Abstract
Cardiovascular disease is the leading cause of death in Western countries. A major limitation of current treatments is the inability to efficiently repair or replace dead myocardium. Recently, stem cell based therapies have been explored as an avenue to circumvent current therapeutic limitations. Overall, these therapies appear to result in small improvements in the contractile function of the heart. The exact mechanism(s) of action that underlie these improvements remain unknown and it is believed that paracrine effects play a significant role. Previously, we had reported that an extract derived from bone marrow cells (BMC), in the absence of any live cell, contained cardio-protective soluble factors. In this study, we identify IL-15 as a putative cardio-protectant within the BMC paracrine profile. Using an in vitro culture system, we assessed the ability of IL-15 to protect cardiomyocytes under hypoxic conditions. For the first time, we have identified IL-15 receptors on the surface of cardiomyocytes and delineated the signaling system by which hypoxic cardiomyocytes may be protected from cellular death and rescued from oxidative stress with IL-15 treatment.
Keywords: IL-15, cardiomyocyte, survival, stem cell
INTRODUCTION
Cardiovascular disease is the leading cause of death in Western countries.1 Despite major therapeutic advances, the global burden remains substantial. A major limitation of current treatments, particularly in ischemic heart disease, is the inability to efficiently repair or replace dead myocardium. Currently, heart transplantation remains the ultimate approach to treating end-stage heart failure. More recently, stem cells have given hope that cell based therapies may hold therapeutic potential; however, experimental studies in both animals and humans have shown variable results when adult stem cells are injected into the infarcted heart. Overall, these therapies appear to result in small improvements in the contractile function of the heart. The potential mechanism(s) of action that underlie these cell therapies remain controversial and include several reported possibilities: cardiomyocyte (CM) regeneration,2 cell fusion,3 enhanced neo-vascularization,4 and paracrine mechanisms.5-9
Previously, we have shown that paracrine secretion is an underlying mechanism of successful cell therapy. We demonstrated that bone marrow (BM) derived cell extract, in the absence of any live cells, could improve cardiac function post-MI, resulting in smaller left ventricular systolic and diastolic volumes and limiting the extent of the infarct scar, enhancing vascularity and reducing CM apoptosis.5 One of these factors in the extract is the immunostimulatory cytokine interleukin-15 (IL-15).
IL-15 is a cytokine that is expressed in both immune and non-immune cells 10-13. It induces proliferation, inhibits apoptosis, and initiates cellular differentiation in hematopoietic cells. Additionally, it has been shown to protect against cell death in several non-immune cell types.12, 14 IL-15 inhibits TNF-alpha mediated apoptosis within L929 murine fibroblasts,15 and functions as a pro-survival autocrine factor in renal epithelial cells14 and murine microglia.12 To reduce apoptosis IL-15 signals though the IL-15 receptor system.16
The IL-15 receptor is a multimeric complex comprised of three subunits: IL-15 receptor alpha (IL-15Rα), IL-2 receptor beta (IL-2Rβ), and the IL-2 receptor gamma (IL-2Rγ) common chain.11 Each subunit chain has a unique binding specificity and signaling capacity. Moreover, all three subunits mediate survival, anti-apoptotic, and proliferative pathways. They function in concert to enhance survival, and a variety of other responses, in both immune and non-immune cell types.16
The effects of IL-15 on cardiomyocytes are unknown. The present study is the first to characterize the IL-15 system in cardiac cells. In this report, we demonstrate that IL-15 and its receptors are present on CMs. Furthermore, we show that administration of supplemental IL-15 increases the survival of CMs under oxidative stress. Lastly, we report on the specific pro-survival and anti-apoptotic pathways through which IL-15 results in the rescue of CMs under hypoxic conditions.
METHODS
Materials
Recombinant murine IL-15 was obtained from PeproTech (Rocky Hill, NJ), wortmannin from Sigma-Aldrich (St. Louis, MO) and WP1066 from EMD Millipore (Billerica, MA). Antibodies directed against phosphorylated ERK and STAT3 were purchased from Cell Signaling Technology (Danvers, MA). Anti-GAPDH was obtained from Stressgen (Farmingdale, NY). Biotinylated goat anti-IL-15Rα was obtained from R&D Systems (Minneapolis, MN), rabbit polyclonal anti-IL-2Rβ and IL-2Rγ (M-20) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA), rabbit polyclonal anti-IL-2Rβ from Sigma-Aldrich (St. Louis, MO) and rat monoclonal anti-IL-2Rβ (clone A95-1) from BD Pharmingen (San Jose, CA).
Animals
Male C57BL/6J mice (20–25 g) were purchased from Jackson Laboratories (Sacramento, CA). All studies were approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Adult mouse CM isolation and culture
Adult mouse CMs were isolated and cultured using a modification of the collagenase dissociation method of Zhou et al.13 as previously described in our laboratory.17-19 Mice were treated with heparin (50 units) and anesthetized by intraperitoneal injection with pentobarbital sodium (200 mg/kg). The heart was quickly excised, and the aorta was cannulated for retrograde perfusion in a Langendorff apparatus at a constant flow rate of 3 ml/min at 37°C. The heart was perfused for 2 min with isolation buffer [120 mM NaCl, 5.4 mM KCl, 1.2 mM MgSO4, 1.2 mM NaH2PO4, 5.6 mM glucose, 5 mM NaHCO3, 10 mM HEPES, 50 μM CaCl2, 10 mM 2, 3-butanedione monoxime (BDM) and 5 mM taurine], followed by digestion for 9 min with collagenase II (1.5 mg/ml; Worthington, Lakewood, NJ) with 50 μM Ca2+ in isolation buffer. After digestion, the soft and flaccid heart was removed, and myocytes were suspended in isolation buffer. A series of four centrifugations (40 × g, 1 min) and resuspensions were used for stepwise Ca2+ reintroduction from 50 μM to 1.0 mM, which was the final medium Ca2+ concentration. Isolated CMs were plated on 35 mm tissue culture dishes or 12 well tissue culture plates coated with laminin for 2 h. The cells were suspended in minimum essential medium (MEM) with Hanks’ buffered salt solution (HBSS), 10 μg/ml penicillin, 1.5 μM vitamin B12, 10 mM BDM and 5% bovine calf serum. After this period of myocyte attachment, the medium was changed to MEM-HBSS containing 10 μg/ml penicillin, 1.5 μM vitamin B12, and 1 mM BDM and was incubated overnight at 37°C in a humidified atmosphere of 2% CO2 and air. The culture protocol yielded an average of 80% rod-shaped myocytes at a plating density of 50 cells/mm2 that were viable at pH 7 for 72 h. Experiments were performed the day following isolation and culture.
Hypoxia-reoxygenation protocol
On the day after isolation and culture, cardiomyocytes were rinsed and medium changed to MEM-HBSS containing 10 μg/ml penicillin, 1.5 μM vitamin B12 and no BDM. Cells were subsequently changed to serum-free, glucose-free MEM with HBSS and no BDM. This medium had been pre-equilibrated overnight in an anaerobic chamber containing 1% CO2 and 99% N2. The myocytes were then incubated in a Bactron I anaerobic chamber containing a humidified atmosphere of 1% CO2 and 99% N2 for 3 h. This procedure produced a pO2 of 4 Torr. Normoxic experimental medium also was pre-equilibrated overnight in a water-jacketed incubator in a humidified atmosphere of 1% CO2 and air. After 3 h the media of both normoxic cells and cells in the hypoxia chamber were changed to glucose containing, serum-free MEM with HBSS and were incubated in normoxia for 22 h.
Measurements of CM survival
CM survival was measured by staining cells in tissue culture dishes with trypan blue solution (Sigma Chemical, St. Louis, MO) diluted to a final concentration of 0.04% (wt/vol) as previously described.18 Myocytes were visualized using bright-field microscopy at 100x magnification. The number of viable (unstained) and nonviable (blue stained) CMs in 10 random microscopic fields was recorded, and at least 300 cells were counted in each dish. Percent survival was defined as the number of unstained myocytes counted in each hypoxic treatment dish divided by the number of unstained myocytes counted in each corresponding normoxic control dish. This calculation accounted for the detachment and loss of nonviable cells during the experimental hypoxia protocols.
Immunoprecipitation of receptor proteins
Immunoprecipitation was performed with the following modifications as previously described.20 Fresh adult mouse CMs were lysed in buffer containing 137 mM NaCl, 1% Triton X-100, 20 mM Tris, pH 8.0, 2 mM EDTA with complete mini protease and phosphatase inhibitor cocktails (Roche Applied Science, Indianapolis, IN).
Western blot and signaling analysis
On the day after isolation and culture, CMs were treated with pharmacological inhibitors for 15 min before addition of 5 ng/ml IL-15 for 10 min under normoxic conditions for the indicated times. For whole cell extraction, cells were lysed in buffer containing 150 mM NaCl, 1% NP-40, 0.25%, deoxycholate, 0.1% SDS, 50 mM Tris (pH 8.0), complete mini protease inhibitor cocktail and 0.5% phosphatase inhibitor cocktails (Sigma). Protein concentration was determined using the bicinchoninic acid assay. Equal amounts of protein were resuspended in 1x Laemmli sample buffer, boiled for 5 min, and subjected to sodium dodecyl (lauryl) sulfate-polyacrylamide gel electrophoresis. After transfer to a polyvinylidene difluoride membrane, the extract was blocked in 3% BSA in Tris-buffered saline (TBS) plus 0.1% Tween 20 for 1 h. The membranes were probed overnight with primary antibodies, washed three times with TBS Tween 20 for 5 min, and probed with secondary antibodies for 1 h. The membranes were rinsed three times, and the signal was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions.
RT-PCR and Real-time RT-PCR
Total RNA from cultured CMs was isolated using the TRIzol reagent (Invitrogen). cDNA was generated from 1.5 μg of total RNA by using SuperScript III First-Strand Synthesis kit (Invitrogen). RT-PCR was performed using 0.5 μl of cDNA and Advantage 2 PCR kit (Clontech, Mountain View, CA) with the following program: 95°C 3 min, (95°C 30 s-- 68°C-- 2 min) × 35 cycles, 68°C 10 min. PCR products were separated on 2% agarose gel. Each pair of PCR primers was designed to span one or several introns to distinguish the signals amplified from genomic DNA contamination. The primer sequences of IL-15Rα, IL-2Rβ, IL-2Rγ and internal control hypoxanthine phosphoribosyltransferase (HPRT) are from previous publications. 12, 21
Real-time PCR experiments were performed in duplicate on an ABI 7900 instrument (Applied Biosystems) using Taqman Master Mix (Applied Biosystems). The average threshold cycles (CT) of the duplicates were used to calculate the relative value of IL-2Rβ in CMs. The primers and probes for murine IL-2Rβ and HPRT were purchased from Applied Biosystems; 0.5 μl of cDNA per reaction was used. The CT for HPRT was used to normalize the samples. Expression of IL-2Rβ mRNA relative to HPRT mRNA was calculated based on the CT, ΔCTIL-2Rβ= CTIL-2Rβ-CTHPRT. The relative values of IL-2Rβ were calculated as 2−ΔCTIL-2Rβ.
Statistical analysis
Data are means ± SE. Mean values were compared using one-way analysis of variance and post hoc Student-Newman-Keuls testing. P < 0.05 was considered statistically significant.
RESULTS
IL-15 receptors are present in mouse CMs
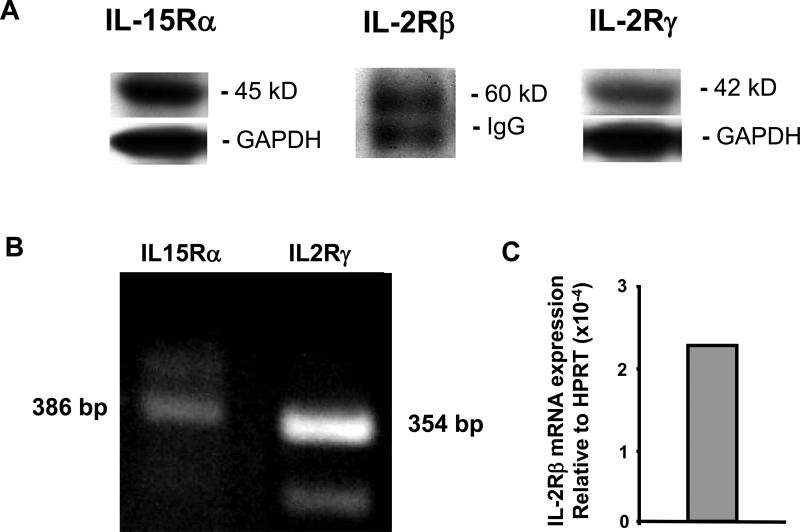
To examine the effects of IL-15 on the heart we used primary mouse CMs as a model system. From gene expression data (http://bgee.unil.ch/bgee/bgee) we knew that IL-15 receptors are expressed in mouse hearts but previously there were no data documenting the presence of these receptors on CMs specifically. Our first step was to determine the expression of the three IL-15 receptors: IL-15Rα, IL-2Rβ and IL-2Rγ on primary CMs. Cultured CMs were harvested as described and the IL-15 receptors were analyzed by immunoblotting except for the IL-2Rβ where the receptor was immunoprecipitated prior to immunoblotting due to low abundance (Figure 1A). The mRNA expression of IL-15Rα and IL-2Rγ in cultured CMs was verified by RT-PCR (Figure 1B). However, IL-2Rβ mRNA in CMs was only detected by real time RT-PCR due to its low expression level (0.0002 relative to HPRT, Figure 1C). For the first time, we have identified all three of these receptors in CMs at the mRNA and protein levels.
Figure 1.
A. Western blots of IL-15 receptor proteins in adult mouse cardiomyocytes (CMs) from cell lysates (IL-15Rα and IL-2Rγ) or after immunoprecipitation (IL2Rβ). B. RT-PCR analysis showed that IL-15Rα and IL-2Rγ are expressed in the CMs. C. IL-2Rβ was only detected in the CMs by real time RT-PCR. HPRT: hypoxanthine phosphoribosyltransferase.
IL-15 protects CMs from cell death after hypoxia/reoxygenation (Hx/Rx) through STAT3 and ERK1/2 pathways
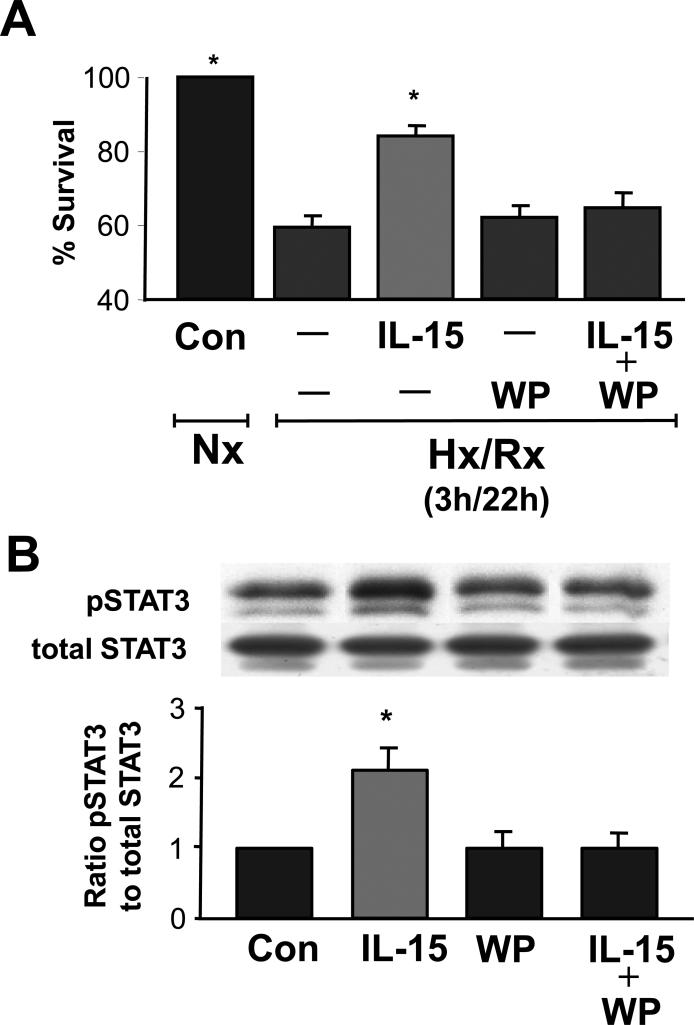
To determine if IL-15 administration acts directly on CMs, we exposed cultured adult murine CMs to 3 h hypoxia and 22 h reoxygenation (Hx/Rx, Figure 2A). Control cells incubated under normoxic conditions for the duration of the experiment were assigned a survival of 100%. Survival of untreated cells exposed to Hx/Rx was reduced to 58% compared to normoxic controls, while treatment with IL-15 at 5 ng/ml during the 22 h reoxygenation period following 3 h of hypoxia improved survival to 84% (p< 0.05 vs. hypoxic controls) (Figure 2A). The concentration of IL-15 we used was based on initial concentration-response experiments in which in which the measured response was cell survival during hypoxia/reoxygenation. There was a steep increase in survival between 1 and 5 ng/ml which plateaued thereafter up to 80 ng/ml.
Figure 2.
IL-15 increases cardiomyocyte survival after Hx/Rx and activates the transcription factor STAT3. These effects can be blocked with WP1066 (a STAT3 inhibitor). A. Addition of IL-15 (5 ng/ml) improves survival of CMs during Hx/Rx. WP1066 inhibits this benefit. WP1066 (1 μM) was added to cultures of adult cardiac myocytes after 3 h of hypoxia and just prior to treatment with IL-15 (5 ng/ml) at the onset of 22 h of re-oxygenation. B. Western blot analysis of IL-15 stimulated cardiomyocytes revealed increased STAT3 phosphorylation during normoxia. Activation was two-fold over untreated cardiomyocyte controls. WP1066 added 15 min prior to stimulation with IL-15 (5 ng/ml) for 10 min blocked activation of STAT3 phosphorylation.
Nx, normoxia; Hx/Rx, hypoxia/re-oxygenation; Con, control; WP, WP1066. A: n = 5 per group. *P < 0.05 vs. control and IL-15. B: n = 5 per group. *P < 0.05 vs. IL-15.
IL-15 activates the transcription factor STAT3. Addition of the STAT3 inhibitor WP1066 at 1 μM reduced the survival of CMs close to the levels of untreated Hx/Rx (Figure 2A). Under normoxic conditions neither this concentration of WP1066 nor IL-15 at 5 ng/ml had any adverse effect on myocyte viability. Western blot analysis of IL-15 stimulated STAT3 phosphorylation in normoxia was two-fold over untreated CMs controls (Figure 2B). WP1066 administered 15 min before IL-15 addition blocked activation of STAT3 phosphorylation (Figure 2B). These results demonstrate that under conditions of oxidative stress, IL-15 promotes survival in CMs through STAT3 phosphorylation.
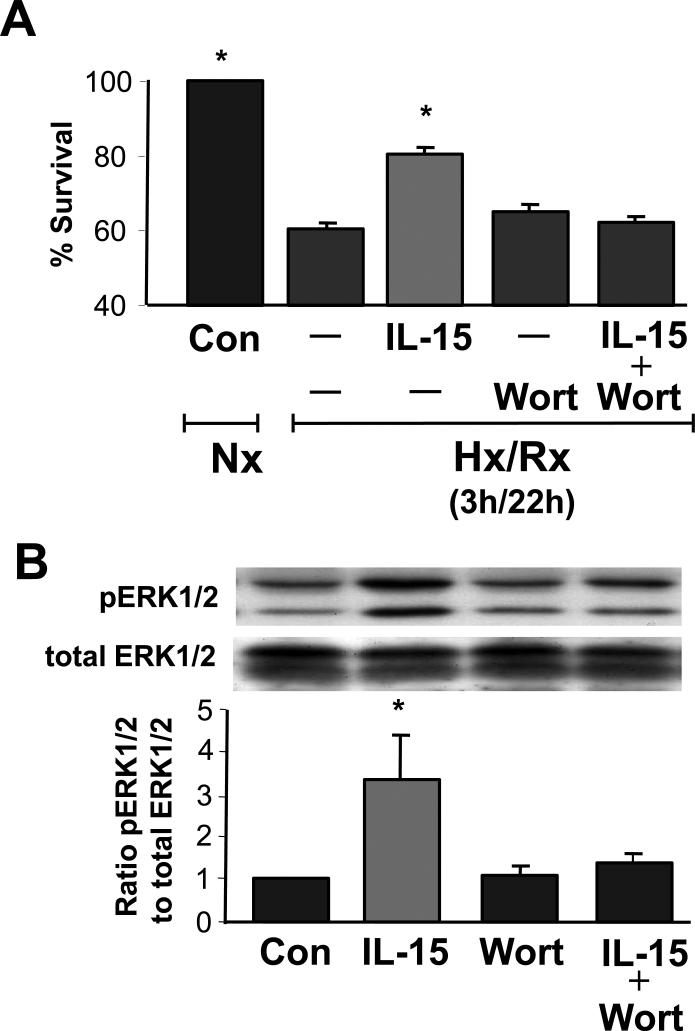
Additionally, we explored whether the PI3K-ERK1/2 pathway was involved in myocyte survival in Hx/Rx (Figure 3). In these experiments, the viability of untreated CMs was 60% of normoxic controls after Hx/Rx and IL-15 treatment improved survival to 81%. The PI3K inhibitor wortmannin reduced CM viability close to levels of untreated CMs exposed to Hx/Rx. IL-15 increased ERK1/2 phosphorylation over four-fold by Western Blot analysis while wortmannin reduced phosphorylation signals to control levels (Figure 3B). These results demonstrate that IL-15 protects CMs from oxidative stress through the PI3K to ERK1/2 pathway.
Figure 3.
IL-15 increases ERK1/2 phosphorylation in cardiomyocytes which can be blocked by wortmannin, a PI3 kinase inhibitor. A. Wortmannin inhibits the beneficial effects of IL-15 on CM survival. Wortmannin (100 nM) was added to cultures of adult cardiac myocytes after 3 h of hypoxia and just prior to treatment with IL-15 (5 ng/ml) at the onset of 22 h of re-oxygenation.
B. Western blot analysis of IL-15 stimulated ERK1/2 phosphorylation in normoxia was 3.3-fold over untreated cardiomyocyte controls. Wortmannin (100 nM) was added 15 min prior to addition of IL-15 (5 ng/ml) for 10 min, which blocked activation of ERK1/2 phosphorylation.
Nx, normoxia: Hx/Rx, hypoxia/re-oxygenation: Con, control: Wort, wortmannin. A: n = 5 per group. *P < 0.05 vs. control and IL-15. B: n = 7 per group. *P < 0.05 vs. IL-15.
DISCUSSION
We have demonstrated for the first time that CMs express IL-15Rα, IL-2Rβ and IL-2Rγ. Additionally, we have shown that administration of supplemental IL-15 can rescue in vitro cultures of CMs subjected to oxidative stress. We have demonstrated that the IL-15 system within CMs signals via PI3K-ERK1/2 and STAT3 pathways. Lastly, we have determined that this system can be activated and employed to protect CMs under hypoxic conditions.
The IL-15 signaling cascade is versatile and can induce a multitude of cellular responses depending on the cell type. For example, previous reports indicate that endothelial cells express IL-15 receptors and that IL-15 signaling is involved in the formation of new vasculature.22, 23 IL-15 has also been shown to exhibit anabolic influences over skeletal muscle cells in vitro.24 Moreover, IL-15 signaling has been shown to function as a protective agent against cell death in several immune and non-immune cell types.11, 14 These studies indicate that the protective capacity of IL-15 is underpinned by the ability of the IL-15 receptor complex to activate pro-survival pathways.25 To this end, each receptor subunit within the IL-15R complex has a distinct binding and signaling capacity. IL-15R-alpha binds IL-15 with high affinity and functions as a chaperone to facilitate the binding of IL-15 to the signal transducing subunits of the receptor complex: IL-2/15R-beta and the gamma chain.11 IL-2/15R-beta is known for stimulating JAK3-, STAT5-, and Akt-dependent signaling pathways that support cellular survival and proliferation.11 And, the gamma common chain, which completes the IL-15 receptor triad, is known to stimulate MAP kinase and PI3 kinase pathways that lead to mitogenic and anti-apoptotic signals.11 Therefore, in light of these findings, the presence of all three of these receptors potentiates the ability of IL-15 to activate pro-survival mechanisms in stressed CMs. We have shown that supplemental IL-15 can protect CMs from cell death under hypoxic conditions. Since IL-15Rs exist on the surface of other cells, the physiologic relevance of IL-15Rs may depend on their density on different cells. Future studies should explore this possibility especially if IL-15 is to be used clinically to treat patients with ischemic cardiac conditions.
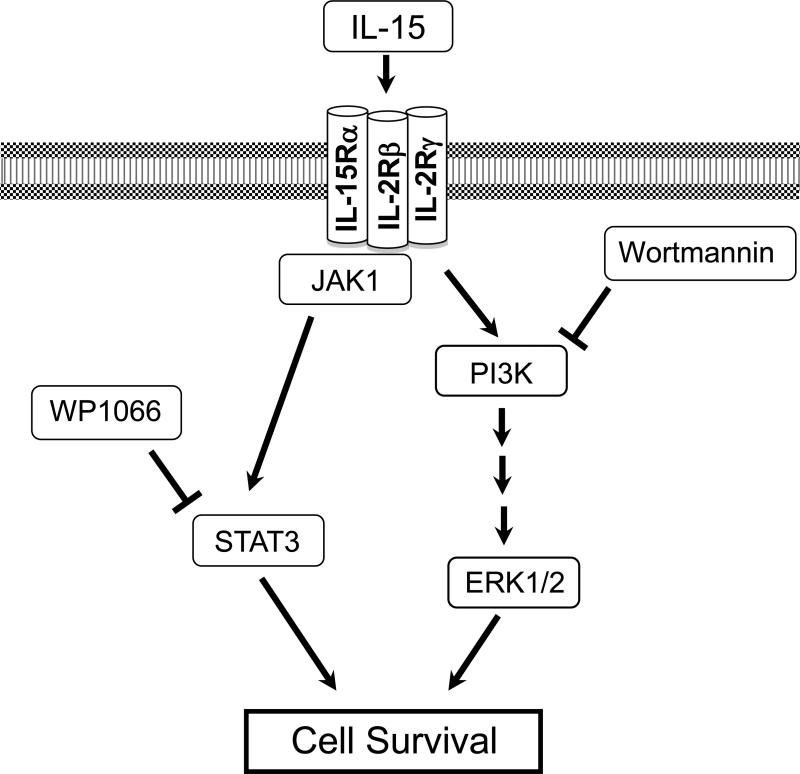
Additionally, we have determined that the IL-15 system utilizes STAT3 and PI3K-ERK1/2 pro-survival pathways to protect stressed CMs from cell death (Figure 4). Both STAT3 and PI3K-ERK1/2 pathways are mediators of cardio-protection that support several cardiac pre-conditioning phenomena. For example, mammalian hearts pre-conditioned with isoflurane have been shown to protect CMs from oxidative stress via activation of the PI3K-ERK1/2 pathway.26 Additionally, sphingosine-1 phosphate (S1P), a biologically active lysophospholipid and reported cardioprotectant, has been shown to guard hypoxic CMs from cell death by stimulating the PI3K-ERK1/2 pathway.27 These data indicate that STAT3 and PI3K-ERK1/2 pathways are mechanisms which many cardio-protective agents utilize to protect CMs. Likewise, IL-15 functions as a cardio- protective agent in hypoxic CMs by engaging the STAT3 and PI3K-ERK1/2 pathways (Figure 4).
Figure 4.
The cartoon depicts IL-15 mediated activation of the PI3K/ERK1/2 and JAK1/STAT3 pathways. The addition of wortmannin inhibits the activation of PI3K and the addition of WP1066 inhibits the activation of STAT3. Note that the binding affinity of IL-15 to each receptor is unknown.
In addition to our understanding of the IL-15 system within CMs, the present study also provides insight into one of the potential cardio-protective mechanisms of BMC treatment post-MI. Previously, we had demonstrated that the extract derived from BMCs results in decreased CM apoptosis under hypoxic conditions.4 In the present study, we extend those findings and report that IL-15 is one of the factors present within the BMC extract that has a cardio-protective role which might contribute to the paracrine benefits of cell based therapies post-MI. Whether in vivo treatment of animals or patients post-MI with IL-15 alone would result in a decrease in CM apoptosis and ultimately lead to a smaller infarct size is unknown and remains the focus of on-going investigation.
CONCLUSIONS
In conclusion, we have shown for the first time that IL-15 mediated signaling provides a novel avenue by which hypoxic CMs may be protected from hypoxia-induced cell death, via activation of STAT3 and PI3K-ERK1/2 pathways.
Acknowledgment
Supported by the UCSF Translational Cardiac Stem Cell Program, the Leone-Perkins Foundation, the Torian Foundation and the Vadasz Foundation (all to YY), and by HL R01 090606, the Foundation for Cardiac Research, and the Chatterjee Center for Cardiac Research (all to JSK). We would also like to thank Dr. Franca Angeli, Dr. Jean-Philippe Coppe and Erika Assoun for their technical expertise and input.
Footnotes
Disclosures: None declared.
REFERENCES
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009. 119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 3.Medvinsky A, Smith A. Stem cells: Fusion brings down barriers. Nature. 2003;422:823–825. doi: 10.1038/422823a. [DOI] [PubMed] [Google Scholar]
- 4.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 5.Yeghiazarians Y, Zhang Y, Prasad M, Shih H, Saini SA, Takagawa J, Sievers RE, Wong ML, Kapasi NK, Mirsky R, Koskenvuo J, Minasi P, Ye J, Viswanathan MN, Angeli FS, Boyle AJ, Springer ML, Grossman W. Injection of bone marrow cell extract into infarcted hearts results in functional improvement comparable to intact cell therapy. Mol Ther. 2009;17:1250–1256. doi: 10.1038/mt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 8.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–1421. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 10.Tsukamoto K, Huang YC, Dorsey WC, Carns B, Sharma V. Juxtacrine function of interleukin-15/interleukin-15 receptor system in tumour derived human B-cell lines. Clin Exp Immunol. 2006;146(5):59–566. doi: 10.1111/j.1365-2249.2006.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma A, Koka R, Burkett P. Annu Rev Immunol. Vol. 24. 657-679: 2006. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. [DOI] [PubMed] [Google Scholar]
- 12.Hanisch UK, Lyons SA, Prinz M, Nolte C, Weber JR, Kettenmann H, Kirchhoff F. Mouse brain microglia express interleukin-15 and its multimeric receptor complex functionally coupled to Janus kinase activity. J Biol Chem. 1997;272:28853–28860. doi: 10.1074/jbc.272.46.28853. [DOI] [PubMed] [Google Scholar]
- 13.Zhou YY, Wang SQ, Zhu WZ, Chruscinski A, Kobilka BK, Ziman B, Wang S, Lakatta EG, Cheng H, Xiao RP. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 14.Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, Kelley VR. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest. 2002;109:951–960. doi: 10.1172/JCI14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulfone-Paus S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. Death deflected: IL-15 inhibits TNF-alpha-mediated apoptosis in fibroblasts by TRAF2 recruitment to the IL-15Ralpha chain. Faseb J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 16.Budagian V, Bulanova E, Paus R, Bulfone-Paus S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 2006;17:259–280. doi: 10.1016/j.cytogfr.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Honbo N, Goetzl EJ, Chatterjee K, Karliner JS, Gray MO. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee K, Zhang J, Tao R, Honbo N, Karliner JS. Vincristine attenuates doxorubicin cardiotoxicity. Biochem Biophys Res Commun. 2008;373:555–560. doi: 10.1016/j.bbrc.2008.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao R, Hoover HE, Honbo N, Kalinowski M, Alano CC, Karliner JS, Raffai R. High-density lipoprotein determines adult mouse cardiomyocyte fate after hypoxia-reoxygenation through lipoprotein-associated sphingosine 1-phosphate. Am J Physiol Heart Circ Physiol. 2010;298:H1022–1028. doi: 10.1152/ajpheart.00902.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imhof I, Gasper WJ, Derynck R. Association of tetraspanin CD9 with transmembrane TGF{alpha} confers alterations in cell-surface presentation of TGF{alpha} and cytoskeletal organization. J Cell Sci. 2008;121(Pt 13):2265–2274. doi: 10.1242/jcs.021717. [DOI] [PubMed] [Google Scholar]
- 21.Su H, Arakawa-Hoyt J, Kan YW. Adeno-associated viral vector-mediated hypoxia response element-regulated gene expression in mouse ischemic heart model. Proc Natl Acad Sci U S A. 2002;99:9480–9485. doi: 10.1073/pnas.132275299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L, Thornton S, Grom AA. Interleukin-15 inhibits sodium nitroprusside-induced apoptosis of synovial fibroblasts and vascular endothelial cells. Arthritis Rheum. 2002;46:3010–3014. doi: 10.1002/art.10610. [DOI] [PubMed] [Google Scholar]
- 23.Angiolillo AL, Kanegane H, Sgadari C, Reaman GH, Tosato G. Interleukin-15 promotes angiogenesis in vivo. Biochem Biophys Res Commun. 1997;233:231–237. doi: 10.1006/bbrc.1997.6435. [DOI] [PubMed] [Google Scholar]
- 24.Quinn LS, Haugk KL, Grabstein KH. Interleukin-15: a novel anabolic cytokine for skeletal muscle. Endocrinology. 1995;136:3669–3672. doi: 10.1210/endo.136.8.7628408. [DOI] [PubMed] [Google Scholar]
- 25.de Jong JL, Farner NL, Widmer MB, Giri JG, Sondel PM. Interaction of IL-15 with the shared IL-2 receptor beta and gamma c subunits. The IL-15/beta/gamma c receptor-ligand complex is less stable than the IL-2/beta/gamma c receptor-ligand complex. J Immunol. 1996;156:1339–1348. [PubMed] [Google Scholar]
- 26.Hu ZY, Abbott GW, Fang YD, Huang YS, Liu J. Emulsified isoflurane postconditioning produces cardioprotection against myocardial ischemiareperfusion injury in rats. J Physiol Sci. 2013;63:251–261. doi: 10.1007/s12576-013-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vessey DA, Li L, Honbo N, Karliner JS. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Am J Physiol Heart Circ Physiol. 2009;297:H1429–1435. doi: 10.1152/ajpheart.00358.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]