Abstract
Cardio-facio-cutaneous syndrome (CFC) is a RASopathy that is characterized by craniofacial, dermatologic, gastrointestinal, ocular, cardiac, and neurologic anomalies. CFC is caused by activating mutations in the Ras/mitogen-activated protein kinase (MAPK) signaling pathway that lies downstream of receptor tyrosine kinase (RTK) signaling. RTK signaling is known to play a central role in craniofacial and dental development, but to date, no studies have systematically examined individuals with CFC to define key craniofacial and dental features. To fill this critical gap in our knowledge, we evaluated the craniofacial and dental phenotype of a large cohort (n=32) of CFC individuals who attended the 2009 and 2011 CFC International Family Conferences. We determined that the craniofacial features common in CFC include macrocephaly, bitemporal narrowing, convex facial profile, and hypoplastic supraorbital ridges. In addition, there is a characteristic dental phenotype in CFC syndrome that includes malocclusion with open bite, posterior crossbite, dental crowding, and a high-arched palate. This thorough evaluation of the craniofacial and dental phenotype in CFC individuals provides a step forward in our understanding of the role of RTK/MAPK signaling in human craniofacial development and will aid clinicians who treat patients with CFC.
Keywords: Cardio-facio-cutaneous syndrome, CFC, craniofacial development, malocclusion, MAPK pathway, occlusion, Ras, RASopathy, receptor tyrosine kinase, signal transduction, tooth development
INTRODUCTION
Cardio-facio-cutaneous syndrome (CFC) is a multiple congenital anomaly disorder characterized by craniofacial malformation, ectodermal abnormalities, congenital heart defects, growth delays, and neurocognitive deficits. CFC is one of the RASopathies, which also include neurofibromatosis type 1 (NF1), Noonan syndrome (NS), NS with multiple lentigines, capillary malformation-AV malformation syndrome, Legius syndrome, and Costello syndrome (CS). The common feature of the RASopathies is that they are caused by germline mutations that result in dysregulation of the Ras/mitogen-activated protein kinase (MAPK) pathway (1). CFC is caused by heterozygous, activating germline mutations in KRAS, BRAF, MAP2K1 (MEK1), or MAP2K2 (MEK2), all of which are components of the Ras/MAPK pathway (2, 3).
Many of the phenotypic features in these syndromes overlap, and the craniofacial phenotypes of several of the RASopathies are in fact so similar that making a definitive syndromic diagnosis can prove difficult. Careful examination of the craniofacial characteristics is critical in order to formulate an accurate diagnostic plan prior to molecular testing. However, to date, a systematic analysis of the craniofacial characteristics of each of the RASopathies in a large cohort is lacking, and such analyses will be essential for identification of unique craniofacial characteristics that may serve as useful diagnostic markers and guide genetic testing.
Receptor tyrosine kinase (RTK) signaling upstream of the Ras/MAPK pathway is known to play a central role in craniofacial and dental development. Fibroblast growth factors (Fgfs), which initiate signaling through RTKs, are involved in the interactions between epithelium and mesenchyme that guide development of almost all structures of the craniofacial complex, including teeth (4, 5). In addition, mice carrying mutations in Sprouty genes, which encode proteins that negatively regulate RTK and Ras/MAPK signaling, have anomalies in both tooth number and morphology (6-8).
Considering the central role that RTK signaling plays in craniofacial development and the value of a detailed characterization of the craniofacial and dental phenotypes present in the different RASopathies, we sought to thoroughly examine the phenotypic features in individuals with CFC. Although previous studies have noted the major craniofacial features in CFC, no studies have systematically characterized both the craniofacial and dental phenotypic features present in CFC in a large cohort of subjects. To fill this critical gap in our knowledge and provide new insight into the effects of activated RTK/MAPK signaling in craniofacial and tooth development, we performed comprehensive craniofacial and dental exams on 32 CFC individuals.
MATERIALS AND METHODS
This study was approved by the UCSF Committee on Human Research. A total of 32 individuals with a clinical diagnosis of CFC were examined during the 5th International CFC Family Conference in Berkeley, California in 2009 (9) and the 6th CFC International Family Conference in Chicago, IL in 2011. The diagnosis was reviewed by a board certified medical geneticist (K.A.R. or O.D.K.) based on clinical features. Of the 32 participants enrolled in our study, 28 of the 32 (88%) had a known mutation in a gene that is causative for CFC, including BRAF (n=21), MEK1 (n=2), MEK2 (n=4), and KRAS (n=1). The cohort consisted of 16 males and 16 females. The average age of the cohort was 8 years, with a range of 2 to 27 years of age. The majority of the cohort reported Caucasian race (84%) but also included were Latino (6%), African (3%), and Middle Eastern (3%) individuals; the race of one subject was not reported. Written informed consent was obtained for all the subjects. Complete intra- and extra-oral exams were preformed by a licensed dentist (A.F.G., S.O. or J.G.). Exams included frontal and side view craniofacial photographs (one patient declined photographs), and, when possible, intra- and extra-oral photographs, review of radiographs (including panoramic, periapical and bitewing radiographs) and dental records provided by the participant, and alginate dental impressions. The total number of patients examined for each dental characteristic is listed in Table 2. When possible, participants’ parents and/or siblings were also examined as controls (n=43). Statistical comparison between the dental phenotype of the CFC cohort and general U.S. population as determined by the NHANES III survey (10) was made using the Fisher’s exact test with 2-tailed p-value. The same statistical test was used to compare the major craniofacial and dental characteristics between individuals with BRAF, MEK1, and MEK2 mutations.
Table 2.
Summary of the dental characteristics in cardio-facio-cutaneous syndrome (CFC).
| Dental Findings | CFC | General Populationb | p-valuec | |||
|---|---|---|---|---|---|---|
| Affected | Total Examineda | % | % | |||
| Malocclusion | ||||||
| Vertical | ||||||
| Open bite | 10 | 27 | 37 | 3 | 0.0001* | |
| Deep bite | 5 | 27 | 19 | 49 | 0.0001* | |
| Transverse | ||||||
| Posterior crossbite | 4 | 21 | 19 | 9 | 0.032* | |
| Anterior/Posterior/Sagittal | ||||||
| Molar relationship | ||||||
| Class I | 7 | 13 | 54 | 41 | 0.089 | |
| Class II | 6 | 13 | 46 | 53 | 0.396 | |
| Class III | 0 | 13 | 0 | 6 | 0.029* | |
| Arch perimeter | ||||||
| Crowding | 8 | 32 | 25 | 60 | 0.0001* | |
| Spacing | 7 | 32 | 22 | N/Ad | ||
| Dental development | ||||||
| Missing teeth | 1 | 31 | 3 | N/A | ||
| Supernumerary teeth | 0 | 31 | 0 | N/A | ||
| Delayed development | 0 | 2 | 0 | N/A | ||
| Delayed eruption | 4 | 32 | 13 | N/A | ||
| Hard tissue | ||||||
| High-arched palate | 16 | 20 | 80 | N/A | ||
| Soft tissue | ||||||
| Frenal attachment | ||||||
| High | 13 | 21 | 62 | N/A | ||
| Low | 8 | 21 | 38 | N/A | ||
| Gingival hyperplasia | 1 | 31 | 3 | N/A | ||
| Pathology | ||||||
| Caries present at exam | 7 | 28 | 25 | N/A | ||
| History of caries | 4 | 7 | 57 | N/A | ||
| Habits | ||||||
| Tongue thrusting | 7 | 31 | 23 | N/A | ||
| Open mouth posture | 9 | 32 | 29 | N/A | ||
| Bruxism | 3 | 31 | 10 | N/A | ||
Number of CFC individuals examined for each dental characteristic since dental exams not completed on every CFC individual in the cohort
Prevalence of dental characteristic in general population as determined by the NHANESIII survey (10)
Comparison of incidence of dental characteristic in CFC cohort compared to general population using the Fisher’s exact test with 2-tailed p-value
significant p-value<0.05
N/A Data not available
RESULTS
Individuals with CFC have a distinct craniofacial phenotype (Fig. 1). The main craniofacial findings observed in ≥50% of subjects examined are summarized in Table 1. The majority of subjects presented with relative macrocephaly (97%), high forehead (84%), and bitemporal narrowing (84%; Fig. 1). Most subjects had a convex (74%) facial profile (Fig. 1). A few subjects (10%) presented with micrognathia or a small mandible, but most appeared to have a proportionally sized mandible. A significant number of subjects had hypoplasia of the superior orbital ridge (52%; Fig. 1). Subjects also commonly had a hyperteloric (65%) and telencanthic (100%) appearance (Fig. 1). Other common features were a short nose (71%), with a depressed nasal bridge (65%) and wide nasal tip (65%), and low-set (90%), posteriorly rotated (84%) ears with upturned lobes (52%; Fig. 1).
Figure 1.
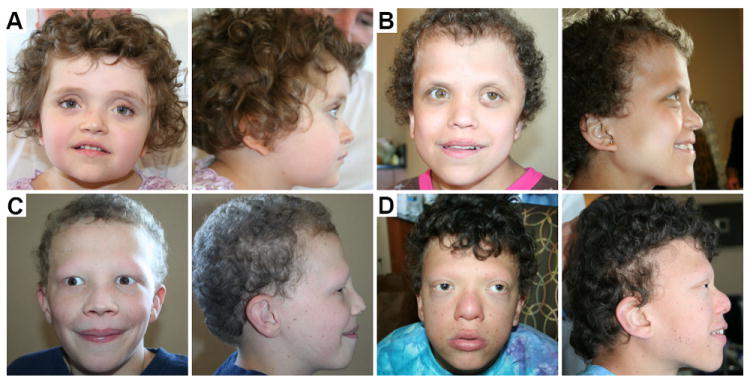
Craniofacial phenotype of CFC. Frontal and profile images of individuals with CFC demonstrate the craniofacial phenotype. Note how craniofacial features change as CFC individuals age. (A) A 3 year-old girl with the common CFC craniofacial features including relative macrocephaly and short nose with depressed nasal bridge and wide nasal tip. (B) A 15 year-old female with high forehead, bitemporal narrowing, and low set, posteriorly rotated ears. (C) A 12 year-old boy with hypoplasia of the superior orbital ridge. (D) A 15 year-old boy with a convex facial profile and hyperteloric and telecanthic appearance typical of CFC.
Table 1.
Summary of craniofacial findings in a cohort of 31 individuals with cardio-facio-cutaneous syndrome (CFC).
| Craniofacial Findings | n | % |
|---|---|---|
| Relative macrocephaly | 30 | 97 |
| High forehead | 26 | 84 |
| Bitemporal narrowing | 26 | 84 |
| Convex facial profile | 23 | 74 |
| Hypoplasia of superior orbital ridge | 16 | 52 |
| Hyperteloric appearing | 20 | 65 |
| Telencanthic appearing | 31 | 100 |
| Depressed nasal bridge | 20 | 65 |
| Short nose | 22 | 71 |
| Wide nasal tip | 20 | 65 |
| Low-set ears | 28 | 90 |
| Posteriorly rotated ears | 26 | 84 |
| Upturned lobes | 16 | 52 |
We next examined the dentition and found that individuals with CFC have a recognizable and characteristic dental phenotype (Table 2; Fig. 2). An open bite, when the anterior teeth are not in contact when the posterior teeth are in occlusion, was a common vertical malocclusion that was observed in 37% of our cohort (Fig. 2C). This incidence is significantly higher than the national average (3%; p=0.0001)(10). In contrast, a deep bite, which is an increased overbite in which the maxillary anterior teeth cover the mandibular teeth by more than 2 mm, was significantly less common among our subjects (19%) than in the general population (49%; p=0.0001; Fig. 2A)(10). Posterior crossbite, a condition in which the maxillary posterior teeth are on the lingual (i.e. toward the tongue) side of the mandibular teeth instead of the normal buccal (i.e. toward the cheek) side, was significantly more common in the CFC cohort (19%) than in the general U.S. population (9%; p=0.032; Fig. 2B)(10).
Figure 2.
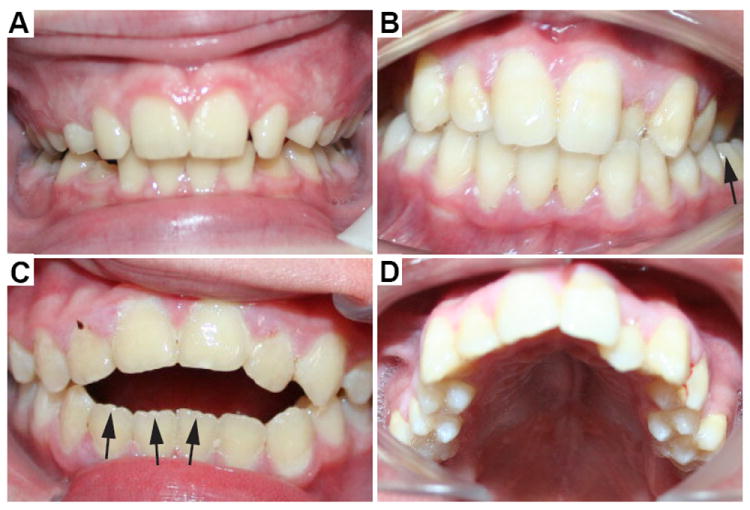
Dental phenotype of CFC. Intra-oral photographs showing the typical dental phenotypes in CFC. (A) A deep bite in which the maxillary incisors cover the mandibular incisors by more than 2 mm. (B) A posterior crossbite on the patient’s left side is marked by the black arrow and is typically seen in CFC. Dental crowding is also a common finding in CFC. (C) Open bite, with space between the anterior teeth while the posterior teeth are in contact. Note the mamelons or ridges on the incisal edges of the mandibular incisors (black arrows) which are normally worn down by abrasion of opposing teeth when the teeth are in contact. (D) High-arched palate.
A majority of subjects had class I molar relationship (54%), which is the ideal molar relationship according to the Angle’s classification system (11). In this relationship the mesiobuccal (anterior, cheek side) cusp of the maxillary first molar aligns with the buccal side groove in the middle of the mandibular first molar so that the maxillary first molar and mandibular first molar are aligned. The percent of subjects with class I molar relationship is not significantly different from the 41% of the U.S. population with class I molar relationship (p=0.089)(10). The percentage of subjects with class II molar relationship (46%), in which the maxillary first molar is positioned mesially (anteriorly in the mouth) to the mandibular first molar, is also similar to the national average (53%; p=0.396)(10). No CFC individuals presented with class III molar relationship, in which the maxillary first molar is positioned distally (posteriorly) to the mandibular first molar; this is significantly less than the U.S. average (6%; p=0.029)(10).
Dental crowding was only seen in 25% of our CFC cohort compared to about 60% of the U.S. population (p=0.0001; Fig. 2B)(10). Only one subject presented with missing teeth (a 17 year-old male missing a maxillary central incisor), and none presented with supernumerary teeth, based on clinical examinations and review of radiographs, including periapical (n=4) and panoramic (n=2) x-rays (Table 2). Examination of panoramic x-rays for two CFC patients indicated that dental development was not delayed, but followed typical timing. Eruption patterns were observed by assessing the teeth present in relationship to the age of the individual examined and comparing to the normal eruption pattern (12). Most CFC individuals (88%) did not show delayed eruption patterns. The enamel appeared clinically normal. The majority of subjects had a constricted high-arched palate (80%; Fig. 2D). The labial frenal attachment was high (62%), at the level of the unattached gingiva near the buccal fold, more often than low (38%), at the attached gingiva near the teeth, and only one subject (3%) presented with gingival hyperplasia defined as overgrowth of gingival tissue. The one subject who presented with gingival hyperplasia reported taking verapamil, a calcium channel blocker that has been reported to cause gingival swelling (13). Twenty-five percent of CFC individuals examined had clinical caries present, and 57% had a history of caries according to dental records. Subjects also presented with habits including a secondary tongue thrust (23%) and open mouth posture (29%). In addition, bruxism, as determined clinically by pathologic wear of the teeth, was present in 10% of our cohort.
We next compared the incidence of the major craniofacial and dental characteristics between individuals with BRAF, MEK1, and MEK2 mutations to determine genotype-phenotype correlations. Individuals with BRAF mutations had a significantly higher incidence (92%) of high-arched palate compared to MEK1- (0%) or MEK2-positive individuals (0%; p=0.03). No other craniofacial or dental characteristic differed significantly between individuals with these mutations.
DISCUSSION
CFC is a RASopathy caused by activating mutations in KRAS, BRAF, MEK1, or MEK2. Ras/MAPK signaling is known to be critical in craniofacial and tooth development, and dysregulation of the Ras/MAPK pathway in these syndromes results in craniofacial dysmorphia. Constitutive activation of the Ras/MAPK pathway affects craniofacial development, yet the mechanism by which this happens is still unclear. It is perplexing that although the RASopathies are caused by mutations in the same pathway, the different syndromes have many unique craniofacial characteristics. For example, Costello Syndrome (CS) is caused by heterozygous de novo germline mutations in the small GTPase HRAS, which is upstream of the kinases BRAF, MEK1, and MEK2, mutations in which cause CFC. However, CS and CFC have distinct craniofacial characteristics, especially as individuals age. These differences are significant enough to be useful to clinically differentiate and diagnose individuals with these syndromes. In this study, we determined that our CFC cohort had common craniofacial features including macrocephaly, bitemporal narrowing, convex facial profile, and hypoplastic supraorbital ridges (Table 1 and Fig. 1), which lays the groundwork for the systematic analysis of the craniofacial features of the various RASopathies and provides insight into the role of Ras/MAPK signaling in craniofacial development.
This study is the first to systematically evaluate the dental phenotype of any RASopathy, and we have determined that Ras/MAPK pathway dysregulation in CFC causes an abnormal oral phenotype. Determining the dental phenotypes associated with each of the RASopathies and correlating these phenotypes with the diverse spectrum of mutations that underlie Ras/MAPK dysregulation will be essential in furthering our understanding of this pathway in tooth development. Like individuals with CFC, mice carrying deletions in Sprouty genes have hyperactive MAPK pathway signaling. In these mice, hyperactive MAPK signaling results in supernumerary teeth (6, 14). Therefore, we expected that individuals with CFC, who have activating mutations in the MAPK pathway, would similarly have supernumerary teeth. However, to our surprise, individuals with CFC did not present with anomalies in tooth number, size or morphology. In addition, these individuals had a normal pattern of tooth development and eruption, and their enamel and gingival architecture appeared normal. There were, however, abnormal dental characteristics more commonly observed in CFC than in the general population, most of which affected occlusion. Individuals with CFC had a fairly normal molar relationship, with a normal distribution of class I and class II but a significantly lower incidence of class III molar relationship compared to the general population (Table 2 and Fig. 2). Individuals with CFC also had a significantly higher incidence of malocclusion than the general population, including anterior open bite and posterior crossbite (Table 2 and Fig. 2). In addition, CFC individuals showed a higher incidence of dental crowding than the general population, and they commonly had a high-arched palate (Fig. 2). Thus, the primary distinguishing dental phenotypic feature in CFC is malocclusion, suggesting that dysregulation of Ras/MAPK signaling disrupts normal craniofacial development, resulting in malocclusion.
Individuals with CFC also presented with abnormal oral habits. Tongue thrusting was observed in a significant number of subjects in our CFC cohort. Also, an open mouth posture was fairly common. Some evidence suggests that a tongue thrust habit may cause an altered tongue position that in turn may produce malocclusion, including open bite, posterior crossbite and vaulting of the palate (15). However, a direct correlation between tongue thrust and malocclusion has not been made, and further research is required to determine how dysregulation of Ras/MAPK signaling results in the malocclusion observed in CFC.
Notably, just as activation of Ras/MAPK in humans results in craniofacial malformation, activation of the same Ras/MAPK pathways in mouse and zebrafish directly affects craniofacial phenotype. A mouse model for CFC expressing a hypomorphic BrafV600E allele (an allele that has only been identified in cancer and not in CFC) displays a rounder and shorter head as well as defects in the shape of the skull vault caused by differences in the shape of the frontal and parietal bones that form the skull vault (16). In addition, we determined that a zebrafish model expressing a kinase-activating BRAFQ257R allele or kinase-inactivating BRAFG596V allele also develops craniofacial anomalies (17). Moreover, these defects were ameliorated by treatment with low doses of MEK inhibitor at early stages of development. These animal models of CFC provide a powerful tool to further understand the role of Ras/MAPK signaling in craniofacial development.
This study, which presents the dental phenotype of CFC, establishes a first step towards understanding the role of Ras/MAPK signaling in dental development, and it provides a tool for clinicians who care for individuals with CFC. CFC individuals do not present with unique dental pathologies requiring specific treatment. Like the general population, patients with CFC require routine dental examinations and appropriate hygiene and restorative care. Careful oral hygiene instructions to patients and their families are necessary, since individuals with CFC may not have meticulous oral hygiene habits. Some individuals with CFC may be anxious dental patients due to cognitive delay and oral aversion, and thus, these individuals should be seen early and often by the dentist to accustom them to dental treatment. In addition, dentists should be aware and monitor the development of malocclusion in individuals with CFC and be prepared to refer patients to an orthodontist for treatment if necessary. In summary, thorough characterization of the craniofacial and dental phenotypes of CFC and other RASopathies will not only help guide clinicians in treating these patients, but will also provide insight into the complex role of the Ras/MAPK pathway during craniofacial development.
Acknowledgments
The authors are grateful to CFC International and all of the participating individuals and their families. We thank all the private and public contributing agencies for the educational grants and awards which made the “2009 Genetic Syndromes of the Ras/MAPK Pathway: From Bedside to Bench and Back” possible, including NIH grant HD061140 (K.A.R.). A.F.G. is supported by an NIDCR fellowship, F30DE022205. L.A.W. is supported by the International Mental Health Research Organization, and this work was funded by the National Institutes of Health through the NIH Director’s New Innovator Award Program, DP2- OD007449 (L.A.W.) and DP2-OD007191 (O.D.K.).
References
- 1.Tidyman WE, Rauen KA. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niihori T, Aoki Y, Narumi Y, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–296. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Viciana P, Tetsu O, Tidyman WE, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–1290. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 4.Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Developmental biology. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 5.Nie X, Luukko K, Kettunen P. FGF signalling in craniofacial development and developmental disorders. Oral diseases. 2006;12:102–111. doi: 10.1111/j.1601-0825.2005.01176.x. [DOI] [PubMed] [Google Scholar]
- 6.Klein OD, Minowada G, Peterkova R, et al. Sprouty genes control diastema tooth development via bidirectional antagonism of epithelial-mesenchymal FGF signaling. Dev Cell. 2006;11:181–190. doi: 10.1016/j.devcel.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein OD, Lyons DB, Balooch G, et al. An FGF signaling loop sustains the generation of differentiated progeny from stem cells in mouse incisors. Development. 2008;135:377–385. doi: 10.1242/dev.015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterkova R, Churava S, Lesot H, et al. Revitalization of a diastemal tooth primordium in Spry2 null mice results from increased proliferation and decreased apoptosis. J Exp Zool B Mol Dev Evol. 2009;312B:292–308. doi: 10.1002/jez.b.21266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauen KA, Schoyer L, McCormick F, et al. Proceedings from the 2009 genetic syndromes of the Ras/MAPK pathway: From bedside to bench and back. Am J Med Genet A. 2010;152A:4–24. doi: 10.1002/ajmg.a.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proffit WR, Fields HW, Sarver DM. Contemporary orthodontics. St. Louis, Mo.: Mosby Elsevier; 2007. [Google Scholar]
- 11.Riolo ML. Essentials for orthodontic practice. Ann Arbor, MI: E.F.O.P. Press/University Lithoprinters; 2002. [Google Scholar]
- 12.Casamassimo PS. Pediatric dentistry : infancy through adolescence. St. Louis, Mo.: Elsevier/Saunders; 2013. [Google Scholar]
- 13.Miller CS, Damm DD. Incidence of verapamil-induced gingival hyperplasia in a dental population. J Periodontol. 1992;63:453–456. doi: 10.1902/jop.1992.63.5.453. [DOI] [PubMed] [Google Scholar]
- 14.Charles C, Hovorakova M, Ahn Y, et al. Regulation of tooth number by fine-tuning levels of receptor-tyrosine kinase signaling. Development. 2011;138:4063–4073. doi: 10.1242/dev.069195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Premkumar S, Avathvadi Venkatesan S, Rangachari S. Altered oral sensory perception in tongue thrusters with an anterior open bite. Eur J Orthod. 2011;33:139–142. doi: 10.1093/ejo/cjq042. [DOI] [PubMed] [Google Scholar]
- 16.Urosevic J, Sauzeau V, Soto-Montenegro ML, et al. Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5015–5020. doi: 10.1073/pnas.1016933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anastasaki C, Rauen KA, Patton EE. Continual low-level MEK inhibition ameliorates cardio-facio-cutaneous phenotypes in zebrafish. Dis Model Mech. 2012 doi: 10.1242/dmm.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]


