Abstract
The RASopathies are a clinically defined group of medical genetic syndromes caused by germline mutations in genes that encode components or regulators of the Ras/mitogen-activated protein kinase (MAPK) pathway. These disorders include neurofibromatosis type 1, Noonan syndrome, Noonan syndrome with multiple lentigines, capillary malformation–arteriovenous malformation syndrome, Costello syndrome, cardio-facio-cutaneous syndrome, and Legius syndrome. Because of the common underlying Ras/MAPK pathway dysregulation, the RASopathies exhibit numerous overlapping phenotypic features. The Ras/MAPK pathway plays an essential role in regulating the cell cycle and cellular growth, differentiation, and senescence, all of which are critical to normal development. Therefore, it is not surprising that Ras/MAPK pathway dysregulation has profound deleterious effects on both embryonic and later stages of development. The Ras/MAPK pathway has been well studied in cancer and is an attractive target for small-molecule inhibition to treat various malignancies. The use of these molecules to ameliorate developmental defects in the RASopathies is under consideration.
Keywords: capillary malformation–arteriovenous malformation syndrome, cardio-facio-cutaneous syndrome, Costello syndrome, Legius syndrome, LEOPARD syndrome, neurofibromatosis, Noonan syndrome, Ras/MAPK, signal transduction pathway
1. INTRODUCTION
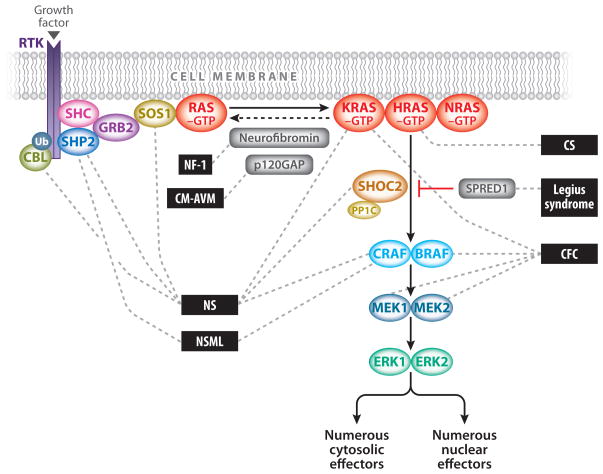
The Ras/mitogen-activated protein kinase (MAPK) pathway plays a vital role in development and is activated by extracellular input in the form of growth factors (Figure 1). RAS genes constitute a multigene family that includes HRAS, NRAS, and KRAS. Ras proteins are small guanosine nucleotide-bound GTPases that function as a critical signaling hub within the cell. They are activated through growth factors binding to receptor tyrosine kinases (RTKs), G-protein-coupled receptors, cytokine receptors, and extracellular matrix receptors. Ras proteins cycle between an active GTP-bound form and an inactive GDP-bound form. Activation through RTKs occurs with the binding of a growth factor that causes RTK autophosphorylation and interaction with the adaptor protein growth factor receptor-bound protein 2 (GRB2). GRB2 is bound to son of sevenless (SOS), which is then recruited to the plasma membrane. SOS proteins are guanosine nucleotide exchange factors (GEFs) that increase the Ras nucleotide exchange rate of GDP for GTP, thereby increasing the level of active GTP-bound Ras.
Figure 1.
The Ras/MAPK signal transduction pathway. The MAPK signaling pathway of protein kinases is critically involved in cellular proliferation, differentiation, motility, apoptosis, and senescence. The RASopathies are medical genetic syndromes caused by mutations in genes that encode components or regulators of the Ras/MAPK pathway (indicated by dashed lines). These disorders include neurofibromatosis type 1 (NF1), Noonan syndrome (NS), Noonan syndrome with multiple lentigines (NSML), capillary malformation–ateriovenous malformation syndrome (CM-AVM), Costello syndrome (CS), cardio-facio-cutaneous syndrome (CFC), and Legius syndrome.
The MAPK pathway is one of several critical downstream signaling cascades of Ras. Activated Ras leads to the activation of Raf (ARAF, BRAF, and/or CRAF), the first MAPK kinase kinase of the pathway. Raf phosphorylates and activates the MAPK kinases MEK1 and/or MEK2; these in turn phosphorylate and activate ERK1 and/or ERK2. ERK1 and ERK2 are the ultimate effectors and exert their function on a large number of downstream molecules, both nuclear and cytosolic. ERK1 and ERK2 substrates include nuclear components, transcription factors, membrane proteins, and protein kinases that in turn control vital cellular functions, including cell cycle progression, cellular differentiation, and cellular growth (69).
The Ras/MAPK pathway has been studied extensively in the context of oncogenesis because its somatic dysregulation is one of the primary causes of cancer. Ras is somatically mutated in approximately 20% of malignancies (8), and BRAF is somatically mutated in approximately 7% of malignancies (for a review, see 39). Because of this, the RASopathies are considered cancer syndromes, with the majority of associated mutations resulting in enhanced pathway activation or dysregulated signaling. However, biochemical studies have demonstrated that a large fraction of the novel germline mutations identified in the pathway are not as robustly activating as those associated with oncogenesis. This is likely due to the embryonic lethality arising from these germline mutations.
2. THE RASOPATHIES
The RASopathies are a class of developmental disorders caused by germline mutations (as opposed to the somatic mutations found in cancer) in genes that encode components or regulators of the Ras/MAPK pathway. The Ras/MAPK pathway is one of the best-studied signal transduction pathways and is critical in regulating the cell cycle and cellular growth, differentiation, and senescence, all of which are essential to normal mammalian development (Figure 1). Therefore, it is not surprising that its dysregulation can have profound consequences in development.
Each RASopathy exhibits a unique phenotype, but owing to the common mechanisms of Ras/MAPK pathway dysregulation, they share many overlapping characteristics, including craniofacial dysmorphology; cardiac malformations; cutaneous, musculoskeletal, and ocular abnormalities; neurocognitive impairment; hypotonia; and an increased cancer risk. Taken together, they are one of the largest known groups of malformation syndromes, affecting approximately 1 in 1,000 individuals. Neurofibromatosis type 1 (NF1) was the first syndrome identified as being caused by mutation of a gene in the Ras/MAPK pathway (NF1) (11, 63, 65), and numerous other syndromes have subsequently been identified. These disorders include (a) Noonan syndrome (NS), caused by activating mutations in PTPN11 (59), SOS1 (46, 60), RAF1 (38, 44), KRAS (51), NRAS (15), SHOC2 (16), and CBL (34, 35); (b) Noonan syndrome with multiple lentigines (NSML), caused by mutations in PTPN11 (18) and RAF1 (38); (c) capillary malformation–arteriovenous malformation syndrome (CM-AVM), caused by haploinsufficiency of RASA1 (20); (d) Costello syndrome (CS), caused by activating mutations in HRAS (5); (e) cardio-facio-cutaneous syndrome (CFC), caused by alteration of MAPK pathway activation by activating mutations in BRAF (36, 48) and MAP2K1 (MEK1) or MAP2K2 (MEK2) (48); and (f) Legius syndrome, caused by inactivating mutations in SPRED1 (10).
2.1. Neurofibromatosis Type 1
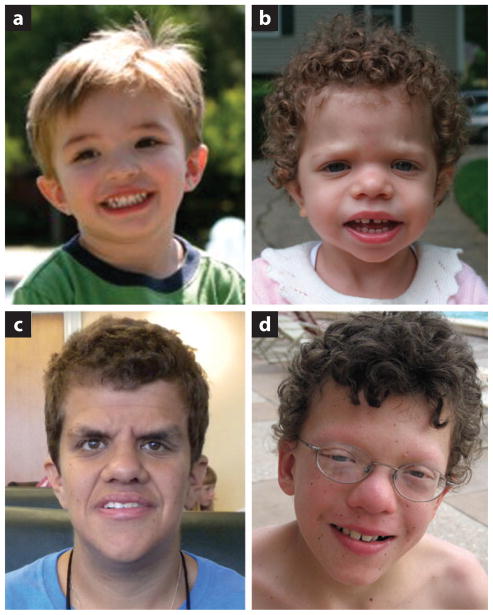
NF1 is an autosomal dominant disorder affecting approximately 1 in 3,000 newborns, with approximately half of NF1 individuals inheriting the mutation from a parent (for a review, see 67). The clinical diagnosis of NF1 is based on the presence of café-au-lait maculae, intertriginous freckling, neurofibromas and plexiform neurofibromas, iris Lisch nodules, osseous dysplasia, optic pathway glioma, and/or a first-degree relative with NF1 (Figure 2, Table 1). Although these are the signs and symptoms most commonly associated with NF1, individuals with NF1 may have other manifestations of the disorder, including cardiac malformations, cardiovascular disease, vasculopathy, hypertension, vitamin D deficiency, brain malformations, and seizures. They may also have dysmorphic craniofacial features reminiscent of NS (27, 57), mild neurocognitive impairment, and a predisposition to developing certain malignancies. Segmental and mosaic forms of NF1 are not uncommon, and gonadal mosaicism has been documented.
Figure 2.
Clinical images of patients with RASopathies. (a) A young boy who has a clinical diagnosis of neurofibromatosis type 1. (b) A young girl with Noonan syndrome who has a PTPN11 mutation. (c) A young adult woman with Costello syndrome who has the common p.G12S HRAS mutation. (d) A school-age boy with cardio-facio-cutaneous syndrome who has a MEK2 mutation.
Table 1.
Genetic syndromes of the Ras/MAPK pathway
| Syndrome | Ras/MAPK pathway gene | Chromosome location | Protein | Protein function | Clinical phenotype |
|---|---|---|---|---|---|
| Neurofibromatosis 1 | NF1 | 17q11.2 | Neurofibromin | RasGAP | Café-au-lait maculae; intertriginous freckling; neurofibromas and plexiform neurofibromas; iris Lisch nodules; osseous dysplasia; optic pathway glioma; normal neurocognitive function or mild impairment; predisposition to other cancers |
| Noonan syndrome |
PTPN11 SOS1 RAF1 KRAS NRAS SHOC2 CBL |
12q24.1 2p22.1 3p25.1 12p12.1 1p13.2 10q25.2 11q23.3 |
SHP2 SOS1 CRAF KRAS NRAS SHOC2 CBL |
Phosphatase RasGEF Kinase GTPase GTPase Scaffolding E3 ubiquitin ligase |
Craniofacial dysmorphic features, including a broad forehead, hypertelorism, down-slanting palpebral fissures, ptosis, a high-arched palate, and low-set, posteriorly rotated ears; congenital heart defects; short stature; undescended testicles; ophthalmologic abnormalities; bleeding disorders; normal neurocognitive function or mild impairment; predisposition to cancer |
| Noonan syndrome with multiple lentigines |
PTPN11 RAF1 |
12q24.1 3p25.1 |
SHP2 RAF1/CRAF |
Phosphatase Kinase |
Same as Noonan syndrome, but with possible development of multiple skin lentigines as individuals age; unclear predisposition to cancer |
| Capillary malformation–arteriovenous malformation | RASA1 | 5q14.3 | p120-RasGAP | RasGAP | Multifocal capillary malformations, which may be associated with arteriovenous malformations and fistulae; unclear predisposition to cancer |
| Costello syndrome | HRAS | 11p15.5 | HRAS | GTPase | Craniofacial features similar to those of Noonan syndrome but potentially more coarse; congenital heart defects; failure to thrive; short stature; ophthalmologic abnormalities; multiple skin manifestations, including papilloma; normal neurocognitive function or mild impairment; hypotonia; predisposition to cancer |
| Cardio-facio-cutaneous syndrome |
BRAF MAP2K1 MAP2K2 KRAS |
7q34 15q22.31 19p13.3 12p12.1 |
BRAF MEK1 MEK2 KRAS |
Kinase Kinase Kinase GTPase |
Craniofacial features similar to those of Noonan syndrome; congenital heart defects; failure to thrive; short stature; ophthalmologic abnormalities; multiple skin manifestations, including progressive formation of nevi; normal neurocognitive function or mild impairment; hypotonia; unclear predisposition to cancer |
| Legius syndrome | SPRED1 | 15q14 | SPRED1 | SPROUTY-related, EVH1 domain–containing protein 1 | Café-au-lait maculae; intertriginous freckling; macrocephaly; normal neurocognitive function or mild impairment; no apparent predisposition to cancer |
NF1 was the first multiple congenital anomaly syndrome to be associated with a germline mutation in the Ras/MAPK pathway. NF1 is caused by mutations in the NF1 gene, with approximately half of the mutations being de novo (12, 63, 65). NF1 encodes neurofibromin, which is a RasGAP, i.e., a GTPase-activating protein that is a negative regulator of Ras (Figure 1, Table 1). NF1 mutations result in neurofibromin loss of function, causing haploinsufficiency within the cell. This in turn reduces RasGTPase activity and therefore results in an overall increase in active GTP-bound Ras. Because of this, NF1 is a cancer predisposition syndrome (for a review, see 9). Individuals with NF1 are at greater risk than the general population for developing malignancies. Pediatric malignancies include optic pathway glioma, rhabdomyosarcoma, neuroblastoma, and juvenile myelomonocytic leukemia, whereas adult malignancies include malignant peripheral nerve sheath tumors, gastrointestinal stromal tumors, somatostatinomas, pheochromocytomas, and breast cancer.
2.2. Noonan Syndrome
NS is an autosomal dominant disorder that affects approximately 1 in 1,000–2,000 newborns. Although it has a variable clinical phenotype, NS is characterized by distinctive craniofacial features, including a broad forehead, hypertelorism, down-slanting palpebral fissures, and low-set, posteriorly rotated ears (Figure 2, Table 1). Other important phenotypic features include congenital cardiac defects, reduced growth, bleeding disorders, and a variable degree of neurocognitive delay (for a review, see 49). In addition, individuals with NS have an increased risk of developing cancer. At present, seven genes have been shown to be associated with NS: PTPN11 (59), SOS1 (46, 60), RAF1 (38, 44), KRAS (51), NRAS (15), SHOC2 (16), and CBL (34, 35). All of these genes harbor heterozygous germline mutations and encode various components of or proteins associated with the Ras/MAPK pathway (Figure 1, Table 1).
The most common gene associated with NS is PTPN11, which accounts for approximately 50% of all cases (59). SHP2, the protein product of PTPN11, is a nonreceptor protein tyrosine phosphatase (PTP) composed of N- and C-terminal SH2 domains and a catalytic PTP domain. The majority of NS-causing missense mutations in PTPN11 cluster in residues involved with the interaction between the N-SH2 and PTP domains. Mutations in this region disrupt the stability of the catalytically inactive form of SHP2, impairing the protein’s ability to switch from the active to the inactive conformation (28, 58) and causing increased signaling of the Ras/MAPK pathway.
SOS1 missense mutations are the second-most-common cause of NS, accounting for approximately 15% of cases (46, 60). SOS1 encodes the Ras guanine nucleotide exchange factor (RasGEF) protein SOS1, which is responsible for stimulating the conversion of Ras from the inactive GDP-bound form to the active GTP-bound form. The majority of SOS1 missense mutations are located in codons encoding residues responsible for stabilizing the protein in an inhibited conformation. These mutations disrupt the autoinhibition of SOS1 RasGEF activity, resulting in a SOS1 gain of function, a subsequent increase in the active form of Ras, and increased Ras/MAPK pathway signaling.
KRAS mutations are a rare cause of NS (51). KRAS encodes two splice variants, KRASA and KRASB; KRASA is expressed in a tissue-specific and developmentally restricted fashion, whereas KRASB is ubiquitously expressed. The novel KRAS mutations that cause NS increase signaling of the Ras/MAPK pathway through one of two distinct mechanisms: mutations that reduce the intrinsic and GAP-stimulated GTPase activity (50, 51) or mutations that interfere with the binding of KRAS and guanine nucleotides. The resultant increased signaling of the Ras/MAPK pathway is, however, less than that which occurs with oncogenic KRAS-activating mutations (51). Although most clinical geneticists would agree that individuals with a KRAS mutation have a clinical phenotype consistent with NS, there are some individuals who have a more severe phenotypic manifestation consistent with CFC (see below).
Mutations in NRAS have also been found in a very small number of individuals with the NS clinical phenotype (15). Mutations have been identified within or near the switch II region of NRAS and are thought to interfere with GTPase function. Mutations cause enhanced phosphorylation of MEK and ERK.
Mutations in RAF1 also cause NS (38, 44). RAF1 encodes the protein RAF1 (a.k.a. CRAF), a serine/threonine kinase that is one of the direct downstream Ras effectors. The majority of RAF1 mutations associated with NS cluster in two regions: conserved region 2 (flanking S259) and conserved region 3 (surrounding the activation segment). These mutations result in a CRAF gain of function because the phosphorylation of residues S259 and S621 are responsible for regulation of CRAF.
A rare subset of NS individuals with a unique phenotypic feature of loose anagen hair has recently been identified as caused by a single mutation in SHOC2, which results in a p.S2G substitution (16). SHOC2 is a homolog of suppressor of clear (SOC-2) in Caenorhabditis elegans, which encodes a protein whose primary structure consists almost entirely of leucine-rich repeats. SHOC2 functions as a scaffold protein linking Ras to RAF1, its downstream effector in the Ras/MAPK pathway. SHOC2 is ubiquitously expressed and serves as the regulatory subunit of protein phosphatase 1C (PP1C) (47). SHOC2 binds RasGTP and mediates PP1C translocation to the cell membrane. This enables PP1C dephosphorylation of residue S259 of RAF1, which is required for RAF1 translocation to the cell membrane and catalytic activity. The unique p.S2G mutation is proposed to cause the abnormal addition of a 14-carbon saturated fatty acid chain, myristate, to the N-terminal glycine of SHOC2. This results in the aberrant translocation of SHOC2 to the cell membrane, prolonged PP1C dephosphorylation of RAF1, and sustained MAPK pathway activation (16).
Another rare cause of NS is mutations in the tumor suppressor gene CBL (34, 35). Individuals may also have myeloproliferative diseases, including juvenile myelomonocytic leukemia, which is seen in individuals with NF1 as well as those with NS. CBL encodes an E3 ubiquitin ligase that negatively regulates the Ras/MAPK signaling downstream of RTK (19). CBL mediates the association of ubiquitin with activated RTK, which is necessary for receptor internalization and degradation (19). Because mutations in CBL reduce the turnover of activated RTK, they have an overall effect of increased ERK activation.
2.3. Noonan Syndrome with Multiple Lentigines
NSML (formerly referred to as LEOPARD syndrome) is a rare autosomal dominant disorder. Individuals have the craniofacial features of NS as well as multiple lentigines, electrocardiogram abnormalities, ocular hypertelorism, pulmonary valve stenosis, abnormal genitalia, growth retardation, and deafness. NSML and NS are allelic disorders, caused by different heterozygous missense mutations in the same genes, PTPN11 (18, 32) and RAF1 (38) (Figure 1, Table 1). The most common NSML-associated PTPN11 mutations affect amino acids in the catalytic PTP domain, which results in reduced SHP2 catalytic activity in vitro, causing a loss of function (18, 29). However, an in vivo Drosophila model has demonstrated that the residual catalytic activity in the NSML mutant SHP2 protein is sufficient to produce a gain-of-function phenotype owing to dysregulation of the protein, causing continuous MAPK pathway activity during development (37).
2.4. Capillary Malformation–Arteriovenous Malformation Syndrome
CM-AVM is an autosomal dominant inherited disorder characterized by multifocal capillary malformations, which may be associated with arteriovenous malformations and fistulas (for a review, see 7). CM-AVM is caused by heterozygous inactivating mutations in RASA1 (20), which, like NF1, encodes a RasGAP, specifically the p120-RasGAP (Figure 1, Table 1). The N terminus contains a Src (sarcoma) homology region, and the C terminus contains a pleckstrin homology domain and the RasGTPase-activating domain. Like neurofibromin, the protein product of NF1, RASA1 switches the active GTP-bound Ras to the inactive GDP-bound form. It is a negative regulator of the Ras/MAPK signal transduction pathway that is important for cellular growth, differentiation, and proliferation.
The major feature of this syndrome is the multifocality of the malformations. Arteriovenous malformations can occur in many tissues, including skin, muscle, bone, and various internal organs, including the heart and the brain. RASA1 mutations have also been associated with individuals diagnosed with Parkes Weber syndrome and vein of Galen malformations (45). Haploinsufficiency of p120-RasGAP reduces the hydrolysis of RasGTP and therefore increases Ras/MAPK pathway signaling. Most individuals with CM-AVM have an affected parent; however, ~30% of cases are caused by a de novo mutation. The types of mutations vary, with the majority being nonsense, frameshift, or splice mutations. In addition to arteriovenous malformations, individuals may have cardiovascular malformations such as tetralogy of Fallot, septal defects, and valve anomalies. Interestingly, CM-AVM patients may be at increased risk of developing tumors similar to those seen in NF1 and NF2, although this is not entirely clear at this point.
2.5. Costello Syndrome
CS, one of the rarer RASopathies, is a multiple congenital anomaly syndrome that has many overlapping features with the other syndromes. CS individuals have characteristic dysmorphic craniofacial features; failure to thrive, especially in the newborn period; cardiac, musculoskeletal, ectodermal, and ocular abnormalities; hypotonia; and variable neurocognitive delay (for a review, see 40) (Figure 2, Table 1). Phenotypic features become apparent in the perinatal period with polyhydramnios in utero, and many children are born prematurely and have an increased birth weight. Facial features are coarse and typically include macrocephaly with a prominent forehead, epicanthal folds, down-slanting palpebral fissures, a short nose with a depressed nasal bridge and broad base, and low-set, posteriorly rotated ears with thickened helices and lobes. The cheeks may be full and the mouth large with full lips. Dermatologic manifestations aid in the clinical diagnosis of CS, and include soft skin with excessive wrinkling and redundancy over the dorsum of the hands and the feet along with deep plantar and palmar creases (53). The majority of CS individuals have cardiac anomalies, which may include hypertrophic cardiomyopathy (HCM), valve anomalies, septal defects, and arrhythmia (33). Failure to thrive and gastrointestinal dysfunctions such as reflux, oral aversion, and constipation are typical in early infancy; most patients require a gastronomy tube for feeding.
CS is caused by heterozygous activating germline mutations in HRAS (5) (Figure 1, Table 1). The distribution of mutations reveals that more than 80% of CS individuals have a p.G12S substitution; the second-most-common substitution is p.G12A (for a review, see 61). These substitutions disrupt guanine nucleotide binding and cause a reduction in intrinsic and GAP-induced GTPase activity, resulting in Ras remaining in the active state (22). Less frequently observed HRAS mutations may also occur, which may result in an atypical phenotype. It is interesting that amino acid positions 12 and 13, the two most common positions mutated in CS, are also the most frequently mutated positions in oncogenic Ras. Ras mutations in codons 12, 13, or 61 are present in approximately 20% of all tumors (8).
As with many of the other RASopathies, individuals with CS are at increased risk of developing neoplasms, both benign and malignant. Benign growths commonly seen in CS are cutaneous papillomas (53), which are observed in ~72% of CS individuals, with age of onset ranging from infancy to 22 years. The most commonly reported location of papillomas is the nose, although they may occur anywhere on the body. Importantly, these neoplasms are not seen in other RASopathies. Of great concern is that approximately 15–20% of individuals with CS develop malignancies, with the most common being rhabdomyosarcoma, transitional cell carcinoma, and neuroblastoma (23). Interestingly, rhabdomyosarcoma and neuroblastoma are common childhood malignancies, whereas transitional cell carcinoma is not. The most commonly reported malignancy in CS is embryonal rhabdomyosarcoma, in which loss of heterozygosity of wild-type HRAS has been reported (21). The origin of constitutional germline mutations causing CS reflects a paternal bias (56, 70). Somatic mosaicism has been identified in CS, although it is rare (25). Also, there has been one case of reported autosomal dominant transmission (55) and one case of reported gonadal mosaicism (24).
2.6. Cardio-Facio-Cutaneous Syndrome
CFC, like CS, is rare, and it has many overlapping phenotypic features with NS and CS. CFC individuals have NS-like facies, including macrocephaly, a broad forehead, bitemporal narrowing, hypoplasia of the supraorbital ridges, down-slanting palpebral fissures with ptosis, a short nose with a depressed nasal bridge and anteverted nares, a high-arched palate, and low-set, posteriorly rotated ears with prominent helices (Figure 2, Table 1). Ectodermal findings typically consist of sparse, curly hair with sparse eyebrows and eyelashes, hyperkeratosis, keratosis pilaris, hemangioma, ichthyosis, and progressively forming nevi (54). Cardiac anomalies are similar in frequency to those of NS and CS, with the most prevalent being pulmonic stenosis, septal defects, and HCM. Musculoskeletal abnormalities are common, as are ocular abnormalities, including strabismus, nystagmus, myopia, hyperopia, and astigmatism. Failure to thrive is typical in infancy, as are gastrointestinal dysfunctions such as reflux, vomiting, oral aversion, and constipation. Neurologic abnormalities are universally present to varying degrees and include hypotonia, motor delay, seizures, speech delay, and/or learning disability (68).
Four genes that encode proteins in the Ras/MAPK pathway downstream of Ras have been associated with CFC syndrome: BRAF (36, 48), MAP2K1 (MEK1) and MAP2K2 (MEK2) (48), and KRAS (36) (Table 1). The role of KRAS in CFC remains unclear because KRAS mutations were also identified in individuals clinically diagnosed with NS (51). Heterozygous BRAF mutations are found in approximately 75% of mutation-positive CFC individuals (for a review, see 62). BRAF is a serine/threonine protein kinase and one of the direct downstream effectors of Ras. It is also a known oncoprotein, with somatic mutations reported in several different types of malignancies, including thyroid, lung, ovarian, and colorectal cancers; however, the majority of CFC-associated mutations are novel. Unlike the cancer-associated mutations, the majority of CFC BRAF mutations cluster in the cysteine-rich domain in exon 6 and in the protein kinase domain, with the most common CFC BRAF mutation being p.Q257R. In vitro functional analyses of the BRAF mutation proteins have demonstrated that most have increased kinase activity; however, a few mutant proteins have kinase-impaired activities (36, 48). BRAF kinase impairment also increases signaling of the MAPK pathway through CRAF (26). Further in vivo studies of CFC mutations have demonstrated that both kinase-active and kinase-impaired mutations result in similar phenotypic dysregulation of MAPK signaling in a zebrafish model (3).
Heterozygous missense mutations in MAP2K1 (MEK1) and MAP2K2 (MEK2) are present in approximately 25% of CFC individuals in which a gene mutation has been identified (for a review, see 62). MEK1 and MEK2 are threonine/tyrosine kinases, with both isoforms having the ability to phosphorylate and activate ERK1 and ERK2. Functional studies of MEK mutant CFC proteins have shown that all are activating (3, 48).
CFC syndrome is transmitted in an autosomal dominant manner (43). Because it is very rare for individuals with CFC to reproduce, the vast majority of cases result from a de novo dominant mutation. Although the mutations that cause CFC are in a well-known oncogenic pathway, it is still unclear whether individuals with CFC are at an increased risk for malignancies. CFC certainly does not appear to have the malignancy risk associated with NF1, NS, and CS (31, 43).
2.7. Legius Syndrome
Legius syndrome (formally called NF1-like syndrome) is a relatively newly described autosomal dominant RASopathy. It shares many phenotypic features with NF1 as well as with other RASopathies. Individuals may have café-au-lait maculae, intertriginous freckling, mild neurocognitive impairment, and macrocephaly, with some having dysmorphic craniofacial features reminiscent of NS. However, the neoplastic features common in NF1, such as neurofibromas, plexiform neurofibromas, iris Lisch nodules, and central nervous system tumors, do not seem to be associated with this syndrome.
Legius syndrome is caused by heterozygous inactivating mutations in SPRED1 (10) (Figure 1, Table 1). SPRED1 encodes SPRED1, which has a SPROUTY-related, N-terminal enabled/VASP homology 1 (EVH1) domain. SPRED1 functions as a negative regulator of Ras by inhibiting phosphorylation of Raf (64). The vast majority of SPRED1 mutations associated with Legius syndrome cause truncation of the protein, resulting in a SPRED1 loss of function and dysregulated signaling of the Ras/MAPK pathway. Although several patient series have been published, it remains unclear whether individuals with germline SPRED1 mutations are at increased risk for developing cancer (17).
3. CONSIDERATIONS FOR SYSTEMIC TREATMENT OF GERMLINE EFFECTS
Work on treating germline phenotypic features of RASopathies with pathway modulation or small-molecule inhibition is under way. One common feature of the RASopathies is some degree of neurocognitive involvement, which is an attractive target for treatment (1). The first randomized, placebo-controlled, double-blind clinical trial examined the effect of a 12-week simvastatin treatment on cognitive function in children with NF1 (30). Sixty-two children from 8 to 16 years old were treated with simvastatin, a 3-hydroxy-3-methylglutamyl coenzyme A reductase inhibitor that interferes with the cholesterol biosynthetic pathway as well as Ras isoprenylation, the latter of which decreases Ras activity. Preliminary and secondary outcomes were designed to test numerous neuropsychologic, neurophysiologic, and neuroradiologic effects. No significant difference was found between the treatment and control groups, and the authors concluded that the treatment did not improve cognitive function of NF1 children as designed in this trial.
Another clinical trial examined lovastatin treatment in children with NF1 (2). Twenty-four children from 10 to 17 years old were treated for three months with escalating doses of lovastatin to evaluate the safety and toxicity of the drug. The primary outcome showed that the vast majority of the children developed decreased cholesterol levels, but no levels fell below the normal range for the age group. Neurocognitive secondary outcomes demonstrated significant improvement in verbal and nonverbal memory and visual attention and efficiency. In an add-on study using functional brain magnetic resonance imaging (MRI), a subset of NF1 children treated with lovastatin showed an increase in long-range positive resting-state functional connectivity within the default network of the brain, similar to that seen in the brain of typically developing children (13).
In addition to the treatment of germline NF1 phenotypic features, other RASopathy treatments are candidates for clinical trials. An NS phase II clinical trial is currently recruiting (http://www.clinicaltrials.gov). This is a proof-of-concept trial to evaluate the effect of the MEK inhibitor MEK162 (Novartis) on adults with NS who also have HCM. The primary outcome measure will be the change of baseline left ventricular mass as determined by MRI; the goals are to examine the safety and tolerability of treatment with MEK162 over six months, evaluate the pharmacokinetics and pharmacodynamics of MEK162, and evaluate the therapeutic concept that MEK inhibition will reduce cardiac hypertrophy in NS individuals with HCM.
Like NF1 and NS, the possible treatment of CS and CFC holds great promise (41). CS and CFC are two of the rarest RASopathies, with estimates of several hundred individuals in each group worldwide. The identification of HRAS mutations as the molecular cause of CS raised the possibility that farnesyl transferase inhibitors may provide clinical benefit to patients. These drugs inhibit the enzyme that transfers the farnesyl group onto Ras and other proteins, and were initially developed as potential anticancer agents targeting Ras function (for a review, see 6). The use of MEK inhibitors to treat phenotypic manifestations in individuals with CFC has sparked much discussion as well (41, 42). MEK inhibitors are generally non-ATP competitive and bind in an interior hydrophobic pocket that is adjacent to but distinct from MEK’s ATP binding site. MEK inhibitors were developed for cancer treatment but have, unfortunately, made little impact in cancer clinical trials to date. Their use in animal models has shown promise in treating germline effects of Ras/MAPK activation (14, 52, 66), and the utilization of low-level MEK inhibition to partially reduce Ras/MAPK activation and achieve moderate developmental effects may be effective (4).
4. CONCLUSIONS
The RASopathies, which are caused by germline mutations in genes encoding components or regulators of the Ras/MAPK pathway, emphasize the essential role that this critical signal transduction pathway plays in embryonic and postnatal development. Mutations identified in these syndromes result in dysregulation of the Ras/MAPK pathway, and functional studies have determined that the vast majority enhance pathway signaling. Therefore, it is not surprising that many of these syndromes exhibit overlapping phenotypic features and share a predisposition for certain malignancies. The number of different genes affected and the variety of mutations within each gene are reflected in the wide spectrum of phenotypic variability associated with these syndromes. Although many of the activating mutations are similar to somatic mutations associated with cancer, they tend to not be as strongly activating. It is likely that the strongly activating oncogenic mutations cannot be tolerated within the germline or in early development.
The initial diagnosis for a patient with a given RASopathy is based on the clinical recognition of phenotypic features, with molecular genetic testing then used to confirm the clinical diagnosis. The correlation between the clinical and molecular diagnoses often depends on the clinical diagnostic criteria. In addition, not all of the genes associated with these syndromes have been identified. The progressive accumulation of genotype-phenotype correlations will increase the importance of molecular diagnosis and help overcome the intrinsic limitations of clinical diagnosis. This will not only improve patient management but also aid in the design of clinical trials to develop potential treatments for these syndromes.
SUMMARY POINTS.
The Ras/MAPK pathway plays a vital role in development and is activated by extracellular input in the form of growth factors. This pathway has been well studied in cancer.
The RASopathies are a clinically defined group of medical genetic syndromes caused by germline mutations in genes that encode components or regulators of the Ras/MAPK pathway. These syndromes include neurofibromatosis type 1, Noonan syndrome, Noonan syndrome with multiple lentigines, capillary malformation–arteriovenous malformation, Costello syndrome, cardio-facio-cutaneous syndrome, and Legius syndrome.
The RASopathies are one of the largest known groups of malformation syndromes, affecting approximately 1 in 1,000 individuals.
Although many of the activating Ras/MAPK pathway mutations are similar to somatic mutations associated with cancer, they tend to not be as strongly activating. It is likely that the strongly activating oncogenic mutations cannot be tolerated in the germline or in early development.
Because the Ras/MAPK pathway has targets for inhibition in cancer treatment, many small-molecule therapeutics are in development or undergoing clinical trials. The use of systemic therapies after birth to reduce Ras/MAPK activity in the RASopathies could potentially ameliorate the progression of signs and symptoms associated with these disorders.
FUTURE ISSUES.
Not all of the genes associated with the RASopathies have been identified; it is therefore critical to continue identifying associated genes.
Additional clinical trials in RASopathies are needed to examine possible treatment of developmental defects.
Although the RASopathies share a predisposition for certain malignancies, it is unclear why some syndromes and individuals have an increased cancer risk compared with others.
As the RASopathies are a newly defined group of medical genetic syndromes, it will be important to continue studying their natural history to assist in defining the best medical practices for each.
Acknowledgments
The author thanks the patients with RASopathies and their families for their ongoing support of research in genetic medicine. This work was supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number R01AR062165. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. The author apologizes for not citing all relevant references owing to space limitations.
Footnotes
DISCLOSURE STATEMENT
The author is not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Acosta MT, Bearden CE, Castellanos XF, Cutting L, Elgersma Y, et al. The Learning Disabilities Network (LeaDNet): using neurofibromatosis type 1 (NF1) as a paradigm for translational research. Am J Med Genet A. 2012;158A:2225–32. doi: 10.1002/ajmg.a.35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acosta MT, Kardel PG, Walsh KS, Rosenbaum KN, Gioia GA, Packer RJ. Lovastatin as treatment for neurocognitive deficits in neurofibromatosis type 1: phase I study. Pediatr Neurol. 2011;45:241–45. doi: 10.1016/j.pediatrneurol.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Anastasaki C, Estep AL, Marais R, Rauen KA, Patton EE. Kinase-activating and kinase-impaired cardio-facio-cutaneous syndrome alleles have activity during zebrafish development and are sensitive to small molecule inhibitors. Hum Mol Genet. 2009;18:2543–54. doi: 10.1093/hmg/ddp186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anastasaki C, Rauen KA, Patton EE. Continual low-level MEK inhibition ameliorates cardio-facio-cutaneous phenotypes in zebrafish. Dis Models Mech. 2012;5:546–52. doi: 10.1242/dmm.008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aoki Y, Niihori T, Kawame H, Kurosawa K, Ohashi H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet. 2005;37:1038–40. doi: 10.1038/ng1641. [DOI] [PubMed] [Google Scholar]
- 6.Basso AD, Kirschmeier P, Bishop WR. Lipid posttranslational modifications: farnesyl transferase inhibitors. J Lipid Res. 2006;47:15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Boon LM, Mulliken JB, Vikkula M. RASA1: variable phenotype with capillary and arteriovenous malformations. Curr Opin Genet Dev. 2005;15:265–69. doi: 10.1016/j.gde.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–89. [PubMed] [Google Scholar]
- 9.Brems H, Beert E, de Ravel T, Legius E. Mechanisms in the pathogenesis of malignant tumours in neurofibromatosis type 1. Lancet Oncol. 2009;10:508–15. doi: 10.1016/S1470-2045(09)70033-6. [DOI] [PubMed] [Google Scholar]
- 10.Brems H, Chmara M, Sahbatou M, Denayer E, Taniguchi K, et al. Germline loss-of-function mutations in SPRED1 cause a neurofibromatosis 1–like phenotype. Nat Genet. 2007;39:1120–26. doi: 10.1038/ng2113. [DOI] [PubMed] [Google Scholar]
- 11.Cawthon RM, O’Connell P, Buchberg AM, Viskochil D, Weiss RB, et al. Identification and characterization of transcripts from the neurofibromatosis 1 region: the sequence and genomic structure of EVI2 and mapping of other transcripts. Genomics. 1990;7:555–65. doi: 10.1016/0888-7543(90)90199-5. [DOI] [PubMed] [Google Scholar]
- 12.Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- 13.Chabernaud C, Mennes M, Kardel PG, Gaillard WD, Kalbfleisch ML, et al. Lovastatin regulates brain spontaneous low-frequency brain activity in neurofibromatosis type 1. Neurosci Lett. 2012;515:28–33. doi: 10.1016/j.neulet.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen PC, Wakimoto H, Conner D, Araki T, Yuan T, et al. Activation of multiple signaling pathways causes developmental defects in mice with a Noonan syndrome–associated Sos1 mutation. J Clin Investig. 2010;120:4353–65. doi: 10.1172/JCI43910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cirstea IC, Kutsche K, Dvorsky R, Gremer L, Carta C, et al. A restricted spectrum of NRAS mutations causes Noonan syndrome. Nat Genet. 2010;42:27–29. doi: 10.1038/ng.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cordeddu V, Di Schiavi E, Pennacchio LA, Ma’ayan A, Sarkozy A, et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat Genet. 2009;41:1022–26. doi: 10.1038/ng.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Denayer E, Chmara M, Brems H, Kievit AM, van Bever Y, et al. Legius syndrome in fourteen families. Hum Mutat. 2011;32:E1985–98. doi: 10.1002/humu.21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Digilio MC, Conti E, Sarkozy A, Mingarelli R, Dottorini T, et al. Grouping of multiple-lentigines/LEOPARD and Noonan syndromes on the PTPN11 gene. Am J Hum Genet. 2002;71:389–94. doi: 10.1086/341528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikic I, Schmidt MH. Malfunctions within the Cbl interactome uncouple receptor tyrosine kinases from destructive transport. Eur J Cell Biol. 2007;86:505–12. doi: 10.1016/j.ejcb.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Eerola I, Boon LM, Mulliken JB, Burrows PE, Dompmartin A, et al. Capillary malformation–arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240–49. doi: 10.1086/379793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estep AL, Tidyman WE, Teitell MA, Cotter PD, Rauen KA. HRAS mutations in Costello syndrome: detection of constitutional activating mutations in codon 12 and 13 and loss of wild-type allele in malignancy. Am J Med Genet A. 2006;140:8–16. doi: 10.1002/ajmg.a.31078. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs JB, Sigal IS, Poe M, Scolnick EM. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci USA. 1984;81:5704–8. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gripp KW. Tumor predisposition in Costello syndrome. Am J Med Genet C. 2005;137C:72–77. doi: 10.1002/ajmg.c.30065. [DOI] [PubMed] [Google Scholar]
- 24.Gripp KW, Stabley DL, Geller PL, Hopkins E, Stevenson DA, et al. Molecular confirmation of HRAS p. G12S in siblings with Costello syndrome. Am J Med Genet A. 2011;155A:2263–68. doi: 10.1002/ajmg.a.34150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gripp KW, Stabley DL, Nicholson L, Hoffman JD, Sol-Church K. Somatic mosaicism for an HRAS mutation causes Costello syndrome. Am J Med Genet A. 2006;140A:2163–69. doi: 10.1002/ajmg.a.31456. [DOI] [PubMed] [Google Scholar]
- 26.Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209–21. doi: 10.1016/j.cell.2009.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hüffmeier U, Zenker M, Hoyer J, Fahsold R, Rauch A. A variable combination of features of Noonan syndrome and neurofibromatosis type I are caused by mutations in the NF1 gene. Am J Med Genet A. 2006;140A:2749–56. doi: 10.1002/ajmg.a.31547. [DOI] [PubMed] [Google Scholar]
- 28.Keilhack H, David FS, McGregor M, Cantley LC, Neel BG. Diverse biochemical properties of Shp2 mutants: implications for disease phenotypes. J Biol Chem. 2005;280:30984–93. doi: 10.1074/jbc.M504699200. [DOI] [PubMed] [Google Scholar]
- 29.Kontaridis MI, Swanson KD, David FS, Barford D, Neel BG. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–92. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 30.Krab LC, de Goede-Bolder A, Aarsen FK, Pluijm SM, Bouman MJ, et al. Effect of simvastatin on cognitive functioning in children with neurofibromatosis type 1: a randomized controlled trial. JAMA. 2008;300:287–94. doi: 10.1001/jama.300.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kratz CP, Rapisuwon S, Reed H, Hasle H, Rosenberg PS. Cancer in Noonan, Costello, cardiofaciocutaneous and LEOPARD syndromes. Am J Med Genet C. 2011;157C:83–89. doi: 10.1002/ajmg.c.30300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legius E, Schrander-Stumpel C, Schollen E, Pulles-Heintzberger C, Gewillig M, Fryns JP. PTPN11 mutations in LEOPARD syndrome. J Med Genet. 2002;39:571–74. doi: 10.1136/jmg.39.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin AE, Alexander ME, Colan SD, Kerr B, Rauen KA, et al. Clinical, pathological, and molecular analyses of cardiovascular abnormalities in Costello syndrome: a Ras/MAPK pathway syndrome. Am J Med Genet A. 2011;155A:486–507. doi: 10.1002/ajmg.a.33857. [DOI] [PubMed] [Google Scholar]
- 34.Martinelli S, De Luca A, Stellacci E, Rossi C, Checquolo S, et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am J Hum Genet. 2010;87:250–57. doi: 10.1016/j.ajhg.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niemeyer CM, Kang MW, Shin DH, Furlan I, Erlacher M, et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat Genet. 2010;42:794–800. doi: 10.1038/ng.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niihori T, Aoki Y, Narumi Y, Neri G, Cavé H, et al. Germline KRAS and BRAF mutations in cardio-facio-cutaneous syndrome. Nat Genet. 2006;38:294–96. doi: 10.1038/ng1749. [DOI] [PubMed] [Google Scholar]
- 37.Oishi K, Zhang H, Gault WJ, Wang CJ, Tan CC, et al. Phosphatase-defective LEOPARD syndrome mutations in PTPN11 gene have gain-of-function effects during Drosophila development. Hum Mol Genet. 2009;18:193–201. doi: 10.1093/hmg/ddn336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pandit B, Sarkozy A, Pennacchio LA, Carta C, Oishi K, et al. Gain-of-function RAF1 mutations cause Noonan and LEOPARD syndromes with hypertrophic cardiomyopathy. Nat Genet. 2007;39:1007–12. doi: 10.1038/ng2073. [DOI] [PubMed] [Google Scholar]
- 39.Pritchard C, Carragher L, Aldridge V, Giblett S, Jin H, et al. Mouse models for BRAF-induced cancers. Biochem Soc Trans. 2007;35:1329–33. doi: 10.1042/BST0351329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauen KA. HRAS and the Costello syndrome. Clin Genet. 2007;71:101–8. doi: 10.1111/j.1399-0004.2007.00743.x. [DOI] [PubMed] [Google Scholar]
- 41.Rauen KA, Banerjee A, Bishop WR, Lauchle JO, McCormick F, et al. Costello and cardio-facio-cutaneous syndromes: moving toward clinical trials in RASopathies. Am J Med Genet C. 2011;157C:136–46. doi: 10.1002/ajmg.c.30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauen KA, Schoyer L, McCormick F, Lin AE, Allanson JE, et al. Proceedings from the 2009 genetic syndromes of the Ras/MAPK pathway: from bedside to bench and back. Am J Med Genet A. 2010;152A:4–24. doi: 10.1002/ajmg.a.33183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauen KA, Tidyman WE, Estep AL, Sampath S, Peltier HM, et al. Molecular and functional analysis of a novel MEK2 mutation in cardio-facio-cutaneous syndrome: transmission through four generations. Am J Med Genet A. 2010;152A:807–14. doi: 10.1002/ajmg.a.33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razzaque MA, Nishizawa T, Komoike Y, Yagi H, Furutani M, et al. Germline gain-of-function mutations in RAF1 cause Noonan syndrome. Nat Genet. 2007;39:1013–17. doi: 10.1038/ng2078. [DOI] [PubMed] [Google Scholar]
- 45.Revencu N, Boon LM, Mulliken JB, Enjolras O, Cordisco MR, et al. Parkes Weber syndrome, vein of Galen aneurysmal malformation, and other fast-flow vascular anomalies are caused by RASA1 mutations. Hum Mutat. 2008;29:959–65. doi: 10.1002/humu.20746. [DOI] [PubMed] [Google Scholar]
- 46.Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007;39:70–74. doi: 10.1038/ng1926. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell. 2006;22:217–30. doi: 10.1016/j.molcel.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Rodriguez-Viciana P, Tetsu O, Tidyman WE, Estep AL, Conger BA, et al. Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome. Science. 2006;311:1287–90. doi: 10.1126/science.1124642. [DOI] [PubMed] [Google Scholar]
- 49.Romano AA, Allanson JE, Dahlgren J, Gelb BD, Hall B, et al. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126:746–59. doi: 10.1542/peds.2009-3207. [DOI] [PubMed] [Google Scholar]
- 50.Schubbert S, Bollag G, Lyubynska N, Nguyen H, Kratz CP, et al. Biochemical and functional characterization of germ line KRAS mutations. Mol Cell Biol. 2007;27:7765–70. doi: 10.1128/MCB.00965-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schubbert S, Zenker M, Rowe SL, Boll S, Klein C, et al. Germline KRAS mutations cause Noonan syndrome. Nat Genet. 2006;38:331–36. doi: 10.1038/ng1748. [DOI] [PubMed] [Google Scholar]
- 52.Shukla V, Coumoul X, Wang RH, Kim HS, Deng CX. RNA interference and inhibition of MEK-ERK signaling prevent abnormal skeletal phenotypes in a mouse model of craniosynostosis. Nat Genet. 2007;39:1145–50. doi: 10.1038/ng2096. [DOI] [PubMed] [Google Scholar]
- 53.Siegel DH, Mann JA, Krol AL, Rauen KA. Dermatological phenotype in Costello syndrome: consequences of Ras dysregulation in development. Br J Dermatol. 2011;164:521–29. doi: 10.1111/j.1365-2133.2011.10744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siegel DH, McKenzie J, Frieden IJ, Rauen KA. Dermatological findings in 61 mutation-positive individuals with cardiofaciocutaneous syndrome. Br J Dermatol. 2011;164:521–29. doi: 10.1111/j.1365-2133.2010.10122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sol-Church K, Stabley DL, Demmer LA, Agbulos A, Lin AE, et al. Male-to-male transmission of Costello syndrome: G12S HRAS germline mutation inherited from a father with somatic mosaicism. Am J Med Genet A. 2009;149A:315–21. doi: 10.1002/ajmg.a.32639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sol-Church K, Stabley DL, Nicholson L, Gonzalez IL, Gripp KW. Paternal bias in parental origin of HRAS mutations in Costello syndrome. Hum Mutat. 2006;27:736–41. doi: 10.1002/humu.20381. [DOI] [PubMed] [Google Scholar]
- 57.Stevenson DA, Viskochil DH, Rope AF, Carey JC. Clinical and molecular aspects of an informative family with neurofibromatosis type 1 and Noonan phenotype. Clin Genet. 2006;69:246–53. doi: 10.1111/j.1399-0004.2006.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tartaglia M, Martinelli S, Stella L, Bocchinfuso G, Flex E, et al. Diversity and functional consequences of germline and somatic PTPN11 mutations in human disease. Am J Hum Genet. 2006;78:279–90. doi: 10.1086/499925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–68. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 60.Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007;39:75–79. doi: 10.1038/ng1939. [DOI] [PubMed] [Google Scholar]
- 61.Tidyman WE, Rauen KA. Noonan, Costello and cardio-facio-cutaneous syndromes: dysregulation of the Ras-MAPK pathway. Expert Rev Mol Med. 2008;10:e37. doi: 10.1017/S1462399408000902. [DOI] [PubMed] [Google Scholar]
- 62.Tidyman WE, Rauen KA. Molecular cause of cardio-facio-cutaneous syndrome. Noonan Syndrome and Related Disorders: A Matter of Deregulated Ras Signaling. In: Zenker M, editor. Monogr Hum Genet. 17. Basel, Switz: Karger; 2009. pp. 73–82. [Google Scholar]
- 63.Viskochil D, Buchberg AM, Xu G, Cawthon RM, Stevens J, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell. 1990;62:187–92. doi: 10.1016/0092-8674(90)90252-a. [DOI] [PubMed] [Google Scholar]
- 64.Wakioka T, Sasaki A, Kato R, Shouda T, Matsumoto A, et al. Spred is a Sprouty-related suppressor of Ras signalling. Nature. 2001;412:647–51. doi: 10.1038/35088082. [DOI] [PubMed] [Google Scholar]
- 65.Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–86. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- 66.Wang Y, Kim E, Wang X, Novitch BG, Yoshikawa K, et al. ERK inhibition rescues defects in fate specification of Nf1-deficient neural progenitors and brain abnormalities. Cell. 2012;150:816–30. doi: 10.1016/j.cell.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123:124–33. doi: 10.1542/peds.2007-3204. [DOI] [PubMed] [Google Scholar]
- 68.Yoon G, Rosenberg J, Blaser S, Rauen KA. Neurological complications of cardio-facio-cutaneous syndrome. Dev Med Child Neurol. 2007;49:894–99. doi: 10.1111/j.1469-8749.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 69.Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006;24:21–44. doi: 10.1080/02699050500284218. [DOI] [PubMed] [Google Scholar]
- 70.Zampino G, Pantaleoni F, Carta C, Cobellis G, Vasta I, et al. Diversity, parental germline origin, and phenotypic spectrum of de novo HRAS missense changes in Costello syndrome. Hum Mutat. 2007;28:265–72. doi: 10.1002/humu.20431. [DOI] [PubMed] [Google Scholar]