Abstract
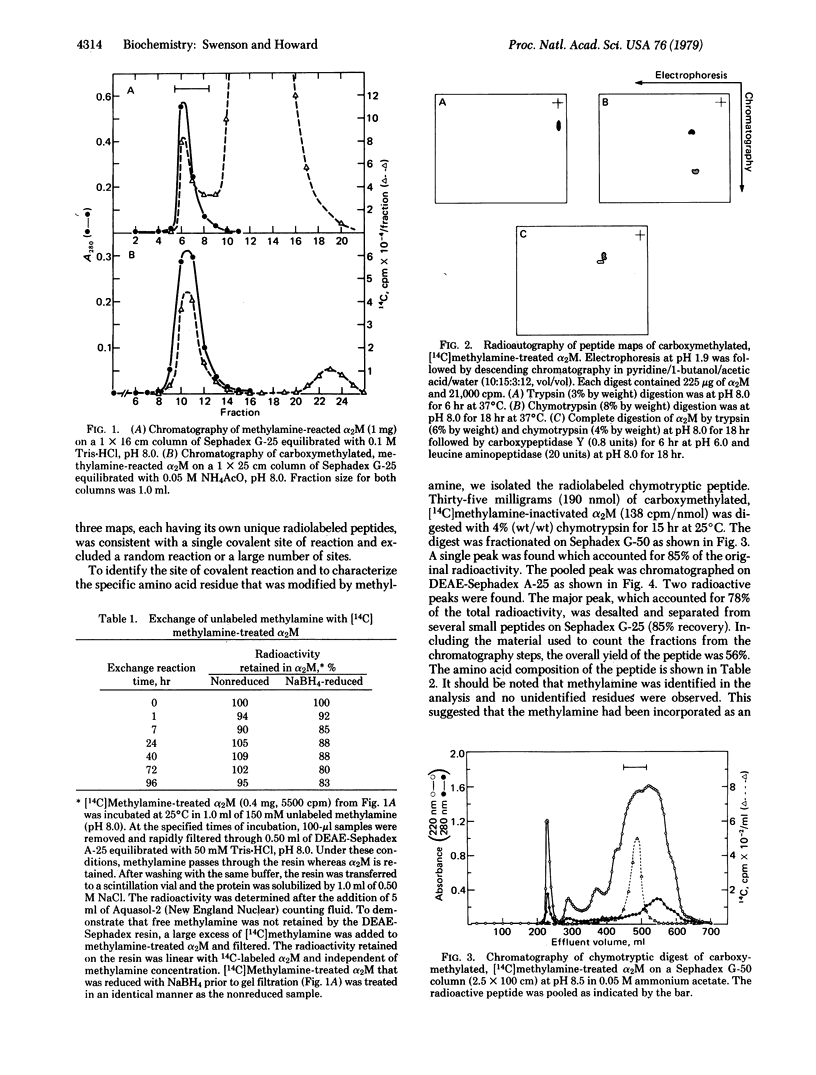
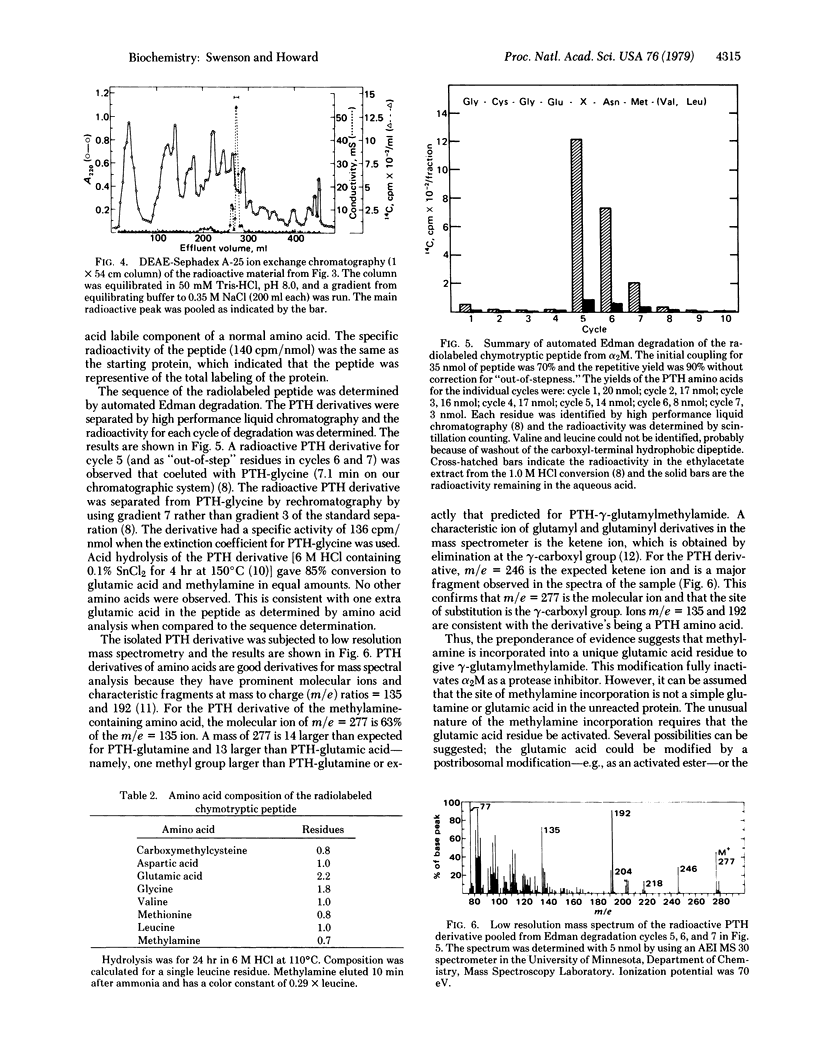
Methylamine reacts with the plasma protease inhibitor, alpha 2-macroglobulin, to form an irreversible, covalent modification. Quantitation of the reaction indicates 3.9 +/- (SD) 0.4 reactive sites per native tetrameric protein (Mr = 725,000) or one site per subunit. The reaction is selective and specific in that only 1 or 2 labeled peptides are observed on radioautography of peptide maps derived from [14C]methylamine-treated alpha 2-macroglobulin. A single chymotryptic peptide was isolated in 56% overall yield from the labeled protein. The peptide sequence by Edman degradation was found to be Gly-Cys-Gly-Glu-X-Asn-Met-(Val, Leu), in which X was the only radiolabeled phenylthiohydantoin derivative. Amino acid analysis and mass spectral analysis of the derivative suggests that X is gamma-glutamylmethylamide. Because glutamic acid and glutamine residues do not normally react with alkylamines, this work presents presumptive evidence for an alternative activated center in selected proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DALMASSO A. P., MUELLER-EBERHARD H. J. INTERACTION OF AUTOLOGOUS COMPLEMENT WITH RED CELLS IN THE ABSENCE OF ANTIBODY. Proc Soc Exp Biol Med. 1964 Dec;117:643–650. doi: 10.3181/00379727-117-29658. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Chung S. I. Molecular and catalytic properties of transglutaminases. Adv Enzymol Relat Areas Mol Biol. 1973;38:109–191. doi: 10.1002/9780470122839.ch3. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Reich E. A study of proteases and protease-inhibitor complexes in biological fluids. J Exp Med. 1978 Jul 1;148(1):223–234. doi: 10.1084/jem.148.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenmaier H., Ebbighausen W., Nicholson G., Vötsch W. Massenspektrometrische Identifizierung der Phenylthiohydantoin-Derivate von Aminosäuren. Z Naturforsch B. 1970 Jul;25(7):681–689. doi: 10.1515/znb-1970-0705. [DOI] [PubMed] [Google Scholar]
- Jones J. M., Creeth J. M., Kekwick R. A. Thio reduction of human 2 -macroglobulin. The subunit structure. Biochem J. 1972 Mar;127(1):187–197. doi: 10.1042/bj1270187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Howard J. B. Isolation and partial characterization of two different subunits from the molybdenum-iron protein of Azotobacter vinelandii nitrogenase. J Biol Chem. 1978 May 25;253(10):3422–3426. [PubMed] [Google Scholar]
- Mendez E., Lai C. Y. Regeneration of amino acids from thiazolinones formed in the Edman degradation. Anal Biochem. 1975 Sep;68(1):47–53. doi: 10.1016/0003-2697(75)90677-6. [DOI] [PubMed] [Google Scholar]
- Müller-Eberhard H. J. Complement. Annu Rev Biochem. 1975;44:697–724. doi: 10.1146/annurev.bi.44.070175.003405. [DOI] [PubMed] [Google Scholar]
- Nagasawa S., Han B. H., Sugihara H., Suzuki T. Studies on alpha 2-macroglobulin in bovine plasma. II. Interaction of alpha2-macroglobulin and trypsin. J Biochem. 1970 Jun;67(6):821–832. doi: 10.1093/oxfordjournals.jbchem.a129314. [DOI] [PubMed] [Google Scholar]
- Nelsestuen G. L., Zytkovicz T. H., Howard J. B. The mode of action of vitamin K. Identification of gamma-carboxyglutamic acid as a component of prothrombin. J Biol Chem. 1974 Oct 10;249(19):6347–6350. [PubMed] [Google Scholar]
- PILLEMER L., RATNOFF O. D., BLUM L., LEPOW I. H. The inactivation of complement and its components by plasmin. J Exp Med. 1953 Apr;97(4):573–589. doi: 10.1084/jem.97.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochon F., Amand B., Lavalette D., Bieth J. Rotational relaxation of free and protease-bound alpha2-macroglobulin. J Biol Chem. 1978 Oct 25;253(20):7496–7499. [PubMed] [Google Scholar]
- RATNOFF O. D., LEPOW I. H., PILLEMER L. The multiplicity of plasmin inhibitors in human serum, demonstrated by the effect of primary amino compounds. Bull Johns Hopkins Hosp. 1954 Apr;94(4):169–179. [PubMed] [Google Scholar]
- Steinbuch M., Pejaudier L., Quentin M., Martin V. Molecular alteration of alpha-2-macroglobulin by aliphatic amines. Biochim Biophys Acta. 1968 Jan 22;154(1):228–231. doi: 10.1016/0005-2795(68)90277-8. [DOI] [PubMed] [Google Scholar]
- Swenson R. P., Howard J. B. Structural characterization of human alpha2-macroglobulin subunits. J Biol Chem. 1979 Jun 10;254(11):4452–4456. [PubMed] [Google Scholar]



