortical thinning.24 TBI-induced neural changes may serve to lower individuals' cognitive reserve and thus increase their propensity to exhibit cognitive decline in older adulthood, particularly when brain changes associated with normal aging start to occur in a neural system that has already undergone damage.31 Alternatively, TBI may initiate a neuropathologic process that more directly leads to dementia. Animal and human studies indicate that, after TBI, there is a buildup of amyloid precursor protein and even formation of diffuse β-amyloid plaques in some cases, resembling the neuropathology of AD.28–30
However, other studies have suggested that TBI-related dementia is distinct from AD, both in the clinical presentation32 and the associated neuropathology. The term chronic traumatic encephalopathy has become widely accepted to describe a dementia syndrome attributable to a history of multiple TBIs with presentations including cognitive impairment, behavioral and psychiatric symptoms, and parkinsonism.28,33 The neuropathology of chronic traumatic encephalopathy, whether seen in athletes with repeated sports injuries or veterans with repeated blast injuries,34 includes prominent accumulation of tau in the form of neurofibrillary tangles, which are located and distributed differently from the neurofibrillary tangles seen in AD.28 Furthermore, yet other work has suggested that TBI may predispose individuals to FTD more than other dementias,27 possibly related to alterations in levels of the protein progranulin that occur after TBI and in FTD.35 In our study, the risk of dementia was significantly elevated for all dementia subtypes, although we did not have an adequate number of cases to examine the association between TBI and FTD.
Despite recent findings from this and other studies, the association between TBI and dementia remains somewhat controversial, particularly for mild TBI. It is possible that the association observed in these studies is not causal and is attributable to uncontrolled confounding or other factors.
Strengths of our study include the large, nationally representative sample of older veterans and the use of VHA medical record data, which capture information on all inpatient and outpatient VHA encounters nationwide. However, several potential limitations also should be considered. It is likely that the TBI and dementia diagnoses captured in the VHA medical system reflect more severe disease, and that veterans with mild TBIs or early-stage dementia cases were not detected. It also was not possible to ascertain how long ago individuals sustained a TBI or to classify TBI severity based on the available data. If the time between TBI and onset of dementia was short, the possibility that the injury resulted from subclinical motor or cognitive dysfunction cannot be excluded. In addition, visits outside the VHA system are not included. Assuming that misclassification associated with these issues was nondifferential, any resulting bias should be toward the null (i.e., an underestimate of the true association). In addition, our study sample was restricted primarily to male veterans, so it is not clear whether results are generalizable to women and nonveterans. We also do not know whether these TBIs were sustained in military or civilian settings, so it is not clear whether results are generalizable to civilian or sport-related injuries. Finally, this study focused on older veterans over 9 years of follow-up, and it is possible that the association between TBI and dementia may vary over the life course.
Our primary finding was that, in a nationally representative sample of more than 188,000 older veterans, TBI was associated with approximately a 60% increase in the risk of developing dementia over 9 years of follow-up after accounting for mortality and adjusting for medical and psychiatric conditions, with age at onset 2 years earlier in those with TBI compared with those without TBI. In addition, an additive association was observed between TBI and other dementia risk factors. These results are consistent with recent studies in civilian populations and professional athletes and support the growing body of literature suggesting that TBIs throughout the lifespan are associated with adverse effects on the brain. Furthermore, these findings raise concern about the consequences of blast-related injuries in today's veterans36,37 as well as the growing rate of TBIs in the civilian population.38,39
ACKNOWLEDGMENT
Two additional individuals are acknowledged as contributors to this manuscript: W. John Boscardin, PhD, Professor of Medicine and Epidemiology & Biostatistics, University of California, San Francisco, who provided analytic supervision, and Kristen R. Krueger, PhD, Staff Neuropsychologist, John H. Stroger Jr. Hospital of Cook County, Chicago, IL, who provided assistance with the literature review and background.
GLOSSARY
- AD
Alzheimer disease
- FTD
frontotemporal dementia
- ICD-9-CM
International Classification of Diseases, ninth revision, Clinical Modification
- PTSD
posttraumatic stress disorder
- TBI
traumatic brain injury
- VaD
vascular dementia
- VHA
Veterans Health Administration
Footnotes
Editorial, page 298
AUTHOR CONTRIBUTIONS
Dr. Barnes oversaw the design of the study, obtained funding, oversaw data analyses, and drafted and revised the manuscript. Dr. Kaup contributed to the drafting and critical revision of the manuscript for content. Ms. Kirby performed the data analyses and contributed to the interpretation of the data. Dr. Byers contributed to the design of the study, analysis and interpretation of the data, and critical revision of the manuscript. Dr. Diaz-Arrastia contributed to the interpretation of the data and critical revision of the manuscript. Dr. Yaffe conceived the study, obtained funding, and contributed to the design of the study, analysis and interpretation of the data, and critical revision of the manuscript.
STUDY FUNDING
Supported by the Department of Defense/NCIRE (Barnes: W81XWH-11-2-0189, project 1610; Byers: W81XWH-11-2-0189, project 1614; Yaffe: W81XWH-12-1-0581) and the NIH (Yaffe: K24 AG031155). The funders did not contribute to the design of the study, analysis or interpretation of data, or decision to publish. The views expressed do not necessarily reflect those of the funders.
DISCLOSURE
D. Barnes reports research support from NCIRE/Department of Defense (W81XWH-11-2-0189, role: principal investigator [PI], 2011–2013); Department of Veterans Affairs (REA 01-097, role: scholar-in-residence, 2009–2013; 1IO1HX000694, role: coinvestigator, 2013–2018); NARSAD (role: PI, 2010–2013); S.D. Bechtel Jr., Foundation (role: senior investigator, 2010–2013; role: coinvestigator, 2011–2014); philanthropic support via Osher Center (role: PI, 2010–2014) and UCB Pharma, Inc. (role: study design and analytic consultant, 2012–2014). A. Kaup reports funding from the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs. K. Kirby reports no disclosures relevant to the manuscript. A. Byers reports support from the Department of Defense (W81XWH-11-2-0189, project 1614; role: PI, 2012–2014), the NIH (MD007019; role: PI, 2012–2015), and with resources from the San Francisco Veterans Affairs Medical Center. R. Diaz-Arrastia reports support from National Institute of Neurological Disorders and Stroke (R01 NS061860) and Department of Defense (ERMS 12109006). K. Yaffe reports serving on data safety monitoring boards for Takeda, Inc., and a study sponsored by the NIH and has served as a consultant for Novartis, Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Institute of Medicine of the National Academies. Gulf War and Health: Volume 7: Long-Term Consequences of Traumatic Brain Injury. Washington, DC: National Academies Press; 2008 [PubMed] [Google Scholar]
- 2.Breteler MM, de Groot RR, van Romunde LK, Hofman A. Risk of dementia in patients with Parkinson's disease, epilepsy, and severe head trauma: a register-based follow-up study. Am J Epidemiol 1995;142:1300–1305 [DOI] [PubMed] [Google Scholar]
- 3.Dams-O'Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: a population-based study. J Neurol Neurosurg Psychiatry 2013;84:177–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry 2003;74:857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guskiewicz KM, Marshall SW, Bailes J, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 2005;57:719–726 [DOI] [PubMed] [Google Scholar]
- 6.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PloS One 2013;8:e62422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer's disease. Neurology 1995;45:555–557 [DOI] [PubMed] [Google Scholar]
- 8.Mehta KM, Ott A, Kalmijn S, et al. Head trauma and risk of dementia and Alzheimer's disease: the Rotterdam Study. Neurology 1999;53:1959–1962 [DOI] [PubMed] [Google Scholar]
- 9.Mortimer JA, van Duijn CM, Chandra V, et al. Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 1991;20(suppl 2):S28–S35 [DOI] [PubMed] [Google Scholar]
- 10.Nemetz PN, Leibson C, Naessens JM, et al. Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am J Epidemiol 1999;149:32–40 [DOI] [PubMed] [Google Scholar]
- 11.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 2000;55:1158–1166 [DOI] [PubMed] [Google Scholar]
- 12.Schofield PW, Tang M, Marder K, et al. Alzheimer's disease after remote head injury: an incidence study. J Neurol Neurosurg Psychiatry 1997;62:119–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sundstrom A, Nilsson LG, Cruts M, Adolfsson R, Van Broeckhoven C, Nyberg L. Increased risk of dementia following mild head injury for carriers but not for non-carriers of the APOE epsilon4 allele. Int Psychogeriatr 2007;19:159–165 [DOI] [PubMed] [Google Scholar]
- 14.Wang HK, Lin SH, Sung PS, et al. Population based study on patients with traumatic brain injury suggests increased risk of dementia. J Neurol Neurosurg Psychiatry 2012;83:1080–1085 [DOI] [PubMed] [Google Scholar]
- 15.Byers AL, Covinsky KE, Barnes DE, Yaffe K. Dysthymia and depression increase risk of dementia and mortality among older veterans. Am J Geriatric Psychiatry 2012;20:664–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yaffe K, Vittinghoff E, Lindquist K, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch General Psychiatry 2010;67:608–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J Head Trauma Rehabil 2008;23:123–131 [DOI] [PubMed] [Google Scholar]
- 18.Krishnan LL, Petersen NJ, Snow AL, et al. Prevalence of dementia among Veterans Affairs medical care system users. Dement Geriatr Cogn Disord 2005;20:245–253 [DOI] [PubMed] [Google Scholar]
- 19.VA Dementia Registry Task Force. VA Dementia Registry Methods Report: Summary Data Tables on Demographics, Service Use and Costs. 2006. Available to researchers with VA network access at: http://vaww.arc.med.va.gov/gec/dementia/fy04/dementia2_reg_methods_report_2nov06.doc. Accessed June 3, 2014 [Google Scholar]
- 20.(VIReC) VIRC. VHA Vital Status File [online]. Available to researchers with VA network access at: http://vaww.virec.research.va.gov/VSF/Overview.htm. Accessed June 3, 2014 [Google Scholar]
- 21.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- 23.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res 2007;13:559–565 [DOI] [PubMed] [Google Scholar]
- 24.Tremblay S, De Beaumont L, Henry LC, et al. Sports concussions and aging: a neuroimaging investigation. Cereb Cortex 2013;23:1159–1166 [DOI] [PubMed] [Google Scholar]
- 25.Lehman EJ, Hein MJ, Baron SL, Gersic CM. Neurodegenerative causes of death among retired National Football League players. Neurology 2012;79:1970–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raymont V, Greathouse A, Reding K, Lipsky R, Salazar A, Grafman J. Demographic, structural and genetic predictors of late cognitive decline after penetrating head injury. Brain 2008;131:543–558 [DOI] [PubMed] [Google Scholar]
- 27.Kalkonde YV, Jawaid A, Qureshi SU, et al. Medical and environmental risk factors associated with frontotemporal dementia: a case-control study in a veteran population. Alzheimers Dement 2012;8:204–210 [DOI] [PubMed] [Google Scholar]
- 28.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012;76:886–899 [DOI] [PubMed] [Google Scholar]
- 29.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat Rev Neurol 2013;9:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: pathogenesis and biomarkers. Nat Rev Neurol 2013;9:192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol 2012;11:1103–1112 [DOI] [PubMed] [Google Scholar]
- 32.Sayed N, Culver C, Dams-O'Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma 2013;30:1117–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shively S, Scher AI, Perl DP, Diaz-Arrastia R. Dementia resulting from traumatic brain injury: what is the pathology? Arch Neurol 2012;69:1245–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldstein L, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans. Sci Transl Med 2012;4:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jawaid A, Rademakers R, Kass JS, Kalkonde Y, Schulz PE. Traumatic brain injury may increase the risk for frontotemporal dementia through reduced progranulin. Neurodegener Dis 2010;6:219–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordts PR, Brosch LA, Holcomb JB. Now and then: combat casualty care policies for Operation Iraqi Freedom and Operation Enduring Freedom compared with those of Vietnam. J Trauma 2008;64:S14–S20 [DOI] [PubMed] [Google Scholar]
- 37.DePalma RG, Burris DG, Champion HR, Hodgson MJ. Blast injuries. N Engl J Med 2005;352:1335–1342 [DOI] [PubMed] [Google Scholar]
- 38.Coronado VG, McGuire LC, Sarmiento K, et al. Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J Saf Res 2012;43:299–307 [DOI] [PubMed] [Google Scholar]
- 39.Faul M, Xu L, Wald MM, Coronado V. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths, 2002–2006. Atlanta: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; 2010 [Google Scholar]


