Abstract
Objective:
The goal of this study was to assess whether the APOE ε4 allele and other APOE single nucleotide polymorphisms (SNPs) influence neuropsychological and neuroimaging outcomes in patients with brain tumors.
Methods:
Two hundred eleven patients with brain tumors participated in the study. All patients completed standardized neuropsychological tests and provided a blood sample for APOE genotyping. Ratings of white matter abnormalities were performed on MRI scans. Patients were classified into 2 groups based on the presence (n = 50) or absence (n = 161) of at least one APOE ε4 allele. Additional APOE SNPs were genotyped in a subset of 150 patients.
Results:
Patients with at least one APOE ε4 allele had significantly lower scores in verbal learning and delayed recall, and marginally significant lower scores in executive function, in comparison to noncarriers of an ε4 allele. Patients with at least one ε4 allele and history of cigarette smoking had significantly higher scores in working memory and verbal learning than ε4 carriers who never smoked. Nine additional APOE SNPs were significantly associated with attention and executive and memory abilities. There were no significant differences between ε4 carriers and noncarriers on the extent of white matter abnormalities on MRI.
Conclusions:
The findings suggest that patients with brain tumors who are carriers of the APOE ε4 allele may have increased vulnerability to developing memory and executive dysfunction, and that additional SNPs in the APOE gene may be associated with cognitive outcome.
Cognitive dysfunction in patients with primary brain tumors is associated with the disease and treatment with radiotherapy (RT) and chemotherapy,1 and is the most frequent complication among long-term survivors.2 The cognitive domains sensitive to adverse treatment effects include attention, executive functions, and learning and retrieval of information.1 Marked interpatient variability is recognized clinically, but little is known about individual factors that may increase the vulnerability for treatment-related neurotoxicity.
One potential genetic risk factor for cognitive decline is the presence of APOE ε4 alleles. APOE is polymorphic and has 3 common isoforms, ε2, ε3, and ε4, encoded by 2 single nucleotide polymorphisms (SNPs). Approximately 25% of the population carries at least one APOE ε4 allele.3 The ε4 allele has been associated with increased risk of late-onset Alzheimer disease4 and with poor outcomes after traumatic head injury.5 Preliminary evidence suggests that the APOE ε4 allele may increase the vulnerability to cognitive dysfunction in patients with breast cancer and lymphoma treated with chemotherapy,6 and in patients with low-grade gliomas.7 These initial studies suggest that the APOE gene may moderate the development of treatment-related neurotoxicity in patients with cancer. In this study, we assessed neuropsychological and neuroimaging outcomes in patients with brain tumors with and without at least one ε4 allele of the APOE gene. We investigated additional APOE SNPs in a subset of patients.
METHODS
Subjects.
Two hundred eleven patients diagnosed with a brain tumor were recruited from a cohort of survivors followed in the Department of Neurology at Memorial Sloan-Kettering Cancer Center (MSKCC); 61 patients were recruited between 2001 and 2009, and 150 between 2009 and 2012. Information on a subset of these patients was provided previously.7 Study eligibility included the following: no evidence of active disease on serial MRIs before accrual; completion of treatment with RT or chemotherapy at least 6 months before enrollment, or surgical resection at least 2 months before accrual if no other treatment was administered; no history of psychiatric or other neurologic disorders; and fluency in English.
Sixty-four patients (30%) had a high-grade tumor (i.e., glioblastoma, anaplastic astrocytoma, or anaplastic oligodendroglioma), 66 (31%) had a low-grade tumor (i.e., oligodendroglioma, oligoastrocytoma), 65 (31%) had primary CNS lymphoma, and 16 (8%) had other brain tumors (i.e., meningioma, ependymoma). One hundred thirty-one patients (62%) received treatment with conventional fractionated RT ± chemotherapy, 64 (30%) received chemotherapy-only regimens, and 16 (8%) had no treatment (except for surgical resection). One hundred seven patients (51%) had focal RT and 24 (11%) had whole-brain RT; RT dose ranged from 2,340 to 6,840 cGy. All patients completed a neuropsychological evaluation and provided a blood sample for APOE genotyping.
Measures.
Neuropsychological assessment.
Neuropsychological tests sensitive to the adverse effects of cancer therapy8 were selected to evaluate the following cognitive domains:
Auditory attention: Digit Span subtests of the Wechsler Memory Scale, third edition (Digit Span Forward, Digit Span Backward); Brief Test of Attention (BTA).
Executive function: Trail Making Test (TMT) Parts A and B; Phonemic Verbal Fluency Test.
Memory: Hopkins Verbal Learning Test-Revised–Learning (HVLT-L), –Delayed Recall (HVLT-D), and –Discrimination Index (HVLT-DI).
The test battery was administered by a neuropsychologist (D.D.C.) or a trained research assistant. Raw cognitive test scores were compared with published normative values according to age, and when available, to education, and converted into z scores.
DNA extraction, selection of SNPs, and genotyping.
APOE ε allelic data were available for 61 patients who were previously genotyped by restriction fragment length polymorphism analysis7 or by Serial Invasive Signal Amplification Reaction (Athena Diagnostics, Worcester, MA).
In 150 patients, blood DNA was extracted with the Qiagen FlexiGene DNA kit (Qiagen, Valencia, CA) according to the manufacturer's protocols, and standard quality control procedures were followed throughout. Genotyping was performed with 2 complementary approaches: (1) DNA sequencing, and (2) the GoldenGate genotyping assay, to include the APOE SNPs rs429358 and rs7412, which encode for the polymorphisms ε2, ε3, and ε4.9 We also included the promoter polymorphisms, rs449647 and rs405509, which have also been associated with Alzheimer disease, cognitive dysfunction, and altered cortical expression.10–12 We also targeted additional APOE SNPs that can affect the gene product, transcription, or messenger RNA stability through missense, nonsense, or frameshift mutations, overlap with seed microRNA regions and/or transcription factors binding sites, with >15% minor allele frequency (MAF) in Caucasians as per HapMap_CEU: rs6857, rs405697, rs439401, rs5112, and rs442706; intronic synonymous SNPs conserved across species, or map near 5′ region with >25% MAF among Caucasians: rs446037, rs405509, rs584007, rs769446, and rs3207187. Haplotype tagging SNPs were searched with the HaploView software and none were identified with a correlation (r2) >0.80 with other SNPs.
Genotyping.
In the first approach, the APOE gene was partially sequenced using 4 overlapping amplicons spanning positions hg19: 45408564–45430336. Bidirectional sequencing was performed in the Beene Core at MSKCC following standard procedures. Nucleotide changes were detected using an automated detection pipeline at the MSKCC Bioinformatics Core. To avoid false-positives, only point mutations supported by at least one bidirectional read pair and one sample mutation called by PolyPhred were considered, and all traces were reviewed and compared with a reference visually and with the Mutation Surveyor software (SoftGenetics, State College, PA). Epsilon isoforms ε2, ε3, and ε4 encoded by rs429358 and rs7412 were annotated according to Mahley and Rall.13 In the second approach, additional APOE SNPs were genotyped using previously described procedures.14 SNPs that were monoallelic, had >5% missing data, failed during earlier stages of the assay design, and/or showed poor clustering were excluded from further analysis: rs442706, rs3207187, rs446037. The genomic context and inclusion criteria for the APOE SNPs are described in table e-1 on the Neurology® Web site at Neurology.org.
Neuroimaging.
White matter (WM) abnormalities were rated on clinical brain MRI scans performed within 3 months of the cognitive evaluation. The ratings were performed by 2 radiologists who were blinded to the cognitive test results. WM abnormalities were rated on a fluid-attenuated inversion recovery sequence for most patients, and if not available, T2-weighted sequences were used. Radiographic endpoints were measured according to the modified Fazekas scale,15 and the tumor and surrounding edema were excluded from these measurements.
Standard protocol approvals, registrations, and patient consents.
The MSKCC institutional review board approved the research protocols. All participants provided written informed consent.
Statistical analyses.
The relation between demographic and clinical variables and the outcome measures was assessed using Wilcoxon rank sums tests for neuropsychological test scores, and Fisher exact tests for WM ratings. Of particular interest were associations between the outcome measures and APOE ε4 carrier status, i.e., patients who were carriers of at least one ε4 allele (ε4-positive) vs noncarriers of the ε4 allele (ε4-negative).
Linear regression was used to evaluate the associations between cognitive test scores and ε4 carrier status controlling for age, education, treatment with RT ± chemotherapy, and cigarette smoking history (i.e., any past or current use). These control variables were selected based on evidence for the association between cognitive performance and age, education, and the APOE ε4 allele,16,17 between cognitive outcome and treatment with RT and chemotherapy in patients with brain tumors,1 and between smoking and APOE status.18 Logistic regression was used similarly for WM ratings, which were classified into 2 categories: none/minimal (grade = 0–1) and moderate/severe (grade ≥2).
The joint effects of APOE SNPs on the outcome measures were assessed by backward selection regression analysis. For each outcome, a full model including all APOE SNPs was fit, controlling for age, education, treatment with RT ± chemotherapy, and cigarette smoking history. Starting with this full model, the SNP providing the poorest fit was removed from the model, where “poorest fit” meant that, compared with the other SNPs, removal of the SNP resulted in the largest decrease in Akaike Information Criterion. This process was repeated until removal of any remaining SNPs resulted in an increase in Akaike Information Criterion. Given the exploratory nature of the study, adjustments for multiple testing were not used on any analyses. Statistical analyses were performed using R version 3.0.1.19
RESULTS
APOE ε status.
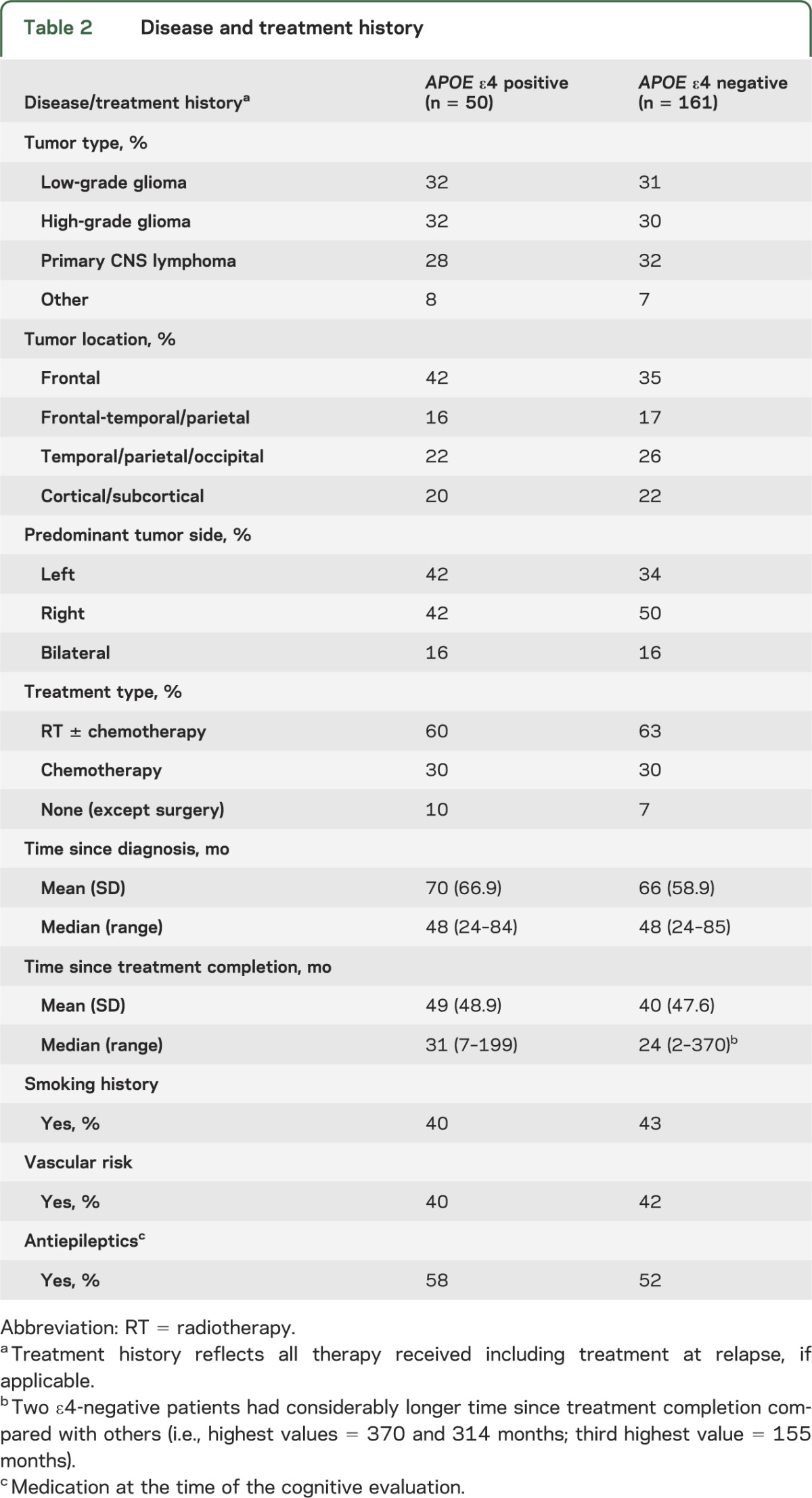
APOE ε allele type results were available for all 211 patients. Fifty patients (24%) carried at least one APOE ε4 allele (ε2/ε4 = 3%, ε3/ε4 = 20%, and ε4/ε4 = 1%), a percentage consistent with the general population distribution (i.e., ε2/ε4 = 3%, ε3/ε4 = 21%, and ε4/ε4 = 2%).20 There were no significant differences between APOE ε4 carriers and noncarriers on any of the demographic, disease, or treatment variables (tables 1 and 2).
Table 1.
Patient demographic characteristics
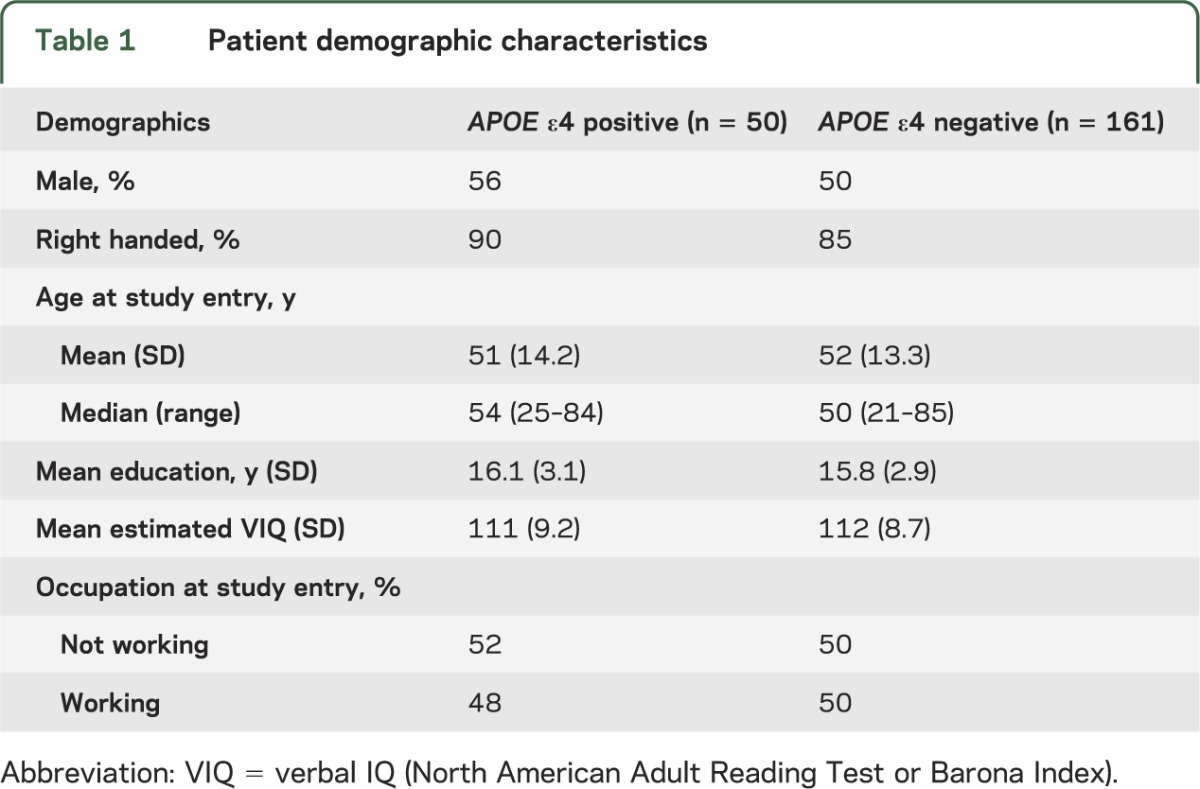
Table 2.
Disease and treatment history
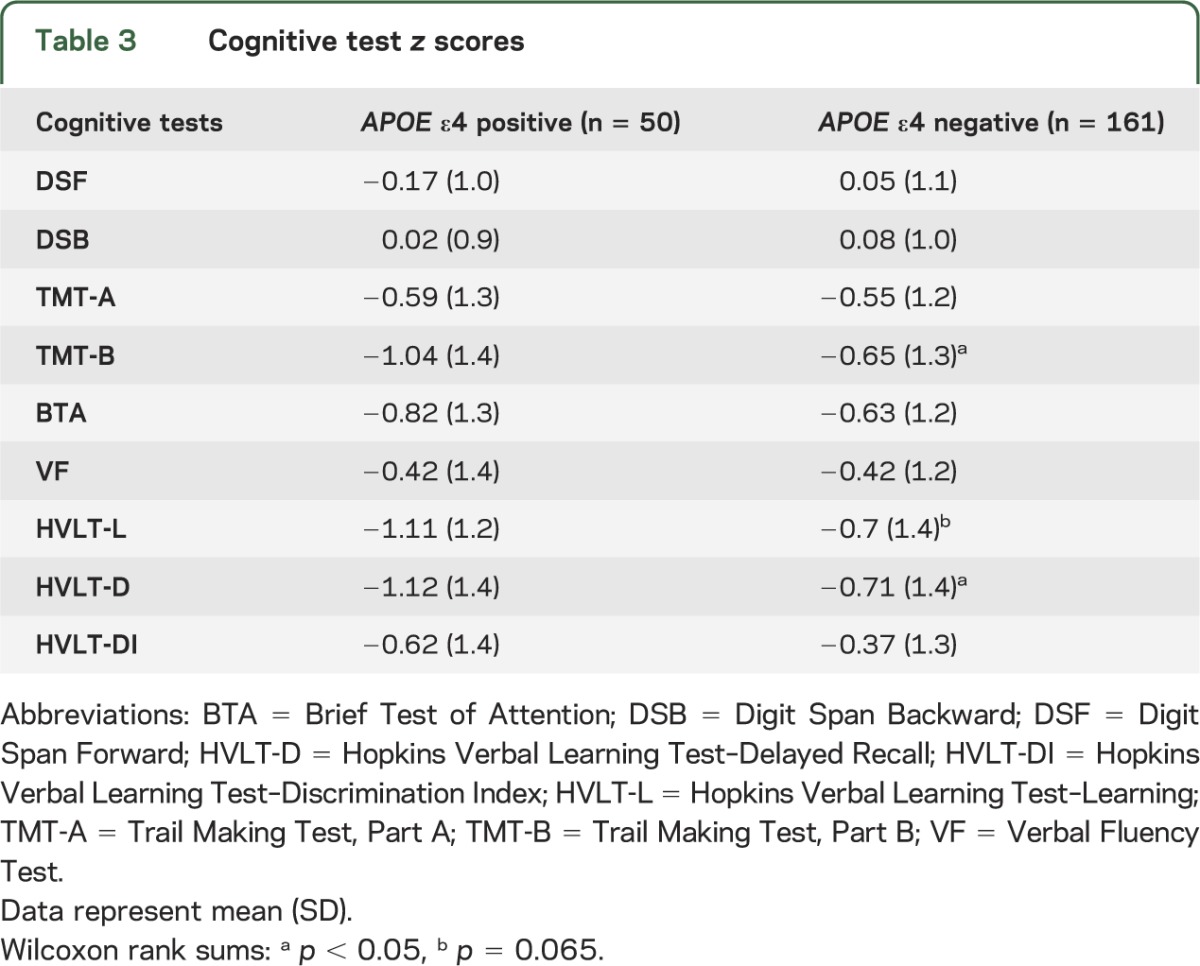
Mean cognitive test scores were within 1 SD below the normative mean on most measures for both groups, but ε4-positive patients had mean z scores more than 1 SD below normative values on the HVLT-L and HVLT-D, and the TMT-B. APOE ε4 carriers had significantly lower mean z scores than noncarriers on the TMT-B and HVLT-D (p < 0.05), and marginally significantly lower HVLT-L scores (p = 0.065) (table 3). These differences remained notable on linear regression analyses adjusting for age, education, treatment with RT ± chemotherapy, and cigarette smoking history; specifically, ε4-positive patients had significantly lower scores in verbal learning (HVLT-L; t203 = −2.11, p = 0.036) and delayed recall (HVLT-D; t203 = −2.12, p = 0.035), and marginally significant lower scores in executive function (TMT-B; t202 = −1.92, p = 0.056), relative to ε4-negative patients.
Table 3.
Cognitive test z scores
We examined the modifying effect of cigarette smoking history on the association between APOE ε4 status and cognitive test performance, adjusting for age, education, and treatment with RT ± chemotherapy. The results showed that ε4-positive patients with a history of cigarette smoking obtained significantly higher scores in attention (Brief Test of Attention, interaction t201 = 2.09, p = 0.038) and verbal learning (HVLT-L, interaction t202 = 2.01, p = 0.046) than ε4-positive patients who never smoked. Marginally significant interactions were seen for delayed recall (HVLT-D, interaction t202 = 1.65, p = 0.099) and phonemic verbal fluency (interaction t191 = 1.84, p = 0.067), with ε4-positive patients with a history of cigarette smoking obtaining higher scores than ε4-positive patients who never smoked (figure). Among ε4-negative patients, cognitive test performance was similar between those with and without a history of smoking. Although there was considerable overlap between history of smoking and other vascular risk factors (e.g., hypertension, hypercholesterolemia, diabetes) among patients, we also examined their possible moderating effects by testing the interaction between ε4 status and a 3-category variable (i.e., no vascular risk factors, nonsmoking vascular risk factors, or smoking history). These results showed no evidence that nonsmoking vascular risk factors moderated the associations between the APOE ε4 allele and cognitive test performance.
Figure. APOE status, smoking history, and cognitive functions.
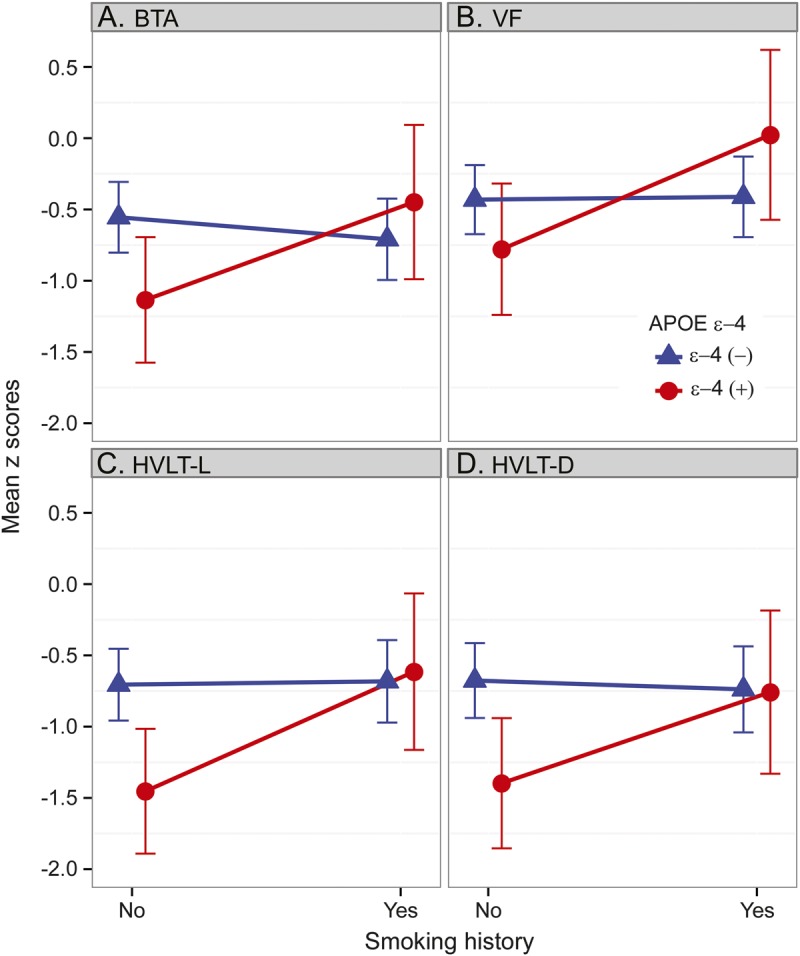
The effects of smoking on the association between APOE status and performance on the (A) BTA (p = 0.038), (B) VF (p = 0.067), (C) HVLT-L (p = 0.046), and (D) HVLT-D (p = 0.099). BTA = Brief Test of Attention; HVLT-D = Hopkins Verbal Learning Test–Delayed Recall; HVLT-L = Hopkins Verbal Learning Test–Learning; VF = Verbal Fluency Test.
Twenty-two ε4-positive patients (44%) were rated as having moderate/severe WM abnormalities, compared with 62 ε4-negative patients (39%) (Fisher exact p = 0.51). The results of logistic regression analyses adjusting for age, education, treatment with RT ± chemotherapy, and smoking history showed no significant differences between ε4-positive and ε4-negative patients on the WM abnormality ratings.
Additional APOE SNPs.
More in-depth genotyping was performed in patients for whom germline DNA was available (n = 150). In addition to the widely reported SNPs rs429358 and rs7412, we genotyped other SNPs described in the literature,10–12 or that were likely functional as per in silico tools: rs449647, rs405509, rs6857, rs405697, rs439401, rs5112, rs584007, and rs769446. Seven additional SNPs were incidentally found during sequencing: rs72654473, rs72654472, hg19:45430118 (C>T), hg19:45430250 (G>A), hg19:45430336 (T>C), hg19:45408815 (C>T), and hg19:45412080 (G>T). Table e-1 provides details on MAF, genomic position, as well as known/predicted SNP features. All incidentally found SNPs were excluded from further analysis because of low MAF (<2%), except for rs72654473. Therefore, a total of 11 APOE SNPs qualified for statistical analyses.
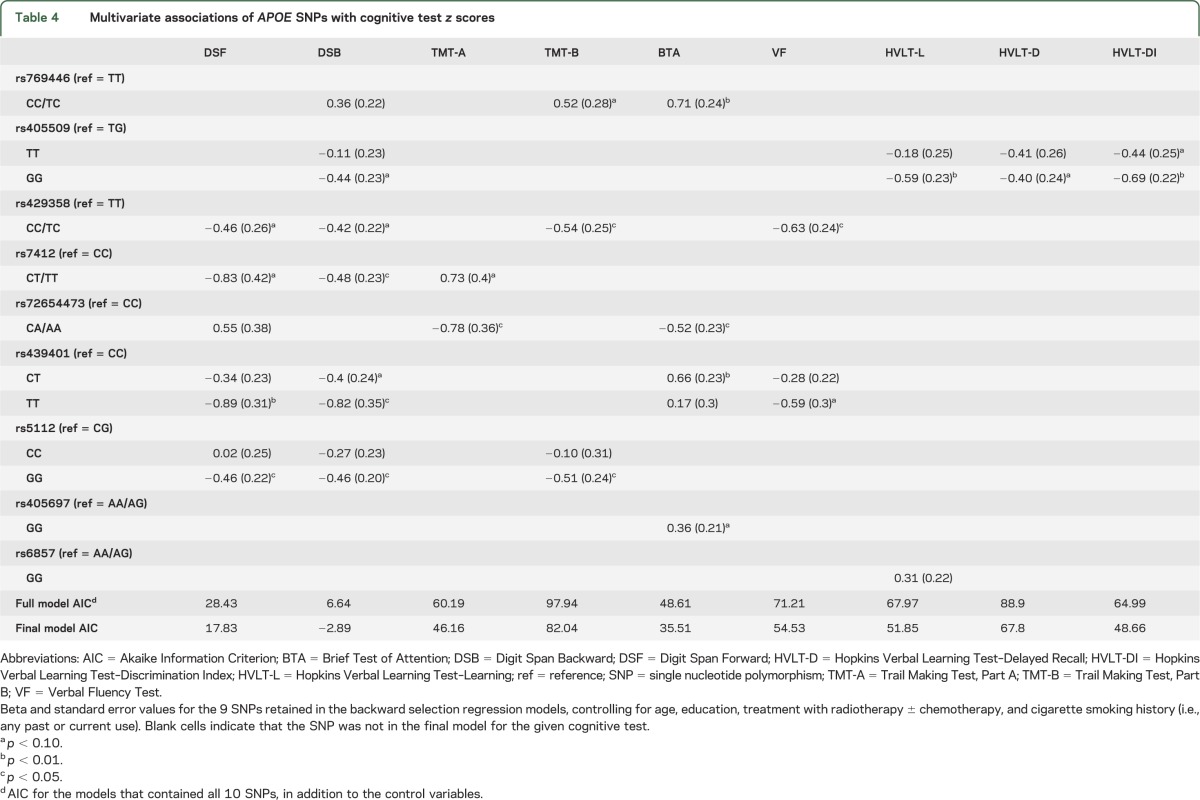
The results of multiple regression analyses using backward selection of SNPs and adjusting for age, education, treatment with RT ± chemotherapy, and cigarette smoking history showed that a total of 9 APOE SNPs were retained in the final models for the cognitive outcomes. SNP rs405509 was retained in the final model for tests of memory including HVLT-L, HVLT-D, and HVLT-DI, and rs6857 for HVLT-L. Two to 6 SNPs were retained for tests of attention and executive functions. The estimated effects of these 9 SNPs, their standard errors, and statistical significance are included in table 4. The results of backward selection adjusting for age, education, treatment with RT ± chemotherapy, and cigarette smoking history showed that none of the APOE SNPs provided a good fit for the WM abnormality ratings.
Table 4.
Multivariate associations of APOE SNPs with cognitive test z scores
DISCUSSION
The study findings provide new evidence that the APOE ε4 allele and additional SNPs in the APOE gene may increase the vulnerability of patients with brain tumors to cognitive dysfunction. Carriers of at least one APOE ε4 allele had significantly lower scores in verbal learning and delayed recall, and a trend toward worse performance in executive functions, relative to noncarriers of the ε4 allele. The presence of the ε4 allele is associated with increased risk of late-onset Alzheimer disease.4 The ε4 allele has been shown to increase the risk and severity of cognitive dysfunction and poor outcomes in traumatic brain injury5 and cardiovascular disease and stroke.21 Several studies described an association between the ε4 allele and diminished memory performance, particularly in encoding and retrieval, and executive functions in middle-aged17 and healthy, older adults,22 and a faster rate of cognitive decline in healthy, older APOE ε4 carriers.16,23
Ahles et al.6 documented worse performance on visual memory, and spatial and psychomotor functions, in APOE ε4 carriers than in noncarriers treated with chemotherapy for breast cancer or lymphoma. We described in a small cohort of patients with low-grade gliomas that carriers of the APOE ε4 allele had relatively lower scores than noncarriers in verbal memory.7 The current study extends these initial findings and provides support for the role of the APOE ε4 allele in moderating memory and executive functions in patients with brain tumors. The APOE ε4 allele may increase amyloid deposition20 and the susceptibility to oxidative stress and mitochondrial damage,24 reduce cholinergic integrity and function,25 disrupt neuronal repair and utilization of glucose,26 and influence the regulation of phospholipid and cholesterol25 after brain injury. It may also accelerate age-related changes in the breakdown of myelin27 and loss of hippocampal volume.28 Preliminary evidence also suggests that RT may be associated with an increase in amyloid deposition.29 It is possible, therefore, that the APOE ε4 allele has an important role in influencing the response to CNS injury from RT or chemotherapy, which may involve vascular damage, depletion of glial progenitor cells, oxidative stress, inflammation, demyelination, and disruption of hippocampal neurogenesis.30,31
The study findings also suggest that the APOE ε4 allele may interact with cigarette smoking and affect cognition. Patients with at least one APOE ε4 allele and a history of smoking had higher scores in attention, learning and delayed recall, and verbal fluency, relative to ε4 carriers who never smoked. A higher risk of dementia and cognitive decline has been described among smokers but only for noncarriers of the APOE ε4 allele.32,33 In a large cohort of healthy, middle-aged adults,18 APOE ε4 carriers with a history of smoking had higher scores in verbal fluency than nonsmokers. However, other studies found no association among APOE ε4 genotype, smoking, and cognitive functions.34 Patients with Alzheimer disease who are carriers of the APOE ε4 allele were reported to have fewer CNS nicotinic binding sites and lower choline acetyltransferase activity than noncarriers.25 Although the mechanisms are poorly understood, cigarette smoking may counterbalance the deficiency in nicotinic receptors in carriers of the ε4 allele by facilitating the release of acetylcholine or increasing the density of nicotinic receptors.35,36 Vascular risk factors have been variably associated with cognitive decline among ε4 carriers,16,37 but this was not observed in our study. This may have been related in part to the overlap between smoking and other vascular risks among patients, but the absence of detailed vascular risk factor information may also have limited a more comprehensive assessment.
We did not identify a significant relationship between APOE ε4 and extent of WM abnormalities. In healthy, older adults, the presence of the ε4 allele has been associated with increased hippocampal atrophy and WM hyperintensity volumes28 and changes in WM integrity.27 It is possible that the sensitivity of the rating scale, which measures global WM abnormalities, was inadequate to detect significant associations between extent and distribution of WM lesions and APOE ε4 status. Advanced neuroimaging methods for measuring regional brain volume and WM integrity may provide greater sensitivity to detect the possible moderating effects of APOE in the development of treatment-related changes in brain structure.
Our findings also suggested that other APOE genetic variants moderate cognitive outcome in patients with brain tumors. The results showed that several SNPs were associated with attention and executive functions, and 2 SNPs, including rs405509 and rs6857, were associated with memory. Previous studies described that 3 APOE SNPs, including rs449647, rs769446, and rs405509, were associated with increased susceptibility to Alzheimer disease.10–12 SNP rs405509 was associated with a poorer recovery after traumatic brain injury.5 Recently, a genome-wide association longitudinal study38 reported that the APOE SNP rs429358 was associated with cognitive decline in older, healthy adults. In our study, rs429358 (TT allele) and rs769446 (TT allele) were associated with lower scores in attention and executive functions. These results suggest that examining APOE genetic variants in addition to identification of the ε4 allele provides relevant information regarding cognitive outcome, and should be considered in future studies.
Given the cross-sectional design of our study, we cannot exclude the possibility that the lower scores in memory and executive functions in ε4-positive patients and in association with several APOE SNPs were related to an interaction with other factors, such as the disease itself, age-related increased vulnerability to cognitive decline observed in ε4 carriers,23,38 or to preexisting differences in hippocampal morphology.39 We also acknowledge that the heterogeneity of the patient population and the variable posttreatment intervals of the neuropsychological evaluations are potential confounding factors. The sample size may have limited the power to detect small to moderate size effects, and to study possible interactions with disease-related factors, such as brain tumor type and location, and the possible contribution of focal vs whole-brain RT. We could not assess a dose effect because there were too few homozygous for the ε4 allele. Nevertheless, this is the largest study of its kind, and our findings provide new evidence that the APOE ε4 allele and other APOE SNPs are important in moderating cognitive outcome in patients with brain tumors. A multisite, prospective, longitudinal study would be warranted to further examine the role of APOE and other relevant genes in this population.
Supplementary Material
GLOSSARY
- HVLT-D
Hopkins Verbal Learning Test–Delayed Recall
- HVLT-DI
Hopkins Verbal Learning Test–Discrimination Index
- HVLT-L
Hopkins Verbal Learning Test–Learning
- MAF
minor allele frequency
- MSKCC
Memorial Sloan-Kettering Cancer Center
- RT
radiotherapy
- SNP
single nucleotide polymorphism
- TMT
Trail Making Test
- WM
white matter
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Correa was responsible for the study concept and design, oversight of the data acquisition and analysis, and for the interpretation of the results and manuscript writing. Dr. Satagopan and Mr. Baser were involved in the overall data analysis and interpretation, and manuscript writing. Mr. Cheung participated in the genetic analyses and interpretation of the results, and critical revision of the manuscript. Dr. Richards participated in data acquisition, organization and interpretation, and critical revision of the manuscript. Dr. Lin was involved in the genetic data analysis and interpretation, and critical revision of the manuscript. Dr. Karimi and Dr. Lyo were involved in the neuroimaging data analysis and interpretation, and critical revision of the manuscript. Dr. DeAngelis was involved in the study concept and design, interpretation of the results, and critical revision of the manuscript. Dr. Orlow was involved in the study concept and design, genetic data analysis and interpretation of the results, and manuscript writing.
STUDY FUNDING
Supported by the Society of MSKCC and the Charles Dana Foundation (Dr. Denise Correa). NCI grant R01CA137420 provided partial support for analytic contributions (Dr. Satagopan).
DISCLOSURE
D. Correa serves on the editorial board of Neuro-Oncology Practice. J. Satagopan serves on the editorial board of Genetic Epidemiology and Sankhya. R. Baser, K. Cheung, E. Richards, M. Lin, S. Karimi, and J. Lyo report no disclosures relevant to the manuscript. L. DeAngelis serves on the editorial board of Neurology, Journal of Neuro-Oncology, Neuro-Oncology, Neuro-Oncology Practice, and The BMJ. She serves as a mentor for CTSC KL2 Scholar Award, KL2TR000458; A Pilot Trial of Enoxaparin vs Aspirin in Patients with Cancer and Stroke. I. Orlow reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Correa DD. Neurocognitive function in brain tumors. Curr Neurol Neurosci Rep 2010;10:232–239 [DOI] [PubMed] [Google Scholar]
- 2.Behin A, Delattre JY. Neurologic sequelae of radiotherapy on the nervous system. In: Schiff D, Wen PY, editors. Cancer Neurology in Clinical Practice. Totowa, NJ: Humana Press; 2003:173–191 [Google Scholar]
- 3.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med 1996;47:387–400 [DOI] [PubMed] [Google Scholar]
- 4.Richard F, Amouyel P. Genetic susceptibility factors for Alzheimer’s disease. Eur J Pharmacol 2001;412:1–12 [DOI] [PubMed] [Google Scholar]
- 5.Jordan BD. Genetic influences on outcome following traumatic brain injury. Neurochem Res 2007;32:905–915 [DOI] [PubMed] [Google Scholar]
- 6.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003;12:612–619 [DOI] [PubMed] [Google Scholar]
- 7.Correa DD, DeAngelis LM, Shi W, Thaler H, Lin M, Abrey LE. Cognitive functions in low-grade gliomas: disease and treatment effects. J Neurooncol 2007;81:175–184 [DOI] [PubMed] [Google Scholar]
- 8.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol 2006;24:1305–1309 [DOI] [PubMed] [Google Scholar]
- 9.Belbin O, Dunn JL, Ling Y, et al. Regulatory region single nucleotide polymorphisms of the apolipoprotein E gene and the rate of cognitive decline in Alzheimer's disease. Hum Mol Genet 2007;16:2199–2208 [DOI] [PubMed] [Google Scholar]
- 10.Bizarro A, Seripa D, Acciarri A, et al. The complex interaction between APOE promoter and AD: an Italian case-control study. Eur J Hum Genet 2009;17:938–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valenza A, Bizarro A, Marra C, et al. The APOE-491 A/T promoter polymorphism effect on cognitive profile of Alzheimer's patients. Neurosci Lett 2010;472:199–203 [DOI] [PubMed] [Google Scholar]
- 12.Xin XY, Ding JQ, Chen SD. Apolipoprotein E promoter polymorphisms and risk of Alzheimer's disease: evidence from meta-analysis. J Alzheimers Dis 2010;19:1283–1294 [DOI] [PubMed] [Google Scholar]
- 13.Mahley RW, Rall SC. Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet 2000;1:507–537 [DOI] [PubMed] [Google Scholar]
- 14.O'Brien KM, Orlow I, Antonescu CR, et al. Gastrointestinal stromal tumors, somatic mutations and candidate genetic risk variants. PLoS One 2013;8:e62119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corn BW, Yousem DM, Scott CB, et al. White matter changes are correlated significantly with radiation dose. Cancer 1994;74:2828–2835 [DOI] [PubMed] [Google Scholar]
- 16.Packard CJ, Westendorp RGJ, Stott DJ, et al. Association between apolipoprotein E4 and cognitive decline in elderly adults. J Am Geriatr Soc 2007;55:1777–1785 [DOI] [PubMed] [Google Scholar]
- 17.Flory JD, Manuck SB, Ferrell RE, et al. Memory performance and the apolipoprotein E polymorphism in a community sample of middle-aged adults. Am J Med Genet 2000;96:707–711 [DOI] [PubMed] [Google Scholar]
- 18.Sabia S, Kivimaki M, Kumari M, et al. Effect of apolipoprotein E ε4 on the association between health behaviors and cognitive function in late midlife. Mol Neurodegener 2010;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. Available at: http://www.R-project.org/ [Google Scholar]
- 20.Relkin NR; National Institute on Aging/Alzheimer's Association Working Group. Apolipoprotein E genotyping in Alzheimer's disease. Lancet 1996;347:1091–1095 [PubMed] [Google Scholar]
- 21.Wagle J, Farner L, Flekkov K, et al. Cognitive impairment and the role of the ApoE epsilon4-allele after stroke: a 13 months follow-up study. Int J Geriatr Psychiatry 2010;8:833–843 [DOI] [PubMed] [Google Scholar]
- 22.Nilsson LG, Adolfsson R, Backman L, et al. The influence of APOE status on episodic and semantic memory: data from a population-based study. Neuropsychology 2006;20:645–657 [DOI] [PubMed] [Google Scholar]
- 23.Caselli RJ, Locke DE, Dueck AC, et al. The neuropsychology of normal aging and preclinical Alzheimer's disease. Alzheimers Dement 2014;10:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibson GE, Haroutunian V, Zhang H, et al. Mitochondrial damage in Alzheimer's disease varies with apolipoprotein E genotype. Ann Neurol 2000;48:297–303 [PubMed] [Google Scholar]
- 25.Poirer J. Apolipoprotein E in the brain and its role in Alzheimer's disease. J Psychiatry Neurosci 1996;21:128–134 [PMC free article] [PubMed] [Google Scholar]
- 26.Nathan BP, Bellosa S, Sanan DA, et al. Differential effects of apolipoprotein E3 and E4 on neuronal growth in vitro. Science 1994;264:850–852 [DOI] [PubMed] [Google Scholar]
- 27.Ryan L, Walther K, Bendlin BB, et al. Age-related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage 2011;54:1565–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cherbuin N, Leach LS, Christensen H, et al. Neuroimaging and APOE genotype: a systematic qualitative review. Dement Geriatr Cogn Disord 2007;24:348–362 [DOI] [PubMed] [Google Scholar]
- 29.Sugihara S, Ogawa A, Nakazaro Y, et al. Cerebral B amyloid deposition in patients with malignant neoplasms: its prevalence with aging and effects of radiation therapy on vascular amyloid. Acta Neuropathol 1995;90:135–141 [DOI] [PubMed] [Google Scholar]
- 30.Greene-Schloesser D, Robbins ME, Pfeifer AM, et al. Radiation-induced brain injury: a review. Front Oncol 2012;2:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 2008;13:1285–1295 [DOI] [PubMed] [Google Scholar]
- 32.Merchant C, Tang MX, Albert S, et al. The influence of smoking on the risk of Alzheimer's disease. Neurology 1999;52:1408–1412 [DOI] [PubMed] [Google Scholar]
- 33.Reitz C, den Heijer T, van Duijn C, Hofman A, Breteler MM. Relation between smoking and risk of dementia and Alzheimer disease: the Rotterdam Study. Neurology 2007;69:998–1005 [DOI] [PubMed] [Google Scholar]
- 34.Kalapatapu RK, Delucchi KL. APOE ε-4 genotype and cigarette smoking in adults with normal cognition and mild cognitive impairment: a retrospective baseline analysis of a national dataset. Am J Drug Alcohol Abuse 2013;39:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ott A, Slooter AJC, Hofman A, et al. Smoking and risk for dementia and Alzheimer's disease in a population-based cohort study: the Rotterdam Study. Lancet 1998;351:1840–1843 [DOI] [PubMed] [Google Scholar]
- 36.van Duijn CM, Havekes LM, Van Broeckhoven C, de Knijff P, Hofman A. Apolipoprotein E genotype and association between smoking and early onset Alzheimer's disease. BMJ 1995;310:627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangen KJ, Beiser A, Delano-Wood L, et al. APOE genotype modifies the relationship between midlife vascular risk factors and later cognitive decline. J Stroke Cerebrovasc Dis 2013;22:1361–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davies G, Harris SE, Reynolds CA, et al. A genome-wide association study implicates the APOE locus in nonpathological cognitive aging. Mol Psychiatry 2014;19:76–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alexopoulos P, Richter-Schmidinger T, Horn M. Hippocampal volume differences between healthy young apolipoprotein E ε2 and ε4 carriers. J Alzheimers Dis 2011;26:207–210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


