Abstract
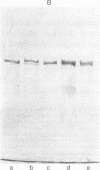
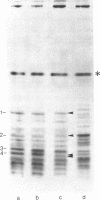
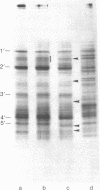
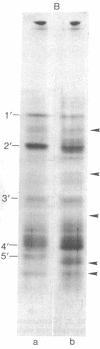
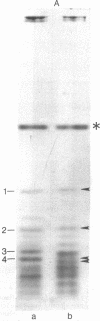
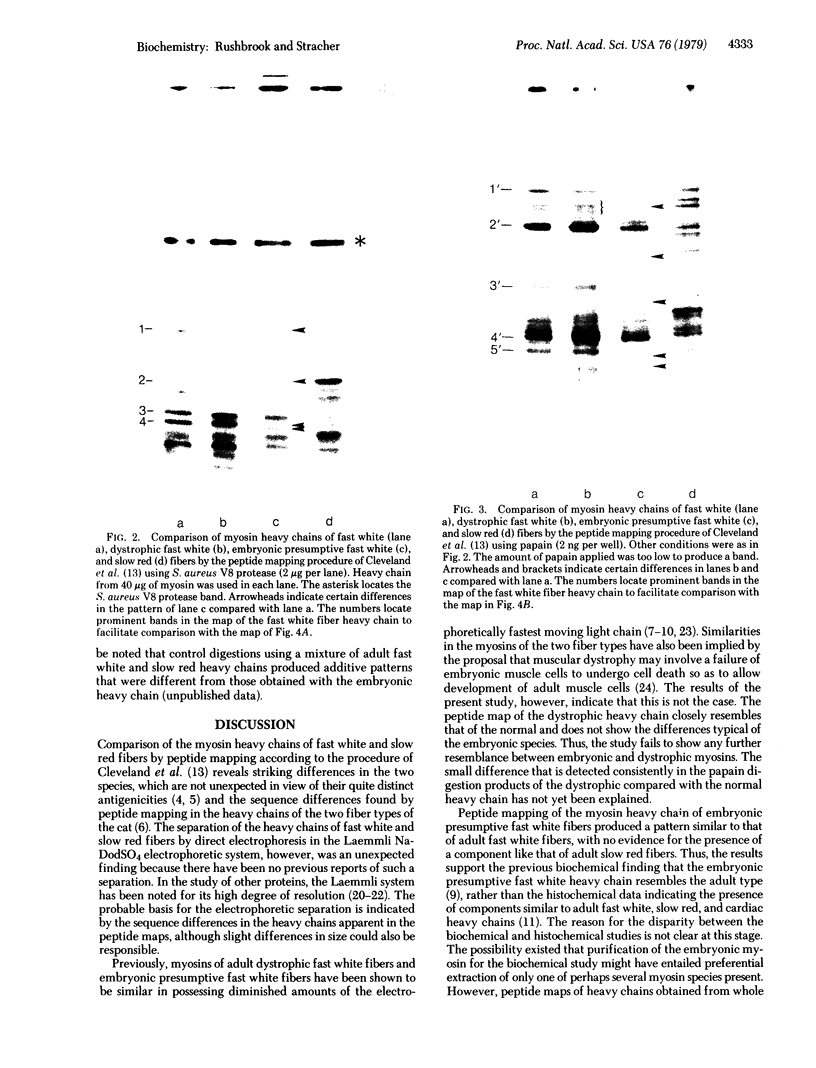
Chicken myosin heavy chains from adult fast white muscle fibers (both normal and dystrophic), adult slow red fibers, and embryonic presumptive fast white fibers were compared by sodium dodecyl sulfate/polyacrylamide gel electrophoresis and by peptide mapping. The heavy chain of slow red myosin migrated electrophoretically more slowly than the heavy chains of the other myosins and differed markedly from them in its peptide maps. The heavy chain of dystrophic fast white myosin was similar to its normal counterpart by peptide mapping but showed slight differences. The peptide map of the heavy chain of embryonic presumptive fast white myosin had the general features of that of the heavy chain of fast white, not slow red, fibers but contained definite differences from the former. The results are consistent with the existence of a separate gene for the heavy chain of embryonic presumptive fast white myosin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arndt I., Pepe F. A. Antigenic specificity of red and white muscle myosin. J Histochem Cytochem. 1975 Mar;23(3):159–168. doi: 10.1177/23.3.47867. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Dehm P., Olsen B. R., Prockop D. J. Antibodies to chick-tendon procollagen. Affinity purification with the isolated disulfide-linded NH2-terminal extensions and reactivity with a component in embryonic serum. Eur J Biochem. 1974 Jul 1;46(1):107–116. doi: 10.1111/j.1432-1033.1974.tb03602.x. [DOI] [PubMed] [Google Scholar]
- Dow J., Stracher A. Identification of the essential light chains of myosin. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1107–1110. doi: 10.1073/pnas.68.6.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Hobbs A. W. Fast and slow myosin in developing muscle fibres. Nature. 1978 Jul 6;274(5666):25–29. doi: 10.1038/274025a0. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S. Polymorphism of myosin among skeletal muscle fiber types. J Cell Biol. 1977 Sep;74(3):760–779. doi: 10.1083/jcb.74.3.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. P., Olsen B. R., Chen H. T., Prockop D. J. Segment-long-spacing aggregates and isolation of COOH-terminal peptides from type I procollagen. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4304–4308. doi: 10.1073/pnas.73.12.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh J. F., Yeoh G. P. Rabbit skeletal myosin isoenzymes from fetal, fast-twitch and slow-twitch muscles. Nature. 1979 Jul 26;280(5720):321–323. doi: 10.1038/280321a0. [DOI] [PubMed] [Google Scholar]
- Huszar G. Developmental changes of the primary structure and histidine methylation in rabbit skeletal muscle myosin. Nat New Biol. 1972 Dec 27;240(104):260–264. doi: 10.1038/newbio240260a0. [DOI] [PubMed] [Google Scholar]
- John H. A. The myosin of developing and dystrophic skeletal muscle. FEBS Lett. 1974 Mar 1;39(3):278–282. doi: 10.1016/0014-5793(74)80130-4. [DOI] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Masaki T. Immunochemical comparison of myosins from chicken cardiac, fast white, slow red, and smooth muscle. J Biochem. 1974 Aug;76(2):441–449. doi: 10.1093/oxfordjournals.jbchem.a130586. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yoshizaki C. Differentiation of myosin in chick embryos. J Biochem. 1974 Jul;76(1):123–131. doi: 10.1093/oxfordjournals.jbchem.a130536. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Suzuyama Y., Maita T., Umegane T. The L-2 light chain of chicken skeletal muscle myosin. FEBS Lett. 1977 Dec 1;84(1):53–56. doi: 10.1016/0014-5793(77)81055-7. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Holtzer H. Fast and slow muscles in tissue culture synthesise only fast myosin. Nature. 1979 Jul 26;280(5720):323–325. doi: 10.1038/280323a0. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Pepe F. A., Holtzer H. Myosin types during the development of embryonic chicken fast and slow muscles. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4524–4527. doi: 10.1073/pnas.74.10.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushbrook J. I., Harvey R. A. Nicotinamide adenine dinucleotide dependent isocitrate dehydrogenase from beef heart: subunit heterogeneity and enzyme dissociation. Biochemistry. 1978 Dec 12;17(25):5339–5346. doi: 10.1021/bi00618a003. [DOI] [PubMed] [Google Scholar]
- Sreter F., Holtzer S., Gergely J., Holtzer H. Some properties of embryonic myosin. J Cell Biol. 1972 Dec;55(3):586–594. doi: 10.1083/jcb.55.3.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracher A., McGowan E. B., Siemankowski L., Molak V., Shafiq S. A. Relationship between myosin structure and muscle degeneration. Ann N Y Acad Sci. 1979;317:349–355. doi: 10.1111/j.1749-6632.1979.tb56546.x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Trayer I. P., Harris C. I., Perry S. V. 3-Methyl histidine and adult and foetal forms of skeletal muscle myosin. Nature. 1968 Feb 3;217(5127):452–453. doi: 10.1038/217452a0. [DOI] [PubMed] [Google Scholar]
- Webb J. N. Muscular dystrophy and muscle cell death in normal foetal development. Nature. 1974 Nov 15;252(5480):233–234. doi: 10.1038/252233a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Burridge K. Myosin from cross-reinnervated cat muscles. Evidence for reciprocal transformation of heavy chains. FEBS Lett. 1975 Sep 15;57(2):203–208. doi: 10.1016/0014-5793(75)80717-4. [DOI] [PubMed] [Google Scholar]