Abstract
Objective
To assess whether acute findings of cerebral arteriopathy, large infarct, and acutely elevated plasma D-dimer levels are independently prognostic of poor long-term neurologic outcome as measured at ≥1 year in children with arterial ischemic stroke (AIS).
Study design
Sixty-one patients with childhood-onset (i.e., >28 days of life) AIS were enrolled in a single-institution cohort study at Children’s Hospital Colorado between February 2006 and June 2011. Data on demographic and diagnostic characteristics, antithrombotic treatments, and outcomes were systematically collected.
Results
Cerebral arteriopathy and D-dimer levels >500 ng/mL (a measure of coagulation activation) were identified acutely in 41% and 31% of the cohort, respectively. Anticoagulation was administered in the acute, sub-acute, and chronic periods post-event in 40%, 43%, and 28% of children. When not receiving anticoagulation, patients were routinely treated with aspirin 2–5 mg/kg once-daily for a minimum of one year. Death, major bleeding (including intracranial hemorrhage [ICH]), and recurrent AIS were infrequent. Pediatric Stroke Outcome Measure at one year demonstrated poor outcome in 54%. Acute cerebral arteriopathy and elevated D-dimer were identified as putative prognostic factors for poor outcome; after adjustment for D-dimer, arteriopathy was an independent prognostic indicator (OR=19.0, 95%CI=1.6–229.8; P=0.02).
Conclusions
Arteriopathy and coagulation activation are highly prevalent acutely in childhood AIS. Although recurrent AIS and ICH were infrequent in our cohort, one-half of children experienced a poor neurologic outcome at one year, the risk for which was increased by acute arteriopathy. Substantiation of these findings in multi-institutional cohort studies is warranted, toward prognostic stratification in childhood-onset AIS.
Arterial ischemic stroke (AIS) is rare in children, with an incidence of approximately 1–2 per 100,000 individuals per year in childhood1–3.It is a serious disorder, with neuroromotor deficits evident in 70% of children, both acutely4;5 and in long-term follow-up.6–8 In addition, recurrence is common, with a cumulative probability of recurrent stroke of 6–15% at approximately one year.1;9 Numerous causal and contributing factors have been identified in childhood-onset AIS, including thrombophilia (ie, hypercoagulability), acute infection, congenital cardiac disease or cardiac catheterization, cerebral/cervical arterial arteriopathy, cerebral angiitis, and other/idiopathic cerebral arteriopathies.7;10–21 Interestingly, preliminary data indicate that plasma D-dimer concentration—a marker of coagulation activation—is often elevated acutely and varies with etiologic subtype in childhood-onset AIS.22 Recently, retrospective and mixed prospective-retrospective cohort studies have shown an important association of cerebral arteriopathy (especially its persistence/progression) with recurrent AIS in childhood.1;23 In addition, cerebral arteriopathy has been identified in the International Pediatric Stroke Study as a prognostic factor for death or neurologic deficit at hospital discharge.5
Whether acute cerebral arteriopathy or elevated D-dimer levels are prognostic of long-term neurologic outcomes remains unknown. Accordingly, we investigated the importance of these and other characteristics in children with AIS in a single-institution mixed cohort study. We hypothesized that acute findings of cerebral arteriopathy, large infarct, and acutely elevated plasma D-dimer levels are independently prognostic of poor long-term neurologic outcome as measured at ≥1 year in children with AIS.
METHODS
Risk factors, treatments, and long-term outcomes were systematically assessed for children diagnosed with AIS (>28 days of age at symptom onset) between April 1, 1999 and June 20, 2011 and followed in the multidisciplinary Stroke Clinic at Children’s Hospital Colorado. All patients were invited to participate in a local prospective cohort study designed to capture and analyze these clinically-derived data (COMIRB #05-0339). For patients diagnosed with acute AIS prior to February 28, 2006 (n=15), data were retrospectively collected prior to this date and prospectively ascertained from this date forward. Signed informed consent was required for study participation.
Diagnostic criteria for acute pediatric AIS consisted of sudden onset of focal neurologic deficit with objective confirmation of arterial-distribution ischemia/infarct by computed tomography (CT) or magnetic resonance imaging (MRI). In the Stroke Clinic, a detailed clinical history and exam as well as follow-up neurovascular imaging were obtained at three-to-six months and one year post-event. Imaging studies were reviewed independently by a pediatric radiologist (L.Z.F. or N.V.S.) and a pediatric neurologist (T.J.B.). In the case of disagreement in radiographic classification between the two primary reviewers, final decision was achieved and recorded via consensus at a multidisciplinary Pediatric Neuroradiology Stroke Conference. Patients were classified according to the Childhood AIS Standardized Classification and Diagnostic Evaluation (CASCADE) criteria.24 Presence of acute arteriopathy was defined by angiographic findings within one week of AIS symptom onset. Infarct size was measured within the clinical software for CT and MRI imaging using axial views, and reported as < versus ≥ ⅓ of the MCA territory. Additional diagnostic evaluation included trans-thoracic echocardiography with peripheral intravenous saline microbubble injection and comprehensive thrombophilia testing (as previously described)25. These were performed during acute hospitalization or the first Stroke Clinic visit.
Acute D-dimer levels were defined as the results of first testing within 24 hours of AIS symptom onset. Abnormal thrombophilia results (other than DNA-based analyses) were followed via serial testing, typically at six weeks, three-to-six months, one year, and then annually. Genetic thrombophilias consisted of one or more of the following: factor V Leiden polymorphism; prothrombin G20210A polymorphism; elevated plasma lipoprotein(a) concentration confirmed at least 6 weeks after acute event and in the absence of recent infection; plasma protein C activity consistently <40% after 6 months of age; plasmas free protein S antigen levels consistently <40%; plasma antithrombin activity consistently <60% after 6 months of age. Transient deficiencies of protein C activity, free protein S antigen, or antithrombin activity, and transient elevation of lipoprotein(a), were considered acquired. Other acquired thrombophilias included antiphospholipid antibodies and elevated plasma FVIII activity (>191 U/dL). Acquired thrombophilias were further classified as acute if the abnormal results was obtained up to 48 hours before or 1 week after stroke date but resolved within 12 weeks on subsequent testing. Those acquired abnormalities persisting at 12 weeks were categorized as chronic.
Antithrombotic therapies were defined as therapeutic anticoagulation or anti-platelet therapy, with/without preceding thrombolytic modalities. Decisions regarding antithrombotic strategies and durations were made by the treating clinicians. Antithrombotic therapy periods were categorized as acute (first week post-event), sub-acute (1 week – 3 months post-event), and chronic (> 3 months post-event). Acute therapeutic anticoagulation, when employed, consisted of either unfractionated heparin by continuous intravenous infusion (adjusted to achieve a target anti-factor Xa activity of 0.3–0.7 U/mL) or low molecular weight heparin (LMWH) given subcutaneously twice-daily (adjusted to achieve a target anti-factor Xa activity of 0.5–1.0 U/mL at 4 hours post-dose). In cases of continuation of anticoagulation into the sub-acute and/or chronic periods, LMWH was dosed as above, or warfarin was administered by mouth once daily and adjusted to maintain an international normalized ratio of 2.0–3.0. Clopidogrel was used in conjunction with LMWH in the rare setting of severe carotid dissection requiring stents, or as an alternative to anticoagulation for recurrence of AIS despite aspirin therapy.
Major bleeding complications of antithrombotic therapy were defined by one or more of the following criteria: (1) symptomatic central nervous system (CNS) hemorrhage confirmed by objective neuroimaging; (2) documented decline in hemoglobin by 2 g/dL in a 24 hour period, in the absence of a defined non-hemorrhagic etiology; and (3) hemorrhage requiring surgical intervention to restore hemostasis. Symptomatic CNS hemorrhage was ascertained by CT findings of hemorrhage in the setting of new neurologic signs or symptoms; this evaluation was clinically-driven and its findings captured in the study. Neurologic outcome was determined by the presence of any neuromotor deficit at one year, as determined by a treating neurologist (T.J.B or J.A.W), and by the Pediatric Stroke Outcomes Measure (PSOM), version October 2003, format revised November 2005. The last PSOM examination performed at ≥1 year post-event was utilized for the PSOM finding of long-term neurologic outcome.
Originally developed by deVeber et al in the Canadian prospective cohort study of AIS and cerebral sinovenous thrombosis (CSVT),8 the PSOM has 115 items that measure neuromuscular and neurocognitive functioning in 4 domains: Sensorimotor Deficit, Language Deficit (Production), Language Deficit (Comprehension), and Cognitive or Behavioral Deficit. Administered at least one year following stroke, each child’s PSOM score was categorized as either a “good” or “poor” outcome, according to criteria used by deVeber et al in 2000.8 Specifically, a score of 0.5 (mild deficit with no impact on function) in more than one domain, or a score of 1 (moderate deficit with some functional limitations) or 2 (severe or profound deficit with missing function) in any domain, constituted a poor outcome.8
Statistical methods in the descriptive analyses involved calculation of proportions for categorical data and median values with ranges for continuous data. Proportions were compared between groups using a chi-square or Fisher’s exact testing, as appropriate. Logistic regression was employed to evaluate relationships between explanatory variables and PSOM outcome; multivariate models included variables for which potentially-significant (P<0.2) associations with outcome were determined in univariate analyses. Alpha was set at 0.05 for all other inferential statistics. All analyses were performed using SAS version 9.1 statistical software (SAS Institute, Cary, NC).
RESULTS
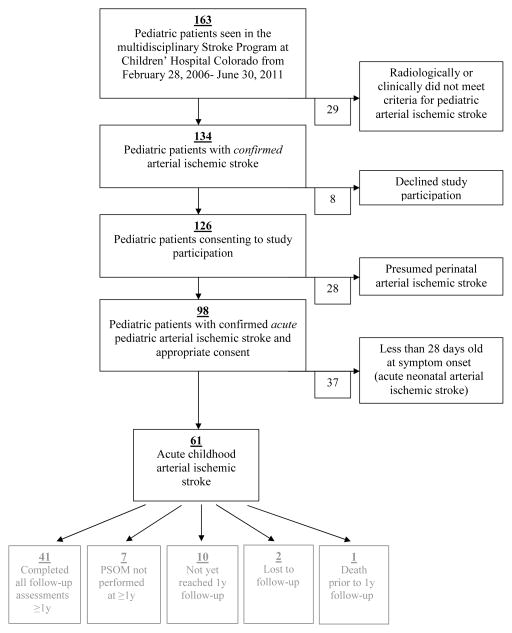
One hundred sixty-three children were evaluated in the multidisciplinary Stroke Clinic at Children’s Hospital Colorado during the 4.5-year study period. Of these, 155 gave informed consent for prospective observational study participation, among whom 61 met eligibility criteria for the cohort of acute childhood-onset AIS (Figure; available at www.jpeds.com). Demographic and clinical characteristics at AIS presentation are shown in Table I for the study population. Eight-seven percent of patients underwent non-invasive angiography (MRA or CTA) acutely.Stroke involved predominantly anterior circulation and, in particular, middle cerebral artery territories (the latter comprising 77% of cases). With regard to CASCADE subtypes, cardioembolism comprised 21% of the cohort, including nine cases related to a cardiac procedure (surgery/catheterization). Arteriopathy was classified in 41% of the cohort, approximately half of which consisted of unilateral large-vessel focal cerebral arteriopathy involving the anterior circulation.
Figure 1.
Cohort recruitment, inclusion and exclusion criteria, and outcome assessment.
Table 1. Demographic characteristics and risk factors in acute childhood-onset AIS.
Number of subjects affected is given in parentheses.
| Median age (range) | 8 y (1mo–19 yrs) |
| Sex % males | 49% (30/61) |
| CASCADE Subtype | |
| Cardioembolic 1,2 | 21% (13/61) |
| Arteriopathy3 | 41% (25/61) |
| Unilateral focal cerebral arteriopathy (anterior circulation)2 | 20% (12/61) |
| Bilateral cerebral arteriopathy (anterior circulation)2 | 10% (6/61) |
| Aortic/Cervical Arteriopathy2 | 11% (7/61) |
| Multifactorial | 2% (1/61) |
| Other4 | 31% (19/61) |
| Thrombophilia5 | 63% (32/51) |
| Genetic | 17% (6/35) |
| Acquired, acute | 28% (8/28) |
| Acquired, chronic | 6% (2/29) |
| APS | 14% (8/57) |
| Acute plasma D-dimer > 500 ng/mL6 | 31% (13/42) |
| Rheumatologic condition7 | 7% (4/61) |
| Chromosomal abnormality8 | 3% (2/61) |
| Congenital metabolic disorder9 | 3% (2/61) |
| Malignancy10 | 2% (1/61) |
Abbreviations: AIS=arterial ischemic stroke; APA= antiphospholipid antibodies; FVIII=factor VIII activity.
Includes hypoplastic left heart syndrome (n=3), hypoplastic left heart syndrome status-post cardiac transplantation (n=1), Ebstein’s anomaly (n=1), single ventricle (n=1), pulmonary artery stenosis (n=1), pulmonary atresia (n=1), Ivemark syndrome (n=1), idiopathic dilated cardiomyopathy status-post cardiac transplantation (n=1), dilated cardiomyopathy due to myocarditis (n=1), transposition of the great arteries and coarctation of the aorta (n=1), and transposition of the great arteries and ventricular septal defect (n=1).
Classified according to criteria of Bernard et al, 2011.24
Defined as arteriopathy visualized within 1 week of AIS onset. Patients with late- onset arteriopathy were excluded. Patients without acute imaging were not included in analysis.
Includes APA syndrome (n=3), elevated plasma lipoprotein(a) (n=1), factor V Leiden (n=2), familial migraine syndrome (n=2), head/neck trauma (n=2, one in the setting of a genetic protein C deficiency), acute factor VIII elevation (n=1), panhypopituitarism in the setting of factor V Leiden (n=1), pericarditis in the setting of an acute mycoplasma infection (n=1), and extracorporeal membrane oxygenation (ECMO) in the setting of acute respiratory distress syndrome (ARDS) secondary to Influenza Type A (n=1). Five strokes were without any risk factors.
Patients without complete genetic or acquired testing were excluded.
Values of 500 ng/mL from 2006–2008 were determined to be equivalent to values of 1.0 ug/mL from 2009–2011 by local laboratory standards, via cross-validation of more recent versus prior assay method for semiquantitative latex agglutination assay. Acute elevation was defined as a positive lab result obtained within 24 hours of stroke. See also Methods.
Includes systemic lupus erythematosus (n=2) and polyarteritis nodosa (n=2).
Includes trisomy 21 (n=1) and Turner’s syndrome (n=1).
Includes Alagille syndrome (n=2).
Includes juvenile pilocytic astrocytoma of the hypothalmus (n=1).
Thrombophilia was present in 63% of patients, with acquired acute thrombophilia being most common (28% overall). D-dimer was acutely elevated in 31% of the cohort. Genetic and persistent acquired thrombophilias (present for ≥ 3 months post-diagnosis) were identified in 17% and 6% of children, respectively. Fourteen percent of children with AIS met recently-proposed consensus criteria for antiphospholipid antibody syndrome (APS),26 with antiphospholipid antibodies detected both acutely and ≥12 weeks following initial testing. Two cases of APS were secondary to systemic lupus erythematosus; the remaining six cases were primary.
Antithrombotic Treatments
Antithrombotic therapy is summarized in Table II. All childhood-onset AIS patients received either anticoagulant or anti-platelet therapy. Anticoagulation was administered in the acute (first week), sub-acute (1 week – 3 months), and chronic (> 3 months) periods post-event in 40%, 43%, and 28% of cases, respectively. Of seventeen patients who received chronic - anticoagulation, six had APS, two had possible APS (had two positive tests less than 12 weeks apart), and two had suffered recurrent AIS/TIA while on aspirin. In the entire cohort, six children suffered their original stroke while on antiplatelet therapy, two while on anticoagulation, and one while on both. Of these nine children who failed initial antithrombotic therapy, six had a serious underlying cardiac condition.
Table 2.
Antithrombotic therapy, follow-up duration, and long-term outcomes of acute childhood-onset AIS.
| Childhood-onset AIS (n=61) | |
|---|---|
| Antithrombotic (AT) therapy | |
| Acute thrombolysis | |
| Systemic | 0% (0) |
| Intra-arterial | 2% (2) |
| Acute AT (0 – 7 d post-diagnosis) | |
| Anti-platelet | 42% (25/60) |
| Anticoagulant | 40% (24/60) |
| Both | 15% (9/60) |
| None | 3% (2/60) |
| Subacute AT (>7 d – < 3 m post-diagnosis) | |
| Anti-platelet | 40% (24/60) |
| Anticoagulant | 43% (26/60) |
| Both | 13% (8/60) |
| None | 3% (2/60) |
| Early chronic AT (3 m – 1 y post-diagnosis) | |
| Anti-platelet | 62% (37/60) |
| Anticoagulant | 23% (14/60) |
| Both | 8% (5/60) |
| None | 7% (4/60) |
| Late chronic AT (> 1 y post-diagnosis) | |
| Anti-platelet | 72% (42/58) |
| Anticoagulant | 7% (4/58) |
| Both | 9% (5/58) |
| None | 12% (7/58) |
| Surgical intervention1 | 26% (16/61) |
| Median follow-up duration | 25 m; range: 2–134 m |
| Cumulative probability of recurrent AIS at 1 y | 6% (3/53) |
| Mortality | 2% (1/61) |
| Major bleeding episodes on therapy2 | 3% (2/61) |
| Prevalence of neuromotor deficit at 1 y | 80% (44/55) |
| Median PSOM3 (scored at or beyond 1 y) | 1.5 (0–7) |
Abbreviations: AIS=arterial ischemic stroke, PSOM=Pediatric Stroke Outcome Measurement Number of subjects affected is given in parentheses.
Includes direct bypass (n=1), indirect bypass (n=5), craniotomy (n=3), closure of shunting cardiac lesion (n=3), stent placement (n=1), coil placement (n=1), and pseudoaneurysm repair via excision and direct anastomosis (n=1).
For definitions, see Methods.
n=41
Outcomes and Prognostic Factors
Median duration of follow-up was 25 months (Table II). Mortality was 2%, representing one child who died of complications from cardiac transplantation for complex cardiac disease. Two major bleeding episodes were observed on antithrombotic therapy. The first episode consisted of the development of chronic subdural hemorrhage in association with aspirin use in a child with congenital cardiac disease. This hemorrhagic complication was disclosed upon neuroimaging for papilledema, and did not require intervention. The second major bleed occurred in a patient with a left ventricular assist device (LVAD) for dilated cardiomyopathy. The patient was noted to have multiple splenic and renal infarcts and so was anticoagulated (acutely with heparin, and then transitioned to warfarin).
The risk (i.e., cumulative incidence) of recurrent AIS at one year in the cohort was 6%; three of these four events occurred within two months post-diagnosis. With regard to neurologic outcomes (Table II), 80% of patients had persistent motor impairment on standard neurologic examination at one year. Of the 41 patients who had a PSOM scored beyond one year, the average PSOM score was 1.5, indicating mild or moderate impairment in at least two domains or severe impairment in a single domain. Fifty-four percent of the 41 patients who underwent PSOM at or beyond one year had poor long-term neurologic outcome. In univariate analyses, acute arteriopathy and acute plasma D-dimer concentration >500 ng/mL each met a priori criteria as potential prognostic factors for inclusion in a multivariate model of poor long-term neurologic outcome, whereas infarct size (> vs. ≤ ⅓ MCA territory) was not a significant prognostic indicator (Table III). After adjustment for D-dimer, acute arteriopathy was independently prognostic of poor outcome (OR=19.0, 95%CI=1.6–229.8; P=0.02).
Table 3.
Predictors of good versus poor long-term neurological outcome in childhood AIS.
| Good outcome1 (n=19) | Poor outcome2 (n=22) | P-value | |
|---|---|---|---|
| Ethnicity | 1.0 | ||
| Hispanic (n=10) | 50% (5) | 50% (5) | |
| Non-Hispanic (n=31) | 45% (14) | 55% (17) | |
| Sex | 0.68 | ||
| Male (n=23) | 43% (10) | 57% (13) | |
| Female (n=18) | 50% (9) | 50% (9) | |
| Acute thrombophilia | 0.80 | ||
| Yes (n=22) | 45% (10) | 55% (12) | |
| No (n=12) | 50% (6) | 50% (6) | |
| Acute D-dimer | 0.13 | ||
| Elevated (n=8) | 25% (2) | 75% (6) | |
| Normal (n=19) | 58% (11) | 42% (8) | |
| Genetic thrombophilia | 0.60 | ||
| Yes (n=4) | 75% (3) | 25% (1) | |
| No (n=19) | 53% (10) | 47% (9) | |
| Acute Arteriopathy3 | 0.08 | ||
| Yes (n=20) | 30% (6) | 70% (14) | |
| No (n= 15) | 60% (9) | 40% (6) | |
| Infarct size >1/3 MCA | 0.50 | ||
| Yes (n=11) | 36% (4) | 64% (7) | |
| No (n=30) | 50% (15) | 50% (15) | |
| Median age | 8 y range: 3 m – 18 y |
7 y range: 1 – 17 y) |
0.62 |
| Median time from stroke to PSOM | 1 y range 1 – 5 y |
1 y range: 1 – 10 y |
0.87 |
For categorical variables, percentages are expressed as number of subjects in a given variable category with good versus poor outcome divided by the total number of subjects in that category. Number of patients affected is given in parentheses. Data in which group-wise comparisons approached statistical significance are in boldface type.
Defined as a PSOM score of 0 in all 5 domains or of 0.5 in only one domain.
Defined as a minimum PSOM score of 0.5 in more than one domain or of 1 or greater in any domain.
Defined as arteriopathy visualized within the 5 days of AIS onset.
DISCUSSION
In this study, cerebral arteriopathy was identified acutely in 41% of children, and acute plasma D-dimer concentration exceeded 500 ng/mL in 31%. Death, major bleeding, and recurrent AIS were infrequent. Although the one-year risk of recurrent AIS of 6% observed here was lower than the 15% in a large retrospective U.S. study,1 it was similar to the 6% previously reported in the German cohort.9 As in our cohort, antithrombotic therapy (and often anticoagulation) was systematically employed in the German study, highlighting the potential importance of addressing differences in antithrombotic therapy when comparing outcomes across studies. Rates of mortality and poor neurologic outcome determined in the present cohort are in line with previous studies6–8, with poor long-term neurologic outcome observed in 54% of children by PSOM, and persistent motor deficits noted beyond one year in 80% of patients. Cerebral arteriopathy and elevated D-dimer were identified here as putative prognostic factors for poor long-term neurologic outcome; after adjustment for D-dimer, arteriopathy was independently prognostic.
This work provides important and unique cohort data on prognostic factors of long-term outcome in childhood-onset AIS, which expand upon prior knowledge in the field. The high prevalence of cerebral arteriopathy in childhood AIS observed in the present work is consistent with recent prior analyses1;6;15. Approximately half of arteriopathy cases were due to unilateral focal large-vessel cerebral arteriopathy, a value consistent with data from the International Pediatric Stroke Study (IPSS) population30. In the past several years, a few cohort studies have identified arteriopathy as an important risk factor for recurrent AIS1,8, and the IPSS study implicated arteriopathy as a predictor of neurologic deficit at hospital discharge, among children with acute AIS. The present work builds upon the prognostic importance of arteriopathy, by uniquely demonstrating acute arteriopathy as a significant independent risk factor for persistent neurologic sequelae at ≥ 1 year following AIS.
The observation that thrombophilia is quite common in childhood-onset AIS confirms findings of prior registry and cohort analyses in pediatric AIS.10–12 Our data demonstrate that acquired thrombophilia is more common than genetic thrombophilia, especially in the acute period. Registries and cohort studies of pediatric AIS have not always distinguished between genetic and acquired thrombophilias, and have not reported acquired thrombophilias according to chronicity. Taken together, our findings support the importance of thrombophilia (both genetic and acquired) as a contributing factor to the development of AIS in childhood. The determination of APS in 14% of childhood-onset AIS patients in this study is a thrombophilia finding worthy of particular emphasis. Atlhough several prior studies have identified the presence of antiphospholipid antibodies in childhood AIS6;10–12;15;27, none to date has systematically collected data on APS. Given that some studies have suggested an increased risk of recurrent thromboembolic events among both adults and children with APS28,29, future work should place emphasis upon evaluation of recurrence risk relative to APS and antithrombotic therapy regimen in childhood-onset AIS.
In addition, markers of acute coagulation activation, such as D-dimer, continue to be attractive candidate biomarkers for prognostic stratification in childhood-onset AIS. Previous work has demonstrated that D-dimer elevation is subtype specific and often seen in the acute phase of this disease.22 Even though acutely elevated D-dimer was not independently prognostic of long-term neurologic outcome after adjustment for arteriopathy in this study, the utility of D-dimer (and other markers of coagulation activation) nevertheless merits attention in multicenter cohorts with larger sample size. The lack of independent significance of D-dimer after adjustment for arteriopathy suggests that acutely elevated D-dimer values may be highly correlated with the presence of acute arteriopathy. It is thought that many cases of cerebral arteriopathy may be related to infectious/inflammatory triggers (such as the classic varicella arteriopathy)21;30, in which ongoing coagulation activation and inflammation could potentiate neuronal damage, leading to a greater risk of neurologic impairment than in non-arteriopathic cases of equivalent infarct size. Alternatively, compromised arterial blood supply to at-risk brain tissue (i.e., ischemic penumbra) in the setting of cerebral arterial stenosis may potentiate damage and its chronic neurologic sequelae. In this manner, D-dimer may serve as a biomarker for the evolution of the arteriopathic process, and/or potentiate its risk for resultant brain injury. Whether D-dimer is also prognostic of recurrent AIS and hence has implications for duration antithrombotic therapies in childhood-onset AIS (as has been seen in the field of venous thromboembolism) remains unknown.
Several limitations of the present work are noteworthy. First, the principal neurologic outcome in this study (PSOM score < vs. ≥0.5), although consistent with that of the prior study of de Veber et al8, is quite sensitive in that the presence of any deficit representing “more than minimal abnormality” is defined as an unfavorable outcome. Whether other outcome measures such as the modified Rankin score should be used instead or in conjunction with the PSOM is an important issue for future studies. Second, with respect to prognostic factors of interest, acute diagnostic radiological evaluation for arteriopathy (e.g. MRA of the brain and neck with brain MRI, rather than brain MRI alone) and acute testing of D-dimer were not clinically obtained in all children, despite a standardized clinical care pathway for childhood AIS. Although this could theoretically have influenced the findings, a systematic bias was unlikely given that the degree of missing data did not vary by such categories as age, sex, or cardioembolic subtype. It should also be noted that our cutoff for acute D-dimer evaluation in the present analysis was quite stringent, at 24 hours, given the very short half-life of this marker; however, future studies may elect to employ a longer time window, such as 72 hours. Third, the CASCADE criteria employed to define childhood AIS subgroups, while serving as a recent international standard, is based upon anatomic classification rather than pathophysiological mechanism; it is possible that with other methods for distinguishing among the arteriopathies, additional prognostic insights will be gained. Perhaps most important among the limitations of this study is its relatively small sample size, such that the present findings should be recognized as preliminary.
Given the aforementioned limitations, and given also the high prevalence of persistent neurologic deficits in childhood-onset AIS, larger cooperative cohort studies should be undertaken to substantiate the present findings and to more robustly evaluate additional putative prognostic factors, such as stroke size and the presence of underlying cardiac disease. In addition, further investigation is needed on the mechanisms of vasculopathy and its role in the extent of brain injury. Such work may lead to the discovery of novel neuroprotective strategies in childhood AIS.
Acknowledgments
Supported by the National Institutes of Health, National Heart Lung and Blood Institute, (1K23HL084055 [to N.G.] and 1K23HL096895 [to T.B.]) and Centers for Disease Control and Prevention (UR6/CCU820552 to M.M.-J.).
The authors thank the patients and their families for generously participating in this research, and Steven Daniels, M.D., Ph.D. for departmental support and vision toward development of an institutional pediatric stroke program.
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 2.Nelson KB, Lynch JK. Stroke in newborn infants. Lancet Neurol. 2004;3:150–158. doi: 10.1016/S1474-4422(04)00679-9. [DOI] [PubMed] [Google Scholar]
- 3.Schoenberg BS, Mellinger JF, Schoenberg DG. Cerebrovascular disease in infants and children: a study of incidence, clinical features, and survival. Neurology. 1978;28:763–768. doi: 10.1212/wnl.28.8.763. [DOI] [PubMed] [Google Scholar]
- 4.Goldenberg NA, Bernard TJ, Gordon A, Fullerton H, DeVeber G. Acute treatments and early outcomes of childhood arterial ischemic stroke: first analysis of the international pediatric stroke study. Blood. 2008;112:1978A. [Google Scholar]
- 5.Goldenberg NA, Bernard TJ, Fullerton HJ, Gordon A, DeVeber G. Antithrombotic treatments, outcomes, and prognostic factors in acute childhood-onset arterial ischaemic stroke: a multicentre, observational, cohort study. Lancet Neurol. 2009;8:1120–1127. doi: 10.1016/S1474-4422(09)70241-8. [DOI] [PubMed] [Google Scholar]
- 6.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. J Child Neurol. 2000;15:290–294. doi: 10.1177/088307380001500504. [DOI] [PubMed] [Google Scholar]
- 7.Steinlin M, Pfister I, Pavlovic J, Everts R, Boltshauser E, Capone MA, Gubser MD, Hanggeli CA, Keller E, Luetschg J, Marcoz J, Ramelli GP, Roulet PE, Schmitt-Mechelke T, Weissert M. The first three years of the Swiss Neuropaediatric Stroke Registry (SNPSR): a population-based study of incidence, symptoms and risk factors. Neuropediatrics. 2005;36:90–97. doi: 10.1055/s-2005-837658. [DOI] [PubMed] [Google Scholar]
- 8.deVeber GA, MacGregor D, Curtis R, Mayank S. Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol. 2000;15:316–324. doi: 10.1177/088307380001500508. [DOI] [PubMed] [Google Scholar]
- 9.Strater R, Becker S, von Eckardstein A, Heinecke A, Gutsche S, Junker R, Kurnik K, Schobess R, Nowak-Gottl U. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002;360:1540–1545. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 10.Kenet G, Sadetzki S, Murad H, Martinowitz U, Rosenberg N, Gitel S, Rechavi G, Inbal A. Factor V Leiden and antiphospholipid antibodies are significant risk factors for ischemic stroke in children. Stroke. 2000;31:1283–1288. doi: 10.1161/01.str.31.6.1283. [DOI] [PubMed] [Google Scholar]
- 11.Nowak-Gottl U, Strater R, Heinecke A, Junker R, Koch HG, Schuierer G, von Eckardstein A. Lipoprotein (a) and genetic polymorphisms of clotting factor V, prothrombin, and methylenetetrahydrofolate reductase are risk factors of spontaneous ischemic stroke in childhood. Blood. 1999;94:3678–3682. [PubMed] [Google Scholar]
- 12.Strater R, Vielhaber H, Kassenbohmer R, von Kries R, Gobel U, Nowak-Gottl U. Genetic risk factors of thrombophilia in ischaemic childhood stroke of cardiac origin. A prospective ESPED survey. Eur J Pediatr. 1999;158 (Suppl 3):S122–S125. doi: 10.1007/pl00014336. [DOI] [PubMed] [Google Scholar]
- 13.Kieslich M, Fiedler A, Heller C, Kreuz W, Jacobi G. Minor head injury as cause and cofactor in the aetiology of stroke in childhood: a report of eight cases. J Neurol Neurosurg Psychiatry. 2002;73:13–16. doi: 10.1136/jnnp.73.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chabrier S, Lasjaunias P, Husson B, Landrieu P, Tardieu M. Ischaemic stroke from dissection of the craniocervical arteries in childhood: report of 12 patients. Eur J Paediatr Neurol. 2003;7:39–42. doi: 10.1016/s1090-3798(02)00138-1. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 16.Shaffer L, Rich PM, Pohl KR, Ganesan V. Can mild head injury cause ischaemic stroke? Arch Dis Child. 2003;88:267–269. doi: 10.1136/adc.88.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sebire G, Fullerton H, Riou E, DeVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–622. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 18.Haywood S, Liesner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90:402–405. doi: 10.1136/adc.2004.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Ann Neurol. 2006;59:620–626. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 20.Kirton A, DeVeber G. Cerebral palsy secondary to perinatal ischemic stroke. Clin Perinatol. 2006;33:367–386. doi: 10.1016/j.clp.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Askalan R, Laughlin S, Mayank S, Chan A, MacGregor D, Andrew M, Curtis R, Meaney B, DeVeber G. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–1262. doi: 10.1161/01.str.32.6.1257. [DOI] [PubMed] [Google Scholar]
- 22.Bernard TJ, Fenton LZ, Apkon SD, Boada R, Wilkening GN, Wilkinson CC, Soep JB, Miyamoto SD, Tripputi M, Armstrong-Wells J, Benke TA, Manco-Johnson MJ, Goldenberg NA. Biomarkers of hypercoagulability and inflammation in childhood-onset arterial ischemic stroke. J Pediatr. 2010;156:651–656. doi: 10.1016/j.jpeds.2009.10.034. [DOI] [PubMed] [Google Scholar]
- 23.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 24.Bernard T, Manco-Johnson M, Lo EH, Mackay MT, Ganesan V, DeVeber G, Goldenberg NA, Armstrong-Wells J, Dowling M, Roach ES, Tripputi M, Fullerton H, Furie K, Benseler SM, Jordan LC, Kirton A, Ichord R. Towards a Consensus-based Classification of Childhood Arterial Ischemic Stroke. Stroke. 2011 doi: 10.1161/STROKEAHA.111.624585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manco-Johnson MJ, Grabowski EF, Hellgreen M, Kemahli AS, Massicotte MP, Muntean W, Peters M, Nowak-Gottl U. Laboratory testing for thrombophilia in pediatric patients. On behalf of the Subcommittee for Perinatal and Pediatric Thrombosis of the Scientific and Standardization Committee of the International Society of Thrombosis and Haemostasis (ISTH) Thromb Haemost. 2002;88:155–156. [PubMed] [Google Scholar]
- 26.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, Derksen RH, DE Groot PG, Koike T, Meroni PL, Reber G, Shoenfeld Y, Tincani A, Vlachoyiannopoulos PG, Krilis SA. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 27.Lanthier S, Kirkham FJ, Mitchell LG, Laxer RM, Atenafu E, Male C, Prengler M, Domi T, Chan AK, Liesner R, DeVeber G. Increased anticardiolipin antibody IgG titers do not predict recurrent stroke or TIA in children. Neurology. 2004;62:194–200. doi: 10.1212/wnl.62.2.194. [DOI] [PubMed] [Google Scholar]
- 28.Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med. 1995;332:993–997. doi: 10.1056/NEJM199504133321504. [DOI] [PubMed] [Google Scholar]
- 29.Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, Laskin C, Fortin P, Anderson D, Kearon C, Clarke A, Geerts W, Forgie M, Green D, Costantini L, Yacura W, Wilson S, Gent M, Kovacs MJ. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med. 2003;349:1133–1138. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 30.Amlie-Lefond C, Bernard TJ, Sebire G, Friedman NR, Heyer GL, Lerner NB, DeVeber G, Fullerton HJ. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]