Abstract
Reactive oxygen species and reactive nitrogen species (ROS/RNS) are often by-products of biochemical reactions, but are increasingly recognized as important second messengers involved in regulation of distinct cellular functions. Mild and reversible oxidation of certain amino acids within protein polypeptide chains is known to precisely control the function of transcription factors, protein kinases and phosphatases, receptors, pumps, ion channels, and so on. Conversely, under pathological conditions, high amounts of oxidants irreversibly oxidize DNA, lipids, and proteins and have deleterious effects on cells, ultimately causing cell death. ROS/RNS can thus be involved in the initiation and progression of many pathological conditions. Within this Forum, seven reviews and one original article summarize the current knowledge regarding redox regulation of various ion channels and ion conducting receptors. These include the recently identified mitochondrial Ca2+ uniporter and Orai Ca2+ channels, as well as selected members of the families of transient receptor potential, voltage-gated Ca2+, P2X, voltage-gated K+, and IP3R/RyR channels. In summary, all authors agree on the functional importance of redox-ion channel interplay. However, it is also clear that this is an emerging field of research where much has to be learned about intra- and extracellular sources, concentrations, and types of oxidants. Given their often short-lived nature and effective cellular buffering systems, the development of tools to measure local ROS production in living cells as well as detailed proteomic approaches to pinpoint protein targets and redox modifications are of importance. Antioxid. Redox Signal. 21, 859–862.
Redox Signaling
Misbalance of cellular redox state due to increased production of reactive oxygen species and reactive nitrogen species (ROS/RNS) and weakened antioxidant defense or environmental oxidative stress can lead to posttranslational oxidative modifications of proteins. Such oxidative events may also occur in small cellular subcompartments where ROS rapidly increase as a result of cellular metabolism thus forming so-called ROS microdomains. The most prominent targets for oxidation within proteins are cysteine residues due to the high reactivity of their thiol groups. Depending on the oxidant concentration, redox potentials, local pH, charge, temperature, and so on, thiols can be oxidized into sulfenic, sulfinic, and sulfonic acids. In addition, cysteines can undergo oxidative modifications such as nitrosylation and glutathionylation. Furthermore, high pathological concentrations of ROS can cause alterations of additional amino acids such as arginine and lysine to aldehydes or of methionine residues to sulfoxides or sulfones. Some of the above-mentioned modifications are not reversible and thereby terminally alter protein function. However, physiologically relevant concentrations of oxidants are known to control the function of transcription factors, protein kinases and phosphatases, receptors, pumps, ion channels, and so on.
Within cells, ROS/RNS can be generated by the NADPH oxidase enzymes and nitric oxide synthases. Furthermore, significant amounts of oxidants are generated by the mitochondrial electron transfer chain during the process of oxidative phosphorylation, upon pathologically increased mitochondrial workload, but also under physiological conditions. Besides these two major sources, ROS are generated as a result of environmental factors by other redox cycling enzymes (1).
Ion Channels
Tight ion homeostasis is critical for cellular functions such as proliferation, migration, cytokine secretion, and apoptosis. Calcium ions, for example, are essential second messengers involved in regulation of many signaling pathways. Perturbations in cellular ion levels usually have deleterious effects. In the past two decades, it has become increasingly apparent that redox signaling and ion transport mechanisms are tightly connected and control each other [for details, see Bogeski et al. (1)]. Increasing number of studies are published reporting redox regulation of ion channels and transporters and vice versa ion control of ROS-producing enzymes and processes. The most prominent in this regard certainly are calcium ions.
The Ca2+ signaling community witnessed two groundbreaking discoveries within the past decade. First, the identity of the elusive Ca2+ release-activated Ca2+ (CRAC) channels was revealed in 2006. The newly identified channels were named Orai1, Orai2, and Orai3. Second, in 2011, the mitochondrial Ca2+ uniporter (MCU) responsible for uptake of Ca2+ across the inner mitochondrial membrane was cloned. Although discovered relatively recently, several studies already indicate that Orai channels and MCU not only control ROS-generating enzymes and processes but are also regulated by redox modifications. Accordingly, this Forum contains one review article discussing the redox control of store-operated Ca2+ channels (Orai) (3) and two articles discussing the redox regulation of mitochondrial Ca2+ homeostasis (4, 5). In addition, within this Forum, selected experts in the field also describe redox regulation of transient receptor potential (TRP) channels (2), voltage-gated Ca2+ channels (9), voltage-gated K+ channels (7), purinergic P2X receptors (8), and inositol trisphosphate/ryanodine receptors (Fig. 1) (6).
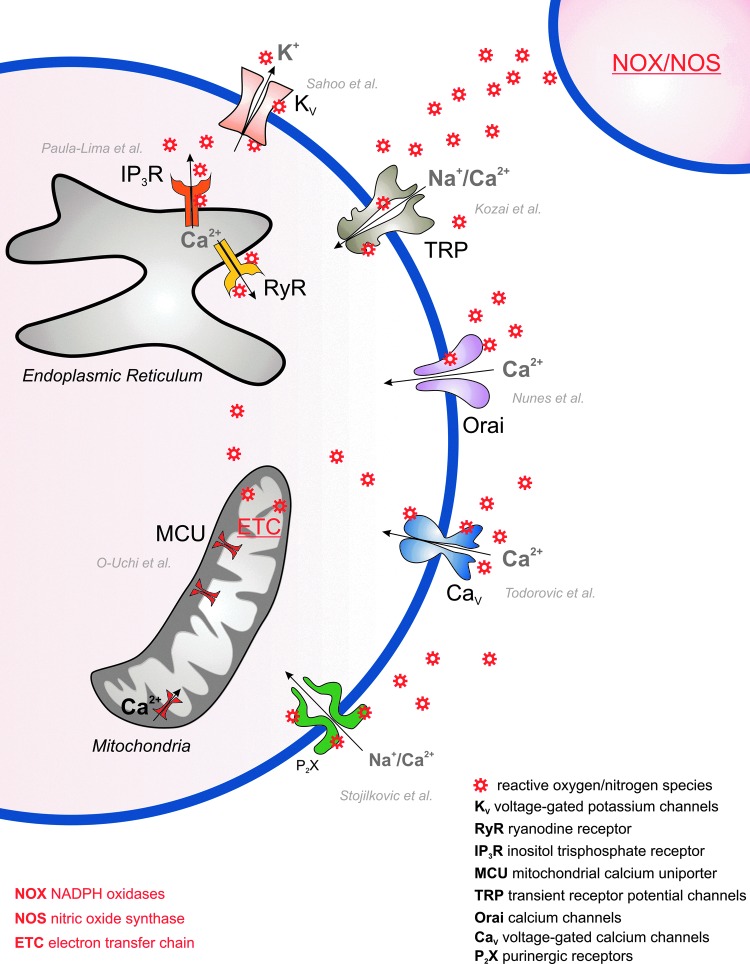
FIG. 1.
Redox regulation of ion channels and transporters. Transient receptor potential (TRP) channels, Orai channels, voltage-gated Ca2+ channels, P2X channels, and voltage-gated K+ channels are all expressed at the plasma membrane of cells and regulated by reactive oxygen species and reactive nitrogen species (ROS/RNS). IP3R/RyR at the endoplasmic reticulum and mitochondrial Ca2+ uniporter (MCU) in the mitochondria are also redox regulated. Major endogenous sources of ROS/RNS are the NADPH oxidases (NOX), nitric oxide synthase (NOS), and the mitochondrial electron transfer chain (ETC). This Forum comprises seven review articles and one original article describing the mechanisms and the functional outcome of redox regulation of these ion channels and transporters. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Orai Channels
The Orai channel family consists of three Orai isoforms: Orai1 as the CRAC channel pore-forming unit, Orai2, and Orai3. These channels are known to be activated by STIM1 and STIM2, Ca2+-sensing proteins within the endoplasmic reticulum (ER), which oligomerize and cluster close to the plasma membrane upon ER Ca2+ store depletion. Nunez and Demaurex elegantly summarize recent studies on the effects of oxidative modification of cysteine residues within Orai channels as well as in STIM1 (3). Moreover, they also discuss the importance of understanding the molecular mechanism of redox regulation of the store-operated Ca2+ entry, to fully decipher the functional implications of the cross talk between redox and Ca2+ signaling.
Mitochondrial Ca2+ Uniporter
The connection between mitochondrial calcium and redox signaling has already been established in the past (1). Many studies have shown that changes in mitochondrial Ca2+ transport across the inner mitochondrial membrane can cause changes in ROS production during oxidative phosphorylation and vice versa that mitochondrial ROS can affect mitochondrial Ca2+ homeostasis. It has, however, been impossible to evaluate the role of particular molecular players in this cross talk until very recently. The reason was the unknown molecular identity of the MCU. Since 2011, however, the identity of the pore forming unit and of other molecular components of the so-called mitochondrial Ca2+ uniplex has been revealed. In one review and one original article, O-Uchi (4, 5) and her colleagues summarize the present knowledge on mitochondrial redox and Ca2+ signaling and their cross talk and uncover novel posttranslational modifications of the MCU. In both articles, the authors indicate the importance of revealing the molecular mechanisms for redox-mediated regulation of mitochondrial ion channels to develop novel therapies.
TRP Channels
TRP family of ion channels is subdivided into seven subgroups (TRPC, TRPM, TRPV, TRPA, TRPN, TRPP, and TRPML). TRP channels are cation permeable and are involved in cell death, chemokine production, pain transduction, and many other functions. Their activating mechanisms include not only direct detection of temperature and/or chemical compounds, extra/intracellular ion concentrations but also detection of second messengers. The expression pattern of TRP channels ranges from ubiquitous to cell-type specific. In their article, Kozai et al. (2) describe how TRP channels sense reactive species and dissect the molecular mechanisms of TRP redox regulation specifically focusing on TRPM2, TRPM7, TRPC5, TRPV1, and TRPA1 channels.
Voltage-Gated Ca2+ Channels
The voltage-gated Ca2+ channel (CaV) family consists of five major subgroups known as L-type (CaV 1.1–1.4), N-type (CaV2.2), P/Q-type (CaV 2.1), R-type (CaV2.3), and T-type (CaV3.1–3.3) Ca2+ channels. These channels are expressed in many cell types and govern cellular functions such as muscle contraction, secretion, and gene expression. CaV channels were one of the first Ca2+ channels to be identified as redox sensitive. In their article (9), Todorovic and Jevtovic-Todorovic describe how both T-type and high-voltage-activated voltage-gated calcium channel subtypes can be redox regulated and explain the implications of CaV channel redox regulation in the development of pain and thalamic oscillations.
Potassium Channels
Voltage-gated K+ channels are a large family of ion channels important for sensing and controlling membrane potential. They are ubiquitously expressed and are hence involved in controlling many vital cellular processes. Sahoo et al. (7) nicely summarize the past findings from not only their laboratories, but also from laboratories of other researchers, in describing how reversible oxidation of K+ channels may play a role in different pathologies. The authors also conclude that the in vivo data concerning redox regulation of K+ channels are almost not existent and that such approaches may reveal many new clues in the treatment of neurodegenerative diseases, for example.
P2X Receptors
As Stojilkovic et al. clearly show (8), the purinergic P2X receptors are part of a superfamily of channels regulated by extracellular ATP. These receptors are ubiquitously expressed and thus regulate a variety of functions in different cells such as muscle, blood, neural, and immune cells. In their review article, the authors summarize the present knowledge on not only redox regulation of these receptors but also indicate the need of further studies to enable a deeper understanding of molecular mechanisms by which P2X receptors control cellular function.
IP3 and Ryanodine Receptors
The physiological roles of these intracellular receptors have been a subject of many studies in the past. IP3R and RyR have also been one of the first ion channels described to be regulated by redox modifications. Accordingly, several reactive cysteine residues have been identified and shown to control receptor function in a redox-dependent manner. In their article, Paula-Lima (6) et al. briefly summarize the present knowledge and then examine the contribution of oxidative modifications of IP3Rs and RyRs in hippocampal spatial memory. Furthermore, the authors point out the need for additional studies to address if and how oxidative modifications contribute to perturbed memory processes during aging and Alzheimer's disease.
Abbreviation Used
- CRAC
Ca2+ release-activated Ca2+
- ER
endoplasmic reticulum
- ETC
electron transfer chain
- MCU
mitochondrial Ca2+ uniporter
- NOS
nitric oxide synthase
- NOX
NADPH oxidases
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- TRP
transient receptor potential
Acknowledgments
I.B. acknowledges financial support by the German Research Foundation (DFG) SFB1027 project C4 and project BO3643/3-1 and by the School of Medicine, University of Saarland, Homburg HOMFOR_excellent program. B.N. acknowledges DFG grants: SFB1027 project C4 and SFB894 project A2. Both editors thank the authors of the Forum articles for their excellent contributions.
References
- 1.Bogeski I, Kappl R, Kummerow C, Gulaboski R, Hoth M, and Niemeyer BA. Redox regulation of calcium ion channels: chemical and physiological aspects. Cell Calcium 50: 407–423, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Kozai D, Ogawa N, and Mori Y. Redox regulation of transient receptor potential channels. Antioxid Redox Signal 21: 971–986, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Nunes P, Demaurex N. Redox regulation of store-operated Ca2+ entry. Antioxid Redox Signal 21: 915–932, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O-Uchi J, Jhun BS, Xu S, Hurst S, Raffaello A, Liu X, Yi B, Zhang H, Gross P, Mishra J, Ainbinder A, Kettlewell S, Smith GL, Dirksen RT, Wang W, Rizzuto R, and Sheu S-S. Adrenergic signaling regulates mitochondrial Ca2+ uptake through Pyk2-dependent tyrosine phosphorylation of the mitochondrial Ca2+ uniporter. Antioxid Redox Signal 21:863–879, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O-Uchi J, Ryu S-Y, Jhun BS, Hurst S, and Sheu S-S. Mitochondrial ion channels/transporters as sensors and regulators of cellular redox signaling. Antioxid Redox Signal 21: 987–1006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paula-Lima AC, Adasme T, and Hidalgo C. Contribution of Ca2+ release channels to hippocampal synaptic plasticity and spatial memory: potential redox modulation. Antioxid Redox Signal 21: 892–914, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Sahoo N, Hoshi T, and Heinemann SH. Oxidative modulation of voltage-gated potassium channels. Antioxid Redox Signal 21: 933–952, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stojilkovic SS, Leiva-Salcedo E, Rokic MB, and Coddou C. Regulation of ATP-gated P2X channels: from redox signaling to interactions with other proteins. Antioxid Redox Signal 21: 953–970, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todorovic SM. and Jevtovic-Todorovic V. Redox regulation of neuronal voltage-gated calcium channels. Antioxid Redox Signal 21: 892–914, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]