Abstract
Patients with schizophrenia show widespread cortical thickness reductions throughout the brain. Likewise, reduced expression of the γ-Aminobutyric acid (GABA) synthesizing enzyme glutamic acid decarboxylase (GAD1) and a single nucleotide polymorphism (SNP) rs3749034 in the corresponding gene have been associated with schizophrenia. We tested whether this SNP is associated with reduced cortical thickness, an intermediate phenotype for schizophrenia. Because of the well known interactions between the GABAergic and dopaminergic systems, we examined whether associations between GAD1 rs3749034 and cortical thickness are modulated by the catechol-O-methyltransferase (COMT) Val158Met genotype. Structural MRI and genotype data was obtained from 94 healthy subjects enrolled in the Mind Clinical Imaging Consortium study to examine the relations between GAD1 genotype and cortical thickness. Our data show a robust reduction of cortical thickness in the left parahippocampal gyrus (PHG) in G allele homozygotes of GAD1 rs3749034. When we stratified our analyses according to the COMT Val158Met genotype, cortical thickness reductions of G allele homozygotes were only found in the presence of the Val allele. Genetic risk variants of schizophrenia in the GABAergic system might interact with the dopaminergic system and impact brain structure and functioning. Our findings point to the importance of the GABAergic system in the pathogenesis of schizophrenia.
Keywords: Cortical thickness, Single nucleotide polymorphism, GAD1, COMT Val/Met polymorphism, Schizophrenia
1. Introduction
1.1. Cortical thickness alterations in schizophrenia
An overwhelming body of evidence supports the neurodevelopmental model of schizophrenia (Fatemi and Folsom, 2009; Insel, 2011; Rapoport et al., 2005). According to this concept schizophrenia is characterized by subtle aberrations in brain development occurring from prenatal to adolescent stages ultimately leading to a variety of positive and negative symptoms as well as cognitive deficits, e.g. reduced working memory (Rapoport et al., 2005; van Os and Kapur, 2009).
During embryonic development neuronal progenitor cells migrating from the marginal zone of the telencephalic vesicle cause the cortex to thicken (Parent and Carpenter, 1995). Cortical thickness is assumed to reflect the arrangement and density of neuronal and glial cells as well as passing axons (Parent and Carpenter, 1995). Postmortem studies in patients with schizophrenia report abnormalities in neuronal migration as well as reductions in neuronal size and arborization compared to healthy control brains (Harrison, 1999; Rapoport et al., 2005). Structural magnetic resonance imaging (sMRI) studies also show regional gray matter changes, most notably reductions of the volume in the medial temporal lobe and ventricular enlargement (Honea et al., 2005; Segall et al., 2009; Shenton et al., 2001;Wright et al., 2000). More recent studies have shown widespread cortical thickness reductions in frontal, temporal and parietal regions as measured by sMRI in patients with schizophrenia compared to healthy controls (Ehrlich et al., 2012; Goldman et al., 2009; Nesvag et al., 2008; Schultz et al., 2010b).
Results from twin studies suggest a heritability of up to 80% for schizophrenia (Sullivan et al., 2003; Tandon et al., 2008). A promising approach for examining the genetic determinants of schizophrenia may be the use of intermediate phenotypes (Meyer-Lindenberg, 2010). These are heritable quantitative traits related to the disease but potentially closer to and more strongly associated with risk genes (Braff et al., 2007; Glahn et al., 2007; Gottesman and Gould, 2003). SMRI studies of patients with schizophrenia and their healthy relatives provide evidence for the heritability of cortical thickness measures (Gogtay et al., 2007; Goldman et al., 2009; Winkler et al., 2010) suggesting that cortical thickness may represent a promising intermediate phenotype and can be used to study the genetic basis of schizophrenia. It has also been proposed to be of greater etiologic relevance than gray matter volume or density (Panizzon et al., 2009; Winkler et al., 2010).
1.2. Glutamic acid decarboxylase 1 as a risk factor in schizophrenia
Abnormalities of the γ-Aminobutyric acid (GABA)-ergic system have long been implicated in the pathophysiology of schizophrenia (Cherlyn et al., 2010; Wassef et al., 2003). Postmortem studies in schizophrenia patients found reductions in GABAergic neuronal density. In addition, abnormalities in receptors and reuptake sites have been identified in cortical and subcortical GABAergic neurons in schizophrenia patients (Wassef et al., 2003). In vitro models using embryonic rat neurons have shown that GABA-receptors modulate cortical migration of neuronal progenitor cells (Behar et al., 2000, 1998; Lopez-Bendito et al., 2003). In line with that, the use of GABA-A agonists and antagonists in a rat model induced prominent alterations of the neuronal migration process in the developing cortex (Heck et al., 2007).
The key enzyme in the synthesis of GABA is glutamic acid decarboxylase (GAD). It is therefore suggested to play a major role in the GABAergic system, to modulate the cortical migration of neurons and to contribute to alterations of the GABAergic system present in patients (Cherlyn et al., 2010; Wassef et al., 2003). The two existing isoforms, GAD65 and GAD67 are encoded by GAD2 and GAD1 respectively. Previous reports suggest that only GAD67 (encoded by GAD1 on chromosome 2) is related to schizophrenia (Addington et al., 2005; Akbarian and Huang, 2006). Reductions of GAD67 mRNA expression levels have been reported consistently in patients with schizophrenia (Akbarian et al., 1995; Guidotti et al., 2000; Veldic et al., 2005; Volk et al., 2000; Woo et al., 2004).
For the purpose of our study we decided to focus on rs3749034, an A/G single nucleotide polymorphism (SNP) that lies in the five prime untranslated region (5′UTR) of GAD1. This SNP is of particular interest, because (i) the G allele of this SNP has been repeatedly found to be associated with schizophrenia (Addington et al., 2005; Straub et al., 2007), (ii) the G allele causes the loss of putative binding sites for two transcription factors that might affect GABAergic neurotransmission: the ATP1a1 regulatory element binding factor 6 and the myoblast determining factor (Addington et al., 2005; Cherlyn et al., 2010) and (iii) the same putative risk allele has been related to alterations of GAD67 mRNA expression levels (Straub et al., 2007).
1.3. Interaction effects of the GABAergic and the dopaminergic systems
A large body of literature suggests an intricate interplay between the GABAergic and the dopaminergic system (Meisenzahl et al., 2007; Seamans et al., 2001; Wassef et al., 2003). Dysfunctions of the latter neurotransmitter system (which might be triggered or modulated by other neurotransmitter systems) are believed to be of crucial relevance to the etiology of schizophrenia (Laruelle and Abi-Dargham, 1999). Recent studies have highlighted the importance of gene–gene interaction models in schizophrenia (Nicodemus et al., 2010a, 2007, 2010b; Nixon et al., 2011; Prata et al., 2009; Sei et al., 2010). One of the most studied genes in the dopaminergic system is the catechol-O-methyltransferase (COMT). COMT degrades catecholamine neurotransmitters such as dopamine. A common COMT gene polymorphism, resulting in a valine (Val)-to-methionine (Met) substitution, leads to a four-fold decrease in enzyme activity (Lachman et al., 1996). The Val allele has been associated with reduced extracellular dopamine levels (Lotta et al., 1995) and is considered a schizophrenia risk-allele although results from meta-analyses have been mixed (Glatt et al., 2003; Munafo et al., 2005). Several recent studies examined effects of variation at COMT Val158Met on cortical gray matter thickness and found increased thickness to be associated with the Met allele (Cerasa et al., 2010; Shaw et al., 2009). The COMT Val158Met polymorphism has also been shown to have an impact on GABA neuron excitability (Seamans et al., 2001) particularly during early development (Tseng et al., 2007). Evidence for epistasis between COMT and GAD1 polymorphisms has been observed previously (Straub et al., 2007) and a more recent study using magnetic resonance spectroscopy demonstrated significant interaction effects of genetic variation in GAD1 and COMT on GABA levels in the brain (Marenco et al., 2010).
1.4. Objective
The goal of this study was to examine the effects of a GAD1 risk variant on cortex-wide gray matter thickness – an established intermediate phenotype of schizophrenia. Because of the aforementioned close interrelationship between the GABAergic and dopaminergic system, and previously demonstrated epistatic mechanisms that might affect neurodevelopmental processes and thus cortical gray matter thickness, we also explored genetic GAD1 × COMT interaction effects on cortical thickness across the entire cortical surface (i.e. without defining regions of interest).
2. Methods
2.1. Participants
Ninety-four healthy volunteers enrolled in the multisite Mind Clinical Imaging Consortium (MCIC) study (Ehrlich et al., 2012, 2010; Roffman et al., 2008) that had complete structural and genotype data were included in this study. After complete description of the study the participants provided written informed consent. The human subjects research committees at each of the four sites (Universities of Iowa (UI), Minnesota (UMN), and New Mexico (UNM) and Massachusetts General Hospital (MGH)) approved the study protocol. All subjects were between the ages of 18 and 60 and spoke English as their native language. General cognitive abilities and achievement were measured using the Reading subtest of the Wide Range Achievement Test – Third Edition (WRAT-3) (Wilkinson, 1993). Participants were excluded if they had a history of neurological or psychiatric disease, history of a head injury, history of substance abuse or dependence within the past month, severe or disabling medical conditions, contraindication to MR scanning or IQ less than 70 (based on the reading subtest from the WRAT-3).
2.2. Genotyping
Genotyping for the GAD1 SNP rs3749034 and COMT Val158Met (rs4680) was conducted with the Taqman platform by using commercially available primer probe assays (Applied Biosystems, Foster City, CA). The assay IDs for rs3749034 and Val158Met are C_2177452_1 and C_25746809_50 respectively. Genotyping rate for GAD1 rs3749034 was 97.2% and for COMT Val158Met 100% respectively. Following the approach taken in previous GAD1 studies (Hyde et al., 2011; Marenco et al., 2010; Straub et al., 2007) we decided to consider homo- and heterozygous carriers of the minor allele as one group. Quality control included removal of subjects that had a genotyping rate of less than 90% across several SNPs obtained within the initial MCIC study. Two subjects were therefore excluded from further analysis.
2.3. Structural image acquisition and data processing
SMRI data was acquired with either a 1.5 T Siemens Sonata (UNM, MGH, and UI) or a 3 T Siemens Trio (UMN). The T1-weighted structural brain scans at each of the four sites were acquired with a coronal gradient echo sequence: TR = 2530 ms for 3 T, TR = 12 ms for 1.5 T; TE = 3.79 ms for 3 T, TE = 4.76 ms for 1.5 T; TI = 1100 ms for 3 T; Bandwidth = 181 for 3 T, Bandwidth = 110 for 1.5 T; 0.625 × 0.625 × 1.5 mm3 voxel size; slice thickness 1.5 mm; FOV, 256 × 256 × 128 cm matrix; FOV = 16 cm; NEX = 1 for the 3 T, NEX = 3 for the 1.5 T.
SMRI processing included volumetric segmentation and cortical surface reconstruction and was performed with FreeSurfer (version 4.0.1) as described previously (Dale et al., 1999; Ehrlich et al., 2012; Fischl et al., 2002, 1999a, 1999b, 2004). Cortical thickness was calculated at each vertex as the closest distance from the gray/white boundary to the gray/cerebrospinal fluid boundary (Fischl and Dale, 2000). Entire cortex vertex wise analyses of cortical thickness were performed contrasting A allele carriers vs. G allele homozygotes. Briefly, spherical registered cortical thickness data from all subjects were mapped to an average subject (http://surfer.nmr.mgh.harvard.edu/fswiki/FsAverage). Cortical thickness maps were smoothed using a 10 mm full-width-at-half-maximum Gaussian kernel. Finally, general linear models were run at all vertices (n = 163 842) per hemisphere. The Desikan–Killiany atlas was used to assign our main finding to the corresponding anatomical surface labels (Desikan et al., 2006). Please find more detailed information about acquisition and processing of the sMRI data in the SM part 1.1.
2.4. Statistical analyses
In line with similar studies, we included age, gender and acquisition site into the models as covariates (Goldman et al., 2009; Kuperberg et al., 2003; Narr et al., 2005; Salat et al., 2004; Schultz et al., 2010b). All cortical thickness results were corrected for multiple comparisons using a Monte-Carlo simulation. This procedure included the following steps: (1) an initial vertex-wise threshold (VWT) was set to p = 0.001 to form spatially contiguous areas of association (referred to as a “cluster”). For our exploratory analyses we used an initial VWT of p = 0.05. (2) The likelihood that a finding (cluster) of this size and magnitude (difference in thickness as specified by the VWT) would appear by chance, i.e. when using repeated random sampling, was tested using Monte-Carlo simulation with 10 000 repeats. This resulted in cluster-wise probabilities (CWP), which are reported using p-values throughout the results section. We set the CWP threshold to p < 0.05. Final statistical maps are shown on the inflated surface of the standard average subject, allowing visualization of data across the entire cortical surface without interference from cortical folding.
In order to control for false positive results due to population stratification or due to brain asymmetry caused by handedness we reran all models on a sample limited to the majority ethnicity (Caucasian participants n = 84) and right handed participants (n = 84). In additional exploratory models we tested the effects of the GAD1 polymorphism in each COMT Val158Met genotype group (n = 25 for Met/Met, n = 44 for Val/Met and n = 25 for Val/Val carriers).
3. Results
3.1. Demographics
The observed genotype frequency of rs3749034 did not deviate from Hardy Weinberg equilibrium (χ2 = 0, df = 1, p = 0.999). Demographic variables such as age, sex, ethnicity, parental socioeconomic status (SES), intelligence as measured by the WRAT-3 and handedness according to the Annett Handedness Scale did not differ across the GAD1 genotype groups (Table 1) and Chi-square statistics did not reveal any relationships between genotype group and acquisition site (χ2 = 1.843, df = 3, p = 0.606). As shown in Table 2 participants from MGH were on average older than those from UI. Participants from UNM showed a lower parental SES than those from MGH and UI. Otherwise, participants did not differ in gender, ethnicity, age, WRAT-3 Score, parental SES and handedness across sites. Chi square statistics did not reveal any relationship between GAD1 and COMT genotype status (χ2 = 2.52, df = 2, p = 0.28). For details about relationships between the COMT Val158Met polymorphism and demographic variables please refer to SM 2.1.
Table 1.
Basic demographics are presented according to GAD1 rs3749034 genotype.
| rs3749034 | A carriers (n = 41) |
G/G homozygotes (n = 53) |
χ2/t-test | df | p | ||
|---|---|---|---|---|---|---|---|
| Sex | female | N | 17 | 23 | 0.035 | 1 | 0.851 |
| % | 41.5 | 43.4 | |||||
| Ethnicity | white | N | 38 | 46 | 1.217 | 1 | 0.270 |
| % | 92.7 | 86.8 | |||||
| Age | Mean (SD) | 34.22 (12.10) | 32.91 (10.63) | 0.559 | 92 | 0.577 | |
| WRAT | Mean (SD) | 49.85 (4.7) | 51.30 (3.69) | −1.674 | 92 | 0.098 | |
| Parental SES | Mean (SD) | 2.59 (0.63) | 2.81 (0.79) | −1.503 | 92 | 0.136 | |
| Handedness | Mean (SD) | 1.12 (2.94) | 1.04 (2.86) | 0.140 | 92 | 0.889 | |
Abbreviations: WRAT, Wide Range Achievement Test; SES, Socioeconomic Status; Handedness, Annett Handedness Scale. T-test did not show any significant main effects of genotype group (A carriers vs. G allele homozygotes) on Age, WRAT Score, Parental SES and Handedness. Chi-square statistics did not reveal any relationships between genotype groups (A carriers vs. G allele homozygotes) and Gender and Ethnicity.
Table 2.
Basic demographics are presented for the 94 healthy volunteers included in this study according to acquisition site.
| Site | N | Gender | Ethnicity | Age | WRAT Score | Parental SES | Handedness | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female | White | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| N | % | N | % | ||||||||||
| UI | 51 | 23 | 45.1 | 48 | 94.1 | 30.92a | 10.51 | 50.20 | 4.24 | 2.84c | 0.42 | 0.92 | 3.04 |
| MGH | 17 | 9 | 52.9 | 11 | 64.7* | 39.65a | 9.27 | 51.47 | 4.32 | 3.00b | 1.06 | 1.41 | 3.36 |
| UMN | 14 | 5 | 35.7 | 14 | 100 | 34.93 | 12.17 | 50.79 | 4.54 | 2.57 | 0.76 | 0.50 | 0.86 |
| UNM | 12 | 3 | 25 | 11 | 91.7 | 33.92 | 13.26 | 51.42 | 3.66 | 1.92b,c | 0.67 | 1.92 | 3.06 |
| Total | 94 | 40 | 42.6 | 84 | 89.4 | 33.48 | 11.25 | 50.67 | 4.20 | 2.71 | 0.73 | 1.07 | 2.88 |
Abbreviations: WRAT, Wide Range Achievement Test; SES, Socioeconomic Status; Handedness, Annett Handedness Scale; UI, University of Iowa; MGH, Massachusetts General Hospital; UMN, University of Minnesota; UNM, University of New Mexico.
One way ANOVA and when appropriate Tamhane post hoc tests were performed to detect significant differences in Age, WRAT Score, Parental SES and Handedness between acquisition sites. Chi-square statistics (Fisher’s exact test in cases of small sample sizes) were performed to detect significant relationships between acquisition site and gender as well as between acquisition site and ethnicity; * – p < 0.05;
– participants from MGH had a significantly higher age than those from UI (p = 0.017);
– participants from MGH had significantly higher parental SES than those from UNM (p = 0.014);
– participants from UI had significantly higher parental SES than those from UNM (p = 0.003).
3.2. Structural MRI
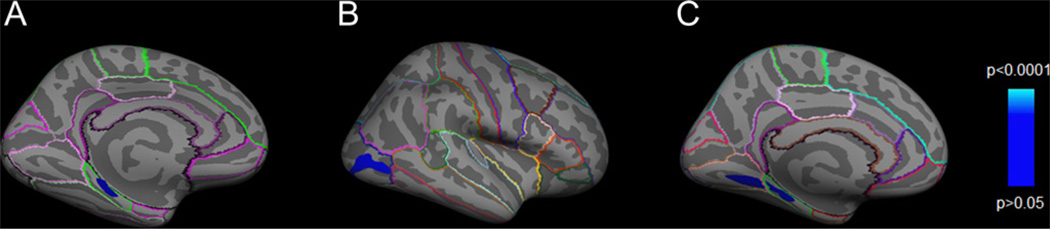
Overall, G allele homozygotes of GAD1 rs3749034 showed reduced cortical gray matter thickness in an area corresponding to the left parahippocampal gyrus (PHG) compared to A carriers (Fig.1A, CWP = 0.0083). The average thickness in this cluster region of A carriers (≈ 2.82 mm) was reduced by 7.54% in G allele homozygotes (≈ 2.61 mm). In order to control for brain asymmetry due to handedness and for population stratification we performed additional analyses restricted to right-handed and white participants. The resulting cortical statistical thickness maps confirmed our main finding in both subanalyses (SM Fig. S1).
Fig. 1.
Cortical statistical maps illustrating the region of reduced cortical thickness in G allele homozygotes compared to A carriers of GAD1 rs3749034 in (A) all participants (B) COMT Val158Met Val/Val carriers and (C) Val/Met carriers. The CWP-values ((A) CWP = 0.0083, (B) CWP = 0.006 and (C) CWP = 0.0285, corrected for multiple comparisons) are represented according to the color code. Surface-based labeling of cortical anatomy is included using the Desikan–Killiany atlas (Desikan et al., 2006). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
When we analyzed the effect of GAD1 rs3749034 by COMT Val158Met polymorphism status we found that within the Val/Met and the Val/Val subgroup GAD1 rs3749034 G allele homozygotes also showed reduced cortical thickness when compared to A carriers in the PHG, lingual gyrus and the lateral occipital gyrus respectively (Fig.1B and C), whereas no associations between GAD1 genotype and cortical thickness could be found in the Met/Met subgroup.
4. Discussion
4.1. Summary of results
The present study used sMRI to explore potential effects of the schizophrenia risk gene variant GAD1 rs3749034 on a schizophrenia-related brain based intermediate phenotype in a healthy population. We found that G allele homozygotes of rs3749034 show regional cortical thinning in the left PHG. We also found gene–gene interaction effects between rs3749034 and the COMT Val158Met polymorphism: cortical thickness reductions of G allele homozygotes were only found in COMT risk-allele carriers.
4.2. GAD1 rs3749034 as a risk variant for schizophrenia
Associations between a GAD1 gene risk variant and the schizophrenia-related intermediate phenotype cortical thickness as demonstrated in this study, complement the existing knowledge about the importance of GAD1 in the pathogenesis of schizophrenia. Previous genetic studies revealed a relationship between variants of GAD1 including the G allele of GAD1 rs3749034 and a diagnosis of schizophrenia (Addington et al., 2005; Du et al., 2008; Straub et al., 2007; Zhao et al., 2007). In particular, the G allele of GAD1 rs3749034 (which disrupts a putative binding site for two transcription factors) has also been associated with reduced GAD1 mRNA expression in the DLPFC and the hippocampus among patients with schizophrenia (Straub et al., 2007). Furthermore quantitative transmission disequilibrium tests of GAD1 variants revealed associations with longitudinal cortical graymatter volume loss in frontal brain regions in a family-based study (Addington et al., 2005). Our finding of reduced cortical thickness in G allele homozygotes of rs3749034 is consistent with these previous expression and association studies and suggest an additional mechanism how this allele may confer genetic risk for schizophrenia.
4.3. PHG in schizophrenia
The association between reduced cortical thickness in the PHG and genetic risk for schizophrenia bodes well with the growing body of literature documenting abnormalities in this brain region in schizophrenia patients. The PHG is a part of the medial temporal lobe (MTL) and provides polysensory input to the hippocampus, a region considered to be crucial in the pathophysiology of schizophrenia (Burwell, 2000; Heckers and Konradi, 2011; Tamminga et al., 2010). Abnormalities of the PHG in schizophrenia patients were first reported in postmortem studies indicating a smaller neuron size and reduced cortical thickness in patients (Arnold, 2000; Brown et al., 1986; Seidman et al., 2003). A meta-analysis of gray matter density as measured by MRI confirmed structural gray matter deficits in the left PHG in schizophrenia patients (Glahn et al., 2008). Additionally, gray matter thickness studies including our own data (Ehrlich et al., 2012) revealed cortical thickness reductions (Jung et al., 2011; Kuperberg et al., 2003) and increased gyrification (a process that occurs mainly in the third trimester of pregnancy) (Armstrong et al., 1995; Schultz et al., 2010a; White et al., 2010) in this area in patients with schizophrenia. The post-mortem and MRI findings corroborate our sMRI results and point to a neurodevelopmental origin of these abnormalities.
The PHG is also a core region of the default mode network (DMN) (Buckner et al., 2008). This network includes interconnected brain regions that are particularly active during periods of internally generated mentation at rest and that decrease their activity during tasks. Patients with schizophrenia have been suggested to be characterized by abnormal activation and connectivity patterns of the DMN (Garrity et al., 2007; Harrison et al., 2007; Meda et al., 2009; Pomarol-Clotet et al., 2008; Shim et al., 2010; Skudlarski et al., 2010; Whitfield-Gabrieli et al., 2009). DMN brain areas in humans and in nonhuman primates are deactivated during cognitive tasks and previous studies have shown that task-induced deactivation can serve as a proxy for DMN activity (Mannell et al., 2010; Watanabe, 2011; Whitfield-Gabrieli and Ford, 2012; Whitfield-Gabrieli et al., 2009). In particular during working memory tasks patients may show reduced deactivation (negative BOLD response as measured by fMRI) in DMN-brain areas compared to healthy controls (Whitfield-Gabrieli et al., 2009). In schizophrenia DMN hyperactivation may reduce the ability to focus on perceiving, thinking about or acting on external stimuli as attentional resources are split or misdirected (Whitfield-Gabrieli et al., 2009). Given the inhibitory nature of cortical GABAergic interneurons and its dysfunction in schizophrenia an association between the potential schizophrenia susceptibility gene GAD1 and the reduced suppression of neural activity in the DMN during task performance is of great interest (Northoff et al., 2007). Therefore we ran additional exploratory models testing for associations between GAD1 and task induced deactivation patterns during a working memory paradigm (the Sternberg Item Recognition Paradigm, SIRP). We found G allele homozygosity at rs3749034 to be associated with reduced task-induced deactivation in Brodmann area 25 bilaterally, i.e. a part of the medial prefrontal cortex (mPFC; for details please refer to SM 1.2, 1.3, 1.4, 2.2 and 3.1). The mPFC is another important hub of the DMN. This post-hoc finding may suggest that GAD1, as a key component of GABAergic functioning, contributes to the disturbed DMN activation.
4.4. Interactions between the GABAergic and dopaminergic systems in schizophrenia
Key schizophrenia-related neuronal circuits, in particular the mesocortical and mesolimbic dopaminergic circuits are thought to be modulated by GABA (Wassef et al., 2003). Dopamine-immunoreactive axons form a greater than random number of contacts with GABAergic interneurons in the mPFC suggesting that these GABAergic interneurons mediate the dopaminergic inhibitory modulation of cortical pyramidal neuronal output in this region (Benes et al.,1993). Much of the evidence implicating the GABAergic system in the etiology of schizophrenia points to dysfunctional inhibitory interaction between the GABAergic and dopaminergic systems. Hence, we tested the interaction effects between GAD1 SNP rs3749034 and the well-characterized COMT Val158Met polymorphism. Although the evidence for an association between the Val allele and increased risk for a diagnosis of schizophrenia is rather weak (Fan et al., 2005; Glatt et al., 2003; Munafo et al., 2005), recent neuroimaging studies revealed decreased cortical thickness in Val compared to Met allele carriers (Cerasa et al., 2010; Shaw et al., 2009). Furthermore, several reports examining this polymorphism revealed gene–gene interaction effects, i.e. genetic polymorphisms of other schizophrenia risk genes including GAD1 were associated with schizophrenia only in subgroups of Val allele carriers of the COMT Val158Met polymorphism (Nicodemus et al., 2007; Straub et al., 2007). Consistent with this data, we found cortical thickness reductions in G allele homozygotes of GAD1 rs3748034 only in the Val/Met and Val/Val subgroups. Interestingly, a recent study examining potential associations between the COMT Val158Met polymorphism and image-encoding and -recognition-related neural activity in patients with schizophrenia and healthy controls found a diagnosis × genotype interaction in the PHG (Di Giorgio et al., 2011). Although the current evidence is preliminary, it cannot be excluded that the joint effect of risk alleles of the COMT Val158Met and GAD1 rs3749034 polymorphisms affects the neural substrates underlying specific brain functions and may thus confer increased risk for schizophrenia.
4.5. Limitations
The findings of our study need to be seen in the light of the following limitations. We focused on the GAD1 SNP rs3749034 that has been previously associated with schizophrenia and affects GABAergic neurotransmission via transcription-factor binding sites and its interactions with COMT. However, variation of GAD1 and accordingly our findings may also be relevant in the etiology of other psychiatric diseases such as bipolar disorder (Cherlyn et al., 2010). It is also possible that rs3749034 is in linkage disequilibrium with other functional GAD1 variants which represent the true underlying genetic determinants responsible for the effects described in our paper. Other SNPs or haplotypes in the same gene or in other genes interacting with GAD1 may also be relevant. Indeed, GAD1 SNP rs10432420, which is in moderate linkage disequilibrium with rs3749034, has been found to be associated with reduced cortical thickness in the PHG in a very large cohort of healthy controls using the same image processing algorithm (personal communication M. N. Smolka). The fact that we only included healthy controls limits the interpretation of our findings. However, a recent review emphasized that genetic imaging studies in healthy subjects are a sensitive and specific method to elucidate pathophysiological processes of schizophrenia (Meyer-Lindenberg, 2010). Moreover, this approach avoids many important environmental confounders present in chronic patients (such as the increased prevalence of smoking, the use of antipsychotics, different lifestyles, poorer somatic health and early institutionalization) that have been shown to impact neuroanatomical and neurofunctional brain phenotypes (Draganski & May, 2008; Meyer-Lindenberg, 2010; Navari and Dazzan, 2009; Scherk and Falkai, 2006; Tomelleri et al., 2009; Tregellas et al., 2007).
4.6. Conclusions
Our study highlights the effects of a schizophrenia risk variant in the GAD1 gene on a brain-based marker of schizophrenia – cortical thickness – as well as genetic interactions with the COMT Val158Met polymorphism. These consistent results further implicate GAD1 in the pathophysiology of schizophrenia and suggest that genetically determined changes in the interplay between the GABAergic and dopaminergic systems may result in subtle changes in brain anatomy and functioning which can be quantified with neuroimaging methods.
Supplementary Material
Acknowledgments
The authors would like to express their gratitude for the personal support from the Deutsche Forschungsgemeinschaft and the NARSAD Young Investigator Award (Research Fellowship and Grant to S Ehrlich), the Biomedical Science Exchange Program (scholarship to S Brauns), the Deutscher Akademischer Austausch Dienst and the Friedrich-Ebert-Stiftung (scholarships to E Walton).
Role of the funding source
This research was supported by grants from NIH/NCRR P41RR14075, Department of Energy DE-FG02-99ER62764, MIND Research Network, Morphometry BIRN 1U24, RR021382A, Function BIRN U24RR021992-01, NIH.NCRR MO1 RR025758-01, the Department of Energy for the Mental Illness and Neuroscience Discovery (MIND) Research Network [DE-FG02-99ER62764], the Deutsche Forschungsgemeinschaft and the NARSAD Young Investigator Award (Research Fellowship and Grant to S Ehrlich), the Biomedical Science Exchange Program (scholarship to S Brauns), the Deutscher Akademischer Austausch Dienst and the Friedrich-Ebert-Stiftung (scholarships to E Walton). These sources had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributors
Author Stefan Brauns analyzed data and wrote the first draft of the manuscript. Author Randy L Gollub was responsible for the study design, supervised subject assessments and data analysis and assisted in manuscript preparation. Author Esther Walton analyzed data and assisted in manuscript preparation. Author Johanna Hass analyzed data and assisted in the preparation of the revised manuscript. Author Michael N Smolka assisted in the preparation of the revised manuscript. Author Tonya White assisted in the study design, conducted and supervised the subject assessments and assisted in the manuscript preparation. Author Thomas H Wassink assisted in the data collection and assisted in manuscript preparation. Author Vince D Calhoun assisted in the study design and assisted in manuscript preparation. Author Stefan Ehrlich assisted in the study design, supervised data analysis and assisted in manuscript preparation. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that there are no conflicts of interest in relation to the subject of this study.
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.jpsychires.2013.03.010.
References
- Addington AM, Gornick M, Duckworth J, Sporn A, Gogtay N, Bobb A, et al. GAD1 (2q31.1), which encodes glutamic acid decarboxylase (GAD67), is associated with childhood-onset schizophrenia and cortical gray matter volume loss. Molecular Psychiatry. 2005;10:581–588. doi: 10.1038/sj.mp.4001599. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Research Reviews. 2006;52:293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Archives of General Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K. The ontogeny of human gyrification. Cerebral Cortex. 1995;5:56–63. doi: 10.1093/cercor/5.1.56. [DOI] [PubMed] [Google Scholar]
- Arnold SE. Cellular and molecular neuropathology of the parahippocampal region in schizophrenia. Annals of the New York Academy of Sciences. 2000;911:275–292. doi: 10.1111/j.1749-6632.2000.tb06732.x. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, Greene CL, Barker JL. GABA receptor antagonists modulate postmitotic cell migration in slice cultures of embryonic rat cortex. Cerebral Cortex. 2000;10:899–909. doi: 10.1093/cercor/10.9.899. [DOI] [PubMed] [Google Scholar]
- Behar TN, Schaffner AE, Scott CA, O’Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells to GABA as a migration stimulus. Journal of Neuroscience. 1998;18:6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Molloy R. Dopamine-immunoreactive axon varicosities form nonrandom contacts with GABA-immunoreactive neurons of rat medial prefrontal cortex. Synapse. 1993;15:285–295. doi: 10.1002/syn.890150405. [DOI] [PubMed] [Google Scholar]
- Braff DL, Freedman R, Schork NJ, Gottesman II. Deconstructing schizophrenia: an overview of the use of endophenotypes in order to understand a complex disorder. Schizophrenia Bulletin. 2007;33:21–32. doi: 10.1093/schbul/sbl049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R, Colter N, Corsellis JA, Crow TJ, Frith CD, Jagoe R, et al. Postmortem evidence of structural brain changes in schizophrenia. Differences in brain weight, temporal horn area, and parahippocampal gyrus compared with affective disorder. Archives of General Psychiatry. 1986;43:36–42. doi: 10.1001/archpsyc.1986.01800010038005. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Burwell RD. The parahippocampal region: corticocortical connectivity. Annals of the New York Academy of Sciences. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- Cerasa A, Cherubini A, Quattrone A, Gioia MC, Tarantino P, Annesi G, et al. Met158 variant of the catechol-O-methyltransferase genotype is associated with thicker cortex in adult brain. Neuroscience. 2010;167:809–814. doi: 10.1016/j.neuroscience.2010.02.040. [DOI] [PubMed] [Google Scholar]
- Cherlyn SY, Woon PS, Liu JJ, Ong WY, Tsai GC, Sim K. Genetic association studies of glutamate, GABA and related genes in schizophrenia and bipolar disorder: a decade of advance. Neuroscience and Biobehavioral Reviews. 2010;34:958–977. doi: 10.1016/j.neubiorev.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Di Giorgio A, Caforio G, Blasi G, Taurisano P, Fazio L, Romano R, et al. Catechol-O-methyltransferase Val(158)Met association with parahippocampal physiology during memory encoding in schizophrenia. Psychological Medicine. 2011;41:1721–1731. doi: 10.1017/S0033291710002278. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Du J, Duan S, Wang H, Chen W, Zhao X, Zhang A, et al. Comprehensive analysis of polymorphisms throughout GAD1 gene: a family-based association study in schizophrenia. Journal of Neural Transmission. 2008;115:513–519. doi: 10.1007/s00702-007-0844-z. [DOI] [PubMed] [Google Scholar]
- Ehrlich S, Brauns S, Yendiki A, Ho BC, Calhoun V, Schulz SC, et al. Associations of cortical thickness and cognition in patients with schizophrenia and healthy controls. Schizophrenia Bulletin. 2012;38:1050–1062. doi: 10.1093/schbul/sbr018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S, Morrow EM, Roffman JL, Wallace SR, Naylor M, Bockholt HJ, et al. The COMT Val108/158Met polymorphism and medial temporal lobe volumetry in patients with schizophrenia and healthy adults. Neuroimage. 2010;53:992–1000. doi: 10.1016/j.neuroimage.2009.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan JB, Zhang CS, Gu NF, LiX W, Sun WW, Wang HY, et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biological Psychiatry. 2005;57:139–144. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophrenia Bulletin. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999b;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant“default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Human Brain Mapping. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatt SJ, Faraone SV, Tsuang MT. Association between a functional catechol O-methyltransferase gene polymorphism and schizophrenia: meta-analysis of case-control and family-based studies. American Journal of Psychiatry. 2003;160:469–476. doi: 10.1176/appi.ajp.160.3.469. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Greenstein D, Lenane M, Clasen L, Sharp W, Gochman P, et al. Cortical brain development in nonpsychotic siblings of patients with childhood-onset schizophrenia. Archives of General Psychiatry. 2007;64:772–780. doi: 10.1001/archpsyc.64.7.772. [DOI] [PubMed] [Google Scholar]
- Goldman AL, Pezawas L, Mattay VS, Fischl B, Verchinski BA, Chen Q, et al. Widespread reductions of cortical thickness in schizophrenia and spectrum disorders and evidence of heritability. Archives of General Psychiatry. 2009;66:467–477. doi: 10.1001/archgenpsychiatry.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Di-Giorgi-Gerevini V, Dwivedi Y, Grayson DR, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Archives of General Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- Harrison BJ, Yucel M, Pujol J, Pantelis C. Task-induced deactivation of midline cortical regions in schizophrenia assessed with fMRI. Schizophrenia Research. 2007;91:82–86. doi: 10.1016/j.schres.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Heck N, Kilb W, Reiprich P, Kubota H, Furukawa T, Fukuda A, et al. GABA-A receptors regulate neocortical neuronal migration in vitro and in vivo. Cerebral Cortex. 2007;17:138–148. doi: 10.1093/cercor/bhj135. [DOI] [PubMed] [Google Scholar]
- Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Current Topics in Behavioral Neurosciences. 2011;4:529–553. doi: 10.1007/7854_2010_43. [DOI] [PubMed] [Google Scholar]
- Honea R, Crow TJ, Passingham D, Mackay CE. Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. American Journal of Psychiatry. 2005;162:2233–2245. doi: 10.1176/appi.ajp.162.12.2233. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Lipska BK, Ali T, Mathew SV, Law AJ, Metitiri OE, et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. Journal of Neuroscience. 2011;31:11088–11095. doi: 10.1523/JNEUROSCI.1234-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. Rethinking schizophrenia. Nature. 2011;468:187–193. doi: 10.1038/nature09552. [DOI] [PubMed] [Google Scholar]
- Jung WH, Kim JS, Jang JH, Choi JS, Jung MH, Park JY, et al. Cortical thickness reduction in individuals at ultra-high-risk for psychosis. Schizophrenia Bulletin. 2011;37:839–849. doi: 10.1093/schbul/sbp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. Journal of Psychopharmacology. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Lopez-Bendito G, Lujan R, Shigemoto R, Ganter P, Paulsen O, Molnar Z. Blockade of GABA(B) receptors alters the tangential migration of cortical neurons. Cerebral Cortex. 2003;13:932–942. doi: 10.1093/cercor/13.9.932. [DOI] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Mannell MV, Franco AR, Calhoun VD, Canive JM, Thoma RJ, Mayer AR. Resting state and task-induced deactivation: a methodological comparison in patients with schizophrenia and healthy controls. Human Brain Mapping. 2010;31:424–437. doi: 10.1002/hbm.20876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenco S, Savostyanova AA, van der Veen JW, Geramita M, Stern A, Barnett AS, et al. Genetic modulation of GABA levels in the anterior cingulate cortex by GAD1 and COMT. Neuropsychopharmacology. 2010;35:1708–1717. doi: 10.1038/npp.2010.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda SA, Stevens MC, Folley BS, Calhoun VD, Pearlson GD. Evidence for anomalous network connectivity during working memory encoding in schizophrenia: an ICA based analysis. PLoS ONE. 2009;4:e7911. doi: 10.1371/journal.pone.0007911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, Moller HJ. The role of dopamine for the pathophysiology of schizophrenia. International Review of Psychiatry. 2007;19:337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- Munafo MR, Bowes L, Clark TG, Flint J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Molecular Psychiatry. 2005;10:765–770. doi: 10.1038/sj.mp.4001664. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cerebral Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nesvag R, Lawyer G, Varnas K, Fjell AM, Walhovd KB, Frigessi A, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophrenia Research. 2008;98:16–28. doi: 10.1016/j.schres.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Callicott JH, Higier RG, Luna A, Nixon DC, Lipska BK, et al. Evidence of statistical epistasis between DISC1, CIT and NDEL1 impacting risk for schizophrenia: biological validation with functional neuroimaging. Human Genetics. 2010a;127:441–452. doi: 10.1007/s00439-009-0782-y. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Kolachana BS, Vakkalanka R, Straub RE, Giegling I, Egan MF, et al. Evidence for statistical epistasis between catechol-O-methyltransferase (COMT) and polymorphisms in RGS4, G72 (DAOA), GRM3, and DISC1: influence on risk of schizophrenia. Human Genetics. 2007;120:889–906. doi: 10.1007/s00439-006-0257-3. [DOI] [PubMed] [Google Scholar]
- Nicodemus KK, Law AJ, Radulescu E, Luna A, Kolachana B, Vakkalanka R, et al. Biological validation of increased schizophrenia risk with NRG1, ERBB4, and AKT1 epistasis via functional neuroimaging in healthy controls. Archives of General Psychiatry. 2010b;67:991–1001. doi: 10.1001/archgenpsychiatry.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon DC, Prust MJ, Sambataro F, Tan HY, Mattay VS, Weinberger DR, et al. Interactive effects of DAOA (G72) and catechol-o-methyltransferase on neurophysiology in prefrontal cortex. Biological Psychiatry. 2011;69:1006–1008. doi: 10.1016/j.biopsych.2010.10.031. [DOI] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, et al. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nature Neuroscience. 2007;10:1515–1517. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cerebral Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A, Carpenter M. Human neuroanatomy. Baltimore, MD: Williams & Wilkins; 1995. [Google Scholar]
- Pomarol-Clotet E, Salvador R, Sarro S, Gomar J, Vila F, Martinez A, et al. Failure to deactivate in the prefrontal cortex in schizophrenia: dysfunction of the default mode network? Psychological Medicine. 2008;38:1185–1193. doi: 10.1017/S0033291708003565. [DOI] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Toulopoulou T, Bramon E, et al. Epistasis between the DAT 3’ UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13600–13605. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Molecular Psychiatry. 2005;10:434–449. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Gollub RL, Calhoun VD, Wassink TH, Weiss AP, Ho BC, et al. MTHFR 677C –> T genotype disrupts prefrontal function in schizophrenia through an interaction with COMT 158Val –> Met. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:17573–17578. doi: 10.1073/pnas.0803727105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Scherk H, Falkai P. Effects of antipsychotics on brain structure. Current Opinion in Psychiatry. 2006;19:145–150. doi: 10.1097/01.yco.0000214339.06507.d8. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Nenadic I, Gaser C, et al. Increased parahippocampal and lingual gyrification in first-episode schizophrenia. Schizophrenia Research. 2010a;123:137–144. doi: 10.1016/j.schres.2010.08.033. [DOI] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, et al. Reduced cortical thickness in first episode schizophrenia. Schizophrenia Research. 2010b;116:204–209. doi: 10.1016/j.schres.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. Journal of Neuroscience. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JM, Turner JA, van Erp TG, White T, Bockholt HJ, Gollub RL, et al. Voxel-based morphometric multisite collaborative study on schizophrenia. Schizophrenia Bulletin. 2009;35:82–95. doi: 10.1093/schbul/sbn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei Y, Li Z, Song J, Ren-Patterson R, Tunbridge EM, Iizuka Y, et al. Epistatic and functional interactions of catechol-o-methyltransferase (COMT) and AKT1 on neuregulin1-ErbB signaling in cell models. PLoS ONE. 2010;5:e10789. doi: 10.1371/journal.pone.0010789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman LJ, Pantelis C, Keshavan MS, Faraone SV, Goldstein JM, Horton NJ, et al. A review and new report of medial temporal lobe dysfunction as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric family study of the parahippocampal gyrus. Schizophrenia Bulletin. 2003;29:803–830. doi: 10.1093/oxfordjournals.schbul.a007048. [DOI] [PubMed] [Google Scholar]
- Shaw P, Wallace GL, Addington A, Evans A, Rapoport J, Giedd JN. Effects of the Val158Met catechol-O-methyltransferase polymorphism on cortical structure in children and adolescents. Molecular Psychiatry. 2009;14:348–349. doi: 10.1038/mp.2008.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophrenia Research. 2001;49:1–52. doi: 10.1016/s0920-9964(01)00163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim G, Oh JS, Jung WH, Jang JH, Choi CH, Kim E, et al. Altered resting-state connectivity in subjects at ultra-high risk for psychosis: an fMRI study. Behavioral and Brain Functions. 2010;6:58. doi: 10.1186/1744-9081-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudlarski P, Jagannathan K, Anderson K, Stevens MC, Calhoun VD, Skudlarska BA, et al. Brain connectivity is not only lower but different in schizophrenia: a combined anatomical and functional approach. Biological Psychiatry. 2010;68:61–69. doi: 10.1016/j.biopsych.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RE, Lipska BK, Egan MF, Goldberg TE, Callicott JH, Mayhew MB, et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Molecular Psychiatry. 2007;12:854–869. doi: 10.1038/sj.mp.4001988. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Archives of General Psychiatry. 2003;60:1187–1192. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Stan AD, Wagner AD. The hippocampal formation in schizophrenia. American Journal of Psychiatry. 2010;167:1178–1193. doi: 10.1176/appi.ajp.2010.09081187. [DOI] [PubMed] [Google Scholar]
- Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophrenia Research. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tomelleri L, Jogia J, Perlini C, Bellani M, Ferro A, Rambaldelli G, et al. Brain structural changes associated with chronicity and antipsychotic treatment in schizophrenia. European Neuropsychopharmacology. 2009;19:835–840. doi: 10.1016/j.euroneuro.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Shatti S, Tanabe JL, Martin LF, Gibson L, Wylie K, et al. Gray matter volume differences and the effects of smoking on gray matter in schizophrenia. Schizophrenia Research. 2007;97:242–249. doi: 10.1016/j.schres.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Tseng KY, Lewis BL, Lipska BK, O’Donnell P. Post-pubertal disruption of medial prefrontal cortical dopamine-glutamate interactions in a developmental animal model of schizophrenia. Biological Psychiatry. 2007;62:730–738. doi: 10.1016/j.biopsych.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Archives of General Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. Journal of Clinical Psychopharmacology. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Watanabe M. Are there internal thought processes in the monkey? – default brain activity in humans and nonhuman primates. Behavioural Brain Research. 2011;221:295–303. doi: 10.1016/j.bbr.2011.02.032. [DOI] [PubMed] [Google Scholar]
- White T, Su S, Schmidt M, Kao CY, Sapiro G. The development of gyrification in childhood and adolescence. Brain and Cognition. 2010;72:36–45. doi: 10.1016/j.bandc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual Review of Clinical Psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. Wide range achievement test. 3rd ed. Wilmington, DE: Wide Range, Inc; 1993. [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo TU, Walsh JP, Benes FM. Density of glutamic acid decarboxylase 67 messenger RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. Archives of General Psychiatry. 2004;61:649–657. doi: 10.1001/archpsyc.61.7.649. [DOI] [PubMed] [Google Scholar]
- 100.Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. American Journal of Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- 101.Zhao X, Qin S, Shi Y, Zhang A, Zhang J, Bian L, et al. Systematic study of association of four GABAergic genes: glutamic acid decarboxylase 1 gene, glutamic acid decarboxylase 2 gene, GABA(B) receptor 1 gene and GABA(A) receptor subunit beta2 gene, with schizophrenia using a universal DNA microarray. Schizophrenia Research. 2007;93:374–384. doi: 10.1016/j.schres.2007.02.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.