Introduction
The indications of Nipple Sparing Mastectomy (NSM) are being broadened, including prophylactic or risk-reduction mastectomy and therapeutic mastectomy for both benign and malignant breast diseases (1-3). Concurrently with the development of better screening protocol and preoperative Magnetic resonance imaging (MRI), now the rate of mastectomy is increasing (4). The vast majority of evidence has confirmed the oncologic and surgical safety of NSM with immediate surgical reconstruction which also increase NSM rate (2,3,5,6). At European Institute of Oncology (EIO), Milan, Italy; the mastectomy rate has increased from 23% to 28% during the past 10 years; this increase in the rate of mastectomy here is due to the interest in NSM combined with the increased use of MRI. More than 2,000 NSM have been carried out for invasive and in situ cancers. Although not evidence by a trial study, our protocol includes an electron intraoperative treatment (ELIOT) on the NAC to decrease the risk of local recurrences (LR). This article authors would like to discuss therapeutic NSM from the perspective of breast and oncoplastic surgeons who involved in breast disease treatment.
Therapeutic NSM candidate
NSM can be offered to patient who suffers from benign or malignant breast disease. Invasive carcinoma or in situ carcinomas of both ductal and lobular cell types are operable by this technique. In the past, NSM were preferably purposed to small breast cancer which tumor located far from the Nipple Areolar Complex (NAC), no clinical lymph node involvement, solitary tumor with favorable pathological features. We started NSM in early 2002 and we have selected our NSM candidate base on preoperative and intraoperative assessment.
Preoperative assessment
Preoperative clinical assessment by meticulous examination of NAC is an integral part of NSM selection. In the case of Paget’s disease of NAC or NAC with significant nipple retraction or NAC edema which are contraindication of NSM procedure. From the literature, the distance from the tumor to NAC is regarded as one of the most important criteria for NSM patient selection. Nonetheless, the margins of the cancer are difficult to evaluate clinically. The more confidence of tumor margin evaluation is advocated by using radiological distance (mammogram or MRI). However preoperative radiologic assessment is not included in the routine NSM protocol. The indication of MRI is more depend on oncologic indication. We recommend NSM in patient who has tumor located outside of the areola area, no nipple retraction, no blood discharge from the nipple, no inflammatory signs, no previous irradiation and no micro calcifications on radiologic assessment. Patients with bilateral cancer, benign disease, preoperative radio- or chemotherapy can be selected for NSM.
Intraoperative assessment
Although we have a proper preoperative selection, the more important and decisive step of NSM is the intraoperative assessment. We relied on frozen section examination of retroareolar tissue. Lohsiriwat et al., performed a pathological study in mastectomy specimens and showed sensitivity of retroareolar frozen section examination of 88.2% and a negative predictive value of 93.3%, which can be interpreted to mean that when the frozen section test yields a negative result it is unlikely that the definite histopathology should have been positive (7). Despite a high specificity and sensibility of frozen section method, there is still a minority of false negative results. In our experience, we observed 8.2% of false-negative results of intraoperative retroareolar frozen section from 1,001 NSM (3). However, our unpublished data showed that the LR in this false negative group is comparable to that without false negative results or close margins.
Surgical technique and intraoperative application
The skin incisions for NSM can be drawn in various designs. The common incisions are radial, curvilinear, peri-areolar, para-areolar or even inframammary fold design. The glandular tissue can be dissected by sharp technique or electric cauterization. The recommend thickness of skin flam is between 3-5 mm in order to preserve the sub-dermal vessels. The breast parenchyma is dissected down to the pectoralis fascia, which can be preserved in around half of the patients.
When the dissection approaches to NAC area, 5 mm thickness is recommended to avoid necrotic complication of NAC. The NAC flap is elevated and the retroareolar specimen is sent to the pathologist for frozen examination. If the retroareolar specimen is confirmed free from tumor cell, then NSM can be completed. Retroareolar sampling should be done in the initial step to avoid hesitancy of the NSM procedure, especially in the operative settings that frozen examination may take long time. At EIO radiotherapy team is called to do the ELIOT when the frozen examination is proved negative for cancer infiltration. A one-shot electron beam 16 Gy is delivered on the NAC by a dedicated linear accelerator. The single dose of has been calculated according to the linear-quadratic model (with a value of alpha/beta ratio of 4 for breast cancer) to be equivalent to a fractionated dose ranging from 40 to 45 Gy. The clinical target volume, fully encompassed by the collimator, is the diameter of the areola plus 1 cm margin around. The entire target is included in the 90% isodose (2,3,8,9). However, NAC irradiation can be postponed or canceled when the blood supply after the subcutaneous mastectomy is critical. Another option if the blood supply of the NAC is very risky is to perform a composite graft of NAC. It can be temporarily banked (e.g., in the groin region) and grafted later on with satisfactory result (10).
The role of ELIOT on decreasing LR was observed in our patients who received ELIOT and also had positive final retroareolar histology. Their NAC were preserved and there was no recurrence was observed with a median follow-up period of 5 years. This is our best argument in favor of the efficacy of intraoperative radiation therapy (3). In contrary, our unpublished data from a series of 30 NSM patients without ELIOT or external radiotherapy found no local recurrence after a median follow-up of 48 months (range, 12-90 months). This result suggests that when NAC blood supply is sub-optimal, ELIOT may be avoided to increase the chances of NAC survival and maintain a good aesthetic and oncologic result.
NSM is almost always completed with immediate breast reconstruction (IBR). After complete the oncologic surgery then IBR is performed by using one of the techniques adapted to the particular case: definitive implant, expander or musculo-cutaneous flaps (11,12). Moreover, the contralateral symmetrical procedure or secondary reconstruction to achieve the best aesthetic result may be needed. One of the arising techniques for refinement or touch-up procedure is lipofilling; so called fat grafting, fat transfer, lipotransfer or fat injection. Despite, the overall safety of this procedure has been demonstrated (13), but a review by Lohsiriwat et al., suggested that the endocrine, paracrine, and autocrine activities of the transplanted fat tissues may interact in the cancerous host (14). In era of tissue engineering and stem cells, adipose tissue is being investigated and the multicentric cancer registration should be employed (15) (Figures 1,2).
Figure 1.
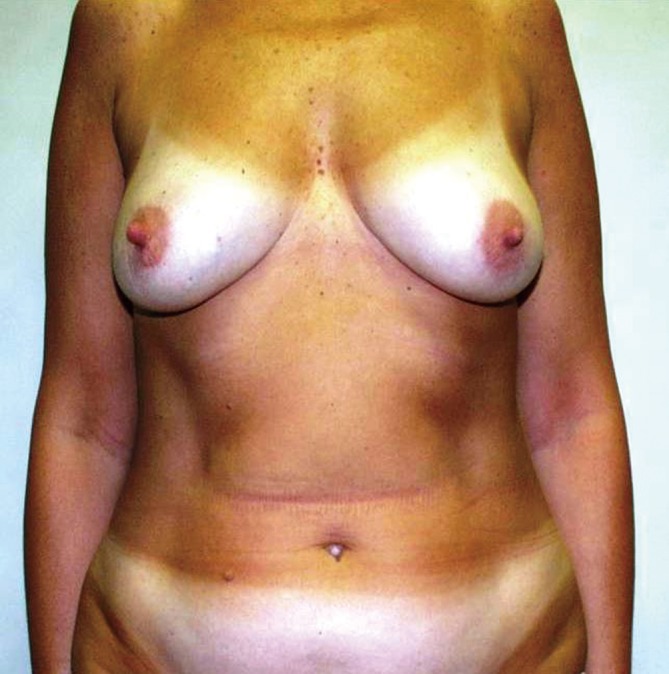
Preoperative, NSM for invasive carcinoma on the right breast.
Figure 2.
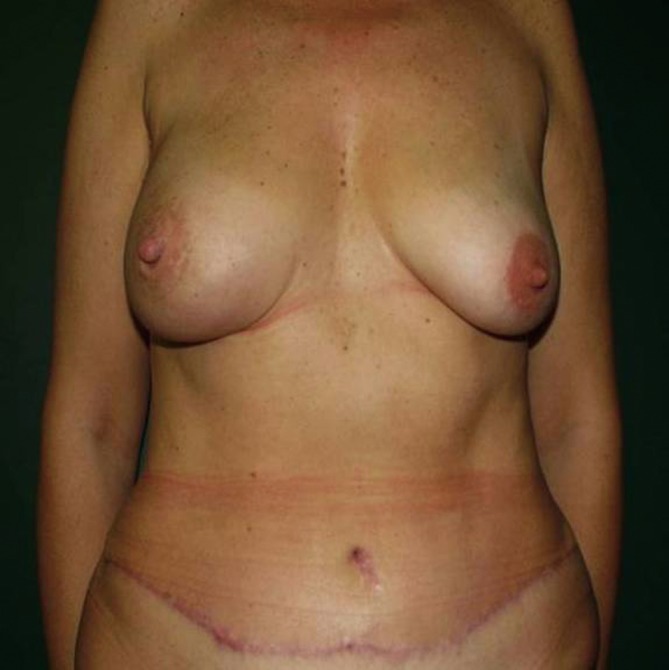
Postoperative, NSM with immediate pedicle transverse rectus abdominis myocutaneous flap reconstruction.
Surgical outcomes
The failure to preserve NAC after NSM leads to the failure of NSM. The most common reasons for which the NAC must be removed post operatively are NAC necrosis and tumor involvement on retroareolar tissue permanent examination. The rate of nipple necrosis varies from 0 to 48%, with most series reporting less than 10%. Our retrospective cohort found NAC removal due to the necrosis was less than 5% and our prospective study of 50 NSM found 26% of partial necrosis without NAC removal (16). NAC necrosis can be affected by patient factors such as BMI, age, concomitant disease, smoking status or breast morphology. Surgical factors such as skin incision location, technique of dissection, skin flap thickness and areola flap thickness, nodal dissection, intra-operative radiotherapy and type of reconstruction may also influence the NAC survival. A study at EIO found that smoking, young age, type of incision and areola flap thickness less than 5 mm are the significant risk factors for NAC necrosis (16). The applicability of the ICG technique may play an important role for evaluating the perfusion of the adjacent skin and NAC (17). The infection rate in our 1,001 NSM was 2% and resulting in 4.3% removal of prostheses. Twenty percent of NAC were found depigmented and 5% of them developed severe radiodystrophy after NSM with ELIOT.
Psychological outcomes
NAC preservation or reconstruction has been approved to be an acceptable technique to preserve the integrity of the body, reduced the feeling of mutilation, improved the breast cosmetic results, and reduced psychological distress regarding the loss of the breast. These impacts were confirmed by a study by sending open questionnaire to 190 women with NAC sparing and 100 patients with successive NAC reconstruction (18). When compare NSM patient with patients with patient who had successive NAC reconstruction, the results were in favour of the NSM group regarding body image, satisfaction with the nipple appearance, the sensitivity of the nipple and regarding the feeling of mutilation. NAC sparing in mastectomy has a positive impact on patient satisfaction, body image and psychological adjustment (19). A study by Didier F et al. also showed that surgeon’s influence is significant factor for patient who accepting NSM (18).
Oncological outcomes
Since the introduction of NSM, there are many publications tried to investigate on the oncological safety of NSM. The literature showed no statistical differences of LR between NSM and SSM or even compared with modified mastectomy. However, the comparison between each institutional experience is difficult due to the difference of patient inclusion and different operative protocol. In our recent study of 934 NSM patients (772 invasive carcinoma and 162 in intraepithelial neoplasia group) with a median follow-up of 50 months. The rate of LR in the breast and in the NAC was 3.6%, and 0.8% respectively for invasive carcinoma. The rate of LR in the breast and in the NAC was 4.9%, and 2.9% respectively in intraepithelial neoplasia group. The significant risk factors of LR in the breast for the invasive carcinoma were grade, overexpression/amplification of HER2/neu and breast cancer molecular subtype Luminal B. In intraepithelial neoplasia group the risk factors of LR in the breast and in the NAC were age (<45 years), absence of estrogen receptors, grade, HER2/neu overexpression and high Ki-67. We conclude that the LR rate after NSM in our series was low but the Biological features of disease and young age should be taken into account when considering NSM in breast cancer patients (5).
When we focus on the preserved NAC, there is also a question if the recurrence can happen inside the NAC itself. A study by Lohsiriwat V et al. (6) found Paget’s disease occurred on NAC as a local recurrences represent in 0.8% of total series (7/861 nipple-sparing mastectomies). Despite a very low incidence of LR on NAC, however, we should pay attention on any suspicious lesion on NAC. Especially in the NAC which had been irradiated or partially necroses, the discoloration or ulceration of LR may mimic to radiodystrophy or fibroses scarring.
Conclusions
Nipple Sparing Mastectomy is a psychological effective and oncological safe procedure. It can be offered to patients who require therapeutic mastectomy including invasive carcinoma, intraepithelial carcinoma and benign breast diseases. A careful preoperative and intraoperative selection protocol should be set to accommodate in each institutional settings. Knowledge and experience of breast reconstruction is mandatory as an essential part of NSM. Oncoplastic training or multidisciplinary team approach with surgical oncologist and reconstructive surgeon is encouraged to maximize the benefit of NSM.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.de Alcantara Filho P, Capko D, Barry JM, et al. Nipple-sparing mastectomy for breast cancer and risk-reducing surgery: the Memorial Sloan-Kettering Cancer Center experience. Ann Surg Oncol 2011;18:3117-22 [DOI] [PubMed] [Google Scholar]
- 2.Petit JY, Veronesi U, Lohsiriwat V, et al. Nipple-sparing mastectomy--is it worth the risk? Nat Rev Clin Oncol 2011;8:742-7 [DOI] [PubMed] [Google Scholar]
- 3.Petit JY, Veronesi U, Orecchia R, et al. Nipple sparing mastectomy with nipple areola intraoperative radiotherapy: one thousand and one cases of a five years experience at the European institute of oncology of Milan (EIO). Breast Cancer Res Treat 2009;117:333-8 [DOI] [PubMed] [Google Scholar]
- 4.Barchie MF, Clive KS, Tyler JA, et al. Standardized pretreatment breast MRI--accuracy and influence on mastectomy decisions. J Surg Oncol 2011;104:741-5 [DOI] [PubMed] [Google Scholar]
- 5.Petit JY, Veronesi U, Orecchia R, et al. Risk factors associated with recurrence after nipple-sparing mastectomy for invasive and intraepithelial neoplasia. Ann Oncol 2012;23:2053-8 [DOI] [PubMed] [Google Scholar]
- 6.Lohsiriwat V, Martella S, Rietjens M, et al. Paget’s Disease as a Local Recurrence after Nipple-Sparing Mastectomy: Clinical Presentation, Treatment, Outcome, and Risk Factor Analysis. Ann Surg Oncol 2012;19:1850-5 [DOI] [PubMed] [Google Scholar]
- 7.Lohsiriwat V, Rojananin S, Bhothisuwan K, et al. Prediction of nipple areolar complex involvement in breast cancer. Thai J Surg 2004;25:71-8 [Google Scholar]
- 8.Petit JY, Veronesi U, Orecchia R, et al. Nipple-sparing mastectomy in association with intra operative radiotherapy (ELIOT): A new type of mastectomy for breast cancer treatment. Breast Cancer Res Treat 2006;96:47-51 [DOI] [PubMed] [Google Scholar]
- 9.Petit JY, Veronesi U, Orecchia R, et al. The nipple-sparing mastectomy: early results of a feasibility study of a new application of perioperative radiotherapy (ELIOT) in the treatment of breast cancer when mastectomy is indicated. Tumori 2003;89:288-91 [DOI] [PubMed] [Google Scholar]
- 10.Ahmed AK, Hahn DE, Hage JJ, et al. Temporary banking of the nipple-areola complex in 97 skin-sparing mastectomies. Plast Reconstr Surg 2011;127:531-9 [DOI] [PubMed] [Google Scholar]
- 11.Petit JY, Rietjens M, Lohsiriwat V, et al. Update on breast reconstruction techniques and indications. World J Surg 2012;36:1486-97 [DOI] [PubMed] [Google Scholar]
- 12.Chen CM, Disa JJ, Sacchini V, et al. Nipple-sparing mastectomy and immediate tissue expander/implant breast reconstruction. Plast Reconstr Surg 2009;124:1772-80 [DOI] [PubMed] [Google Scholar]
- 13.Petit JY, Botteri E, Lohsiriwat V, et al. Locoregional recurrence risk after lipofilling in breast cancer patients. Ann Oncol 2012;23:582-8 [DOI] [PubMed] [Google Scholar]
- 14.Lohsiriwat V, Curigliano G, Rietjens M, et al. Autologous fat transplantation in patients with breast cancer: “silencing” or “fueling” cancer recurrence? Breast 2011;20:351-7 [DOI] [PubMed] [Google Scholar]
- 15.Bertolini F, Lohsiriwat V, Petit JY, et al. Adipose tissue cells, lipotransfer and cancer: A challenge for scientists, oncologists and surgeons. Biochim Biophys Acta 2012;1826:209-14. [DOI] [PubMed]
- 16.Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol 2012;38:125-9 [DOI] [PubMed] [Google Scholar]
- 17.De Lorenzi F, Yamaguchi S, Petit JY, et al. Evaluation of skin perfusion after nipple-sparing mastectomy by indocyanine green dye. Preliminary results. J Exp Clin Cancer Res 2005;24:347-54 [PubMed] [Google Scholar]
- 18.Didier F, Arnaboldi P, Gandini S, et al. Why do women accept to undergo a nipple sparing mastectomy or to reconstruct the nipple areola complex when nipple sparing mastectomy is not possible? Breast Cancer Res Treat 2012;132:1177-84 [DOI] [PubMed] [Google Scholar]
- 19.Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat 2009;118:623-33 [DOI] [PubMed] [Google Scholar]


