1. Introduction
As the most common malignancy among women, breast cancer seriously endangers the physical and mental health of women. So far, the application of multidisciplinary treatment has made breast cancer one of the most treatment-responsive solid tumors.
These guidelines are developed to standardize the diagnosis and treatment protocols for this condition and enhance the related practice at medical institutions, so as to improve the prognosis of breast cancer patients and ensure the quality and safety of healthcare services.
2. Diagnosis
The diagnosis and differential diagnosis of breast cancer should be based on the clinical presentations, physical examinations, imaging studies, and histopathologic findings.
2.1. Clinical manifestations
Due to the lack of typical symptoms and signs, early-stage breast cancer (ESBC) is often neglected by patients and typically found during physical examination or breast cancer screening. The typical signs of breast cancer, as listed below, usually occur in the more advanced stages.
2.1.1. Breast Mass
About 80% of breast cancer cases were first diagnosed with breast masses. Patients often recognize their breast mass unintentionally, and the mass tends to be single and hard, with irregular margins and less smooth surface. Most breast cancers are manifested as a painless mass, and only a few had varied degrees of pain or tingling.
2.1.2. Nipple discharge
Nipple discharge can be sanguineous (bloody), serous, mucinous, milky, or purulent. It can also occur half year after the termination of breastfeeding. Causes for nipple discharge vary but commonly include intraductal papilloma, breast hyperplasia, dilation of mammary duct, and breast cancer. The bloody discharge which is effused from unilateral single hole should be further examined; more attention should be paid if the nipple discharge is accompanied with breast mass.
2.1.3. Skin changes
A variety of skin changes can occur in patients with breast cancer. Most commonly, the tumor invades the Cooper’s ligament and then adheres to the skin, showing a “Dimple sign”. When the cancer cells block the lymphatic vessels, an orange peel like texture of the skin occurs. In patients with advanced breast cancer, the cancer cells can infiltrate into the skin along the lymphatic vessels, glandular vessels, and/or fibrous tissue, and then proliferate to form satellite nodules of skin over breast.
2.1.4. Abnormalities of nipple and areola
If the tumor is located deep inside nipple, it can cause the retraction of nipple. In some patients, although tumor is relatively far away from the nipple, it can invade and shrink the large ducts within the mammary gland, and thus cause the retraction or elevation of nipple. Eczematoid carcinoma of nipple, also known as Paget’s disease, is clinically manifested as the itchy nipple, erosion, ulceration, crusting, scaling, which can be associated with causalgia; in addition, nipple retraction also can be found.
2.1.5. Axillary lymphadenectasis
In patients with occult breast cancer, the mass usually is impalpable during physical examination, with axillary lymphadenectasis as the first symptom. The metastasis of axillary lymph node can be detected in one third or more inpatients. The ipsilateral axillary lymph node(s) may become swollen at the early stage, and some swollen lymph nodes can hard, scattered, and movable. With the progression of the disease, the lymph nodes fuse together gradually and adhere to and even fix with the skin and surrounding tissues. The metastatic lymph nodes can be palpable in the supraclavicular area or contralateral axilla in patients with advanced breast cancer.
2.2. Examination of breast: palpation
Patients should be asked in detail about their medical history of breast, menstrual and marital status, and previous family history of cancer (such as breast cancer and ovarian cancer) before the palpation of breast. For premenopausal women, breast palpation is advised to be conducted after the menstruation ends (Definition of menopause shown in Annex 1).
During the palpation, the patients usually keep a sitting or standing position; patients with sagging breasts or relatively large breast, a supine position is recommended. Breast should be observed with naked eyes first, and then palpated; the healthy side should be examined first, followed by the sick side. The palpation should be implemented using the finger pulp in a certain order; nipple, areola area, and armpit area should be carefully examined. Two hands can be used if necessary.
For most patients with breast cancer, mass can be palpable during the palpation, and therefore the disease can be easily diagnosed. However, some early-stage breast cancer can be negative during palpation. For these patients, the presence of the thickened and stiffened breast glands, nipple erosion, nipple discharge, mild retraction of nipple, mildly inverted skin, mild edema of areola, and breast pain after menopause should be carefully observed during physical examinations. The diagnosis should also refer to the results of imaging studies and histopathologic examinations. If necessary, cytological diagnosis should also be conducted.
2.3. Imaging studies
2.3.1. Mammography
The routine positions for mannography includes mediolateral oblique (MLO) and craniocaudal (CC). For patients in whom the conventional positions provide poor view or can not cover the whole breast parenchyma, position can be adjusted based on the site of lesion. To optimize the visualization of the lesion(s), some special photographic techniques (e.g., spot compression mammography, magnification mammography, or spot compression magnification mammography) may be applied.
-
Indications:
Breast mass/stffness, nipple discharge, skin abnormalities of breast, and local pain or swelling.
Abnormality found in screening.
Short-term follow-up of benign lesions.
After breast repair/reconstruction.
During the treatment of breast cancer.
Other conditions that require radiological examination or radiologist consultations.
For women who are under the age of 35, without specific high risk of breast cancer, or without abnormality in clinical examination, mammography is not recommended.
The basic norm of a diagnostic report is shown in Annex 2.
2.3.2. Breast ultrasound
Breast ultrasound is feasible for populations with suspected breast lesions. It can be applied for the examinations of both breast and axillary lymph nodes. The conventional position for breast ultrasound is supine position. The patients usually are scanned from the top of armpit to the lower bounds of two breasts (including the whole breast area and armpits).
-
Indications:
Ultrasound is a preferred imaging examination for young, pregnant, or lactating women with breast lesions.
It can be used for the confirmation of clinically palpable mass and other suspected lesions.
It can also be used for the assess the breast lesions after prosthesis implantation.
It can guide interventional procedures.
The basic norm of a diagnostic report is shown in Annex 2.
2.3.3. Magnetic resonance imaging (MRI) of the breast
MRI is not a routine imaging technique for breast cancer. Nevertheless, it can be used for evaluating the stage of breast cancer, confirming the scope of ipsilateral breast tumor, and estimating the presence of any multifocal or multicentric tumor. Besides, MRI can be used for the screening of contralateral breast tumor in the initial diagnosis. Furthermore, MRI can help to assess the scope of tumor before and after neoadjuvant therapy, the response after treatment, and the feasibility for breast-conserving treatment.
2.4. Histopathological examination
Histopathological diagnosis is the basis of the confirmative diagnosis and treatment of breast cancer, and also is the final diagnosis based on clinical data and pathomorphological findings. A histopathological diagnosis requires complete and accurate clinical data from the clinicians as well as the timely and adequate tissue specimens.
2.4.1. Standard of tissue fixation
Fixation fluid: 10% neutral formalin.
Fixative volume: the recommended ratio of the tissue volume to the fixative volume is at least 1:2. If the specimen is too thick and/or too large, the fixative may be replaced once during the procedure.
Fixation temperature: room temperature.
Fixation time: depends on the specific specimen.
2.4.2. Sampling and processing of tissue specimens
-
The intra-operative specimens must be quickly frozen and then submit for examination.
Verify the specimens and application forms.
Observe the specimen, measure the three dimensions (length × width × height; in cm), and describe its features. If possible, take morphological photos or draw a diagram for the specimen.
The specimens should be quickly obtained from a typical lesion, and then frozen and sliced; if morphological observation shows malignancy, another 1 to 2 tumor specimens should be fixed immediately for immunohistochemical detection.
After a report is sent, the remaining specimens should be immediately sampled, and then fixed for 12 to 24 hours.
Needle aspiration specimen
Needle (including fine needle and core neelde) aspiration specimens are used for cytological and histological analysis.
Verify the specimens and application forms. Count, observe, and describe the specimens; the specimens are stained with eosin, and then wrapped with tissue paper. All the submitted specimens must be sampled (if clinical serial numbers are available, sample according to these numbers). Do not crush or break the specimens, which should be placed parallel and fixed 6 to 12 hours.
Endoscopic specimens
Verify the specimens and application forms. Sample the specimens according to the submission serial numbers, and the specimens should be placed parallel and fixed 6 to 12 hours.
Sectioned specimens
Verify the specimens and application forms. Observe the specimen, measure the three dimensions (length × width × height; in cm), and describe its features. If possible, take morphological photos or draw a diagram for the specimen. For morphological observation, the abnormal sites should be sampled as possible, and then the specimens should be fiexed for 12-24 hours. All the tumors should be completely sectioned. If the tumor is too large, specimens should be obtained from typical sites; at least, the largest section of the tumor (including the junction between tumor and normal tissue) should be sampled.
-
Specimens from breast-conserving surgery
Specimens obtained around the cutting edge
Verify the specimens and application forms. Observe the specimen, measure the three dimensions (length × width × height; in cm), and describe its features. If possible, take morphological photos or draw a diagram for the specimen. Obtain the specimens according to clinical labels (if possible, around the cutting edge). The orientation of each block is recorded. The remaining specimens are fixed 12 to 24 hours before the post-operative sampling. If the sample is submitted from the clinical departments, the specimens should be obtained and sliced using the cutting edge of the sample, and then note in the report.
Specimens obtained after the breast-conserving surgery
Verify the specimens and application forms. For samples that have not undergone pathological examination for the cutting edge, take morphological photos or draw diagrams to locate the tumor site and the nipple. Section is made every 5 mm along the vertical direction of the connection line between the tumor and the nipple. Specimens are obtained sequentially at each section. The orientation of each block is recorded.
-
Mastectomy specimens
Fresh specimens
Verify the specimens and application forms. Observe, measure, and describe the gross specimens. Take photos or draw diagrams if possible. Submission of groups of lymph nodes according to local anatomy and intraoperative findings is recommended for localization of the lymph drainage area. Or, a pathologist dissect (at least 10) lymph nodes in the specimen, and all the detected lymph nodes shall be sampled. Dissect the specimens along the connecting line between the nipple and tumor center/incisal opening/scar, and ensure the bottom of the specimen is connected (to keep the anatomic position). If the specimen is too large, make several sections parallel to the above section. After rinse the blood and wipe the specimen dry, the specimen shall be fixed 24-48 hours for sampling.
Fixation of specimens
Multiple sections were dissected along the direction parallel to the incisal opening that is made on the first day, and then observe and record the findings. The primary sampling sites include the nipple and the tumor, and the tissue with the largest section should be completely sampled. Meanwhile, all the tumor tissues should be sampled. Sites with morphological abnormality should be sampled.
2.4.3. Pathological classification and pTNM staging of breast cancer
The pathological classification, TNM staging of breast cancer, and pTNM staging of breast cancer are listed in Annex 3, 4 and 5.
2.4.4. Others
Histological grading of breast cancer
Breast cancer is graded based on the infiltrating parts of invasive ductal carcinoma.
Tubule formation: in tumor sections, a score of 1 means greater than 75% of cells are in tubule formation (better), a score of 3 is used when less than 10% of cells are in tubule formation (worse), a score of 2 is in between 10% and 75%.
Nuclear pleomorphism: a score is given from 1 to 3 based on the size, shape, and chromoplasm of the tumor cell nucleus, with 1 being the closest to normal cells (better), 3 being the most variation (worse), and 2 in between.
Mitotic count: the score of 1 means 0-5 per 10 HPF; 2, 6-10 per 10 HPF; and 3, >11 per 10 HPF.
The three scores are then combined for a total score between 3 (1+1+1) and 9 (3+3+3). This score translates to a histological grade: Score of 3-5: Grade 1 (or well differentiated); score of 6 or 7: grade 2 (moderately differentiated); and score of 8 or 9: grade 3 (poorly differentiated).
Cancer tissue invasion and lymph node metastasis
Lymphatic vessel invasion: No lymphatic vessel is invaded in tumor section (–); lymphatic vessel is suspected (±); one lymphatic vessel is invaded (+); two lymphatic vessels are invaded (++); three or more lymphatic vessels are invaded (+++); The whole tumor can not be observed due to the sectioning or incomplete specimens (non-assessable).
Blood vessel invasion: the same criteria as above: (–), (±), (+), (++), (+++), and non-assessable.
Nerve invasion: the same criteria as above: (–), (±), (+), (++), (+++), and non-assessable.
Invasion of other tissues: The invasion of the nipple, skin, fatty tissue, chest muscle, and chest walls, including the morphological and miscroscopic findings.
Scope of tumor: based on the actual site of the tumor, the breast is divided into 6 parts: areola and nipple part (E), upper inner (A), lower inner (B), upper outer (C), lower outer (D), and tail of the galactophore (C'). Morphological (M) and microscopic (m) findings are also included.
Lymph node metastasis: the amount of the microscopically confirmed metastatic lymph nodes and the invasion of the adjacent soft tissues.
Histopathological evaluation of the treatment efficacy
The pathomorphological changes after radiation therapy, chemotherapy, endocrine therapy, and molecular targeted treatment can serve as the histopathological evidences for assessing the treatment efficacy. Because these pathomorphological changes usually are similar, their histopathological evaluation criteria for the efficacy are basically the same. The grades are grade 0 (no response), grade I (partial response), grade II (marked response) and grade III (complete response).
-
Determinations of molecular biological markers and genes and their interpretation
Immunohistochemical examination of steroid receptors (ER and PR)
Positive control (internal and external controls) and negative control are set for each batch of staining. For the same batch of stained sections with expected results, the immunohistochemical findings should be interpreted. Evaluate the percentage of positive cells and their pigmentation intensity (strong, medium, or weak) under microscope. The nucleus of cancer cells with yellow pigment indicates that they are ER (PR) positive cells.
Immunohistochemical examination of HER2/neu protein
Positive control (external control) and negative control (internal and external controls) are set for each batch of staining. Only the same batch of stained sections with expected results is subject to the immunohistochemical analysis, and the cell membran pigmentation of the infiltrative cancer cells were judged. The results are classified as (–), (+), (++), and (+++).
Determination of HER2/neu gene with fluorescence in situ hybridization (FISH)
Cancer cells with uniform nucleus dimensions, intact and non-overlapping nucleus boundaries, and clear green signals are selected from the infiltrative carcinoma area, and then the amounts of red and green signals in at least twenty cancer cells were randomly counted. Calculate the ratio (sum of red signals in the twenty nucli/sum of green signals in the twenty nucli), and the results are classified as negative, positive, threshold value, and uninterpretable (Annex 6).
Due to the heterogeneity of breast cancer and the impacts of factors including detection system, antibody, and detection methodology, the detection results may be inconsistent. Therefore, the detection system, detection methodology (full automatic, semi-automatic, and manual), name and concentration of antibody, and name of probe adopted in the initial examination shall be provided in re-examination.
Pathology report
For the content and format of a pathology report for breast cancer, please refer to Annex 7.
3. Differential diagnosis
Breast cancer shall be differentiated from benign diseases such as hyperplasia of mammary glands, fibroadenoma, cyst, intraductal papilloma, mammary duct ectasia (plasma cell mastitis) and breast tuberculosis, from malignant lymph node tumor, and from metastatic secondary malignant breast tumors from other sites. Medical history-taking and physical examination should be carefully conducted for differential diagnosis, in combination with imaging studies such as breast ultrasound, mammography, and breast MRI. Finally, cytological and/or histopathological findings will confirm the diagnosis.
About 80% of breast cancer has clinically palpable masses, which can be histopathologically confirmed through surgical biopsy or puncture. For clinically non-palpable breast cancer, however, puncture may performed after the lesion is located by imaging technique; or, wire localization biopsy under the guidance of breast X-ray may be performed.
Some patients with breast cancer may have nipple discharge, which should be differentiated from hyperplasia of mammary glands, catheter dilatation, latex retention, intraductal papilloma, and papillomatosis. When possible, the cancer cells should be screened through nipple discharge cytology; lacticiferous vessel endoscopy may also be helpful to identify the space-occupying lesions, which can be confirmed by biopsy, if indicated.
4. Treatment
4.1. Treatment principles
Breast cancer should be managed using multidisciplinary approaches. Based on the biological behaviors of the tumor and the physical condition of a specific patient, multiple therapies (including localized treatment and systemic treatment) can be applied to improve the efficacy and improve the patient’s quality of life.
4.1.1. Treatment of non-invasive breast cancers
Lobular carcinoma in situ (DCIS)
Premenopausal patients with lobular carcinoma in situ usually is treated with tamoxifen for 5 years, and postmenopausal patients patients receive tamoxifen or raloxifene to lower the risk. If the possibility of pleomorphic lobular carcinoma in situ can not be ruled out, mastectomy is recommended, and breast reconstruction may be conducted if necessary.
-
Ductal carcinoma in situ (DCIS)
Extended local lumpectomy plus whole breast radiation.
Mastectomy, and sentinel node biopsy and breast reconstruction if necessary.
For patients with pure ductal carcinoma in situ, complete axillary lymph node dissection is not recommended if no evidence of invasive breast cancer is available or the presence of tumor metastasis has not been confirmed. However, invasive carcinoma may be identified during surgery in some patients who have clinically diagnosed as pure ductal carcinoma in situ. These patients should then treated as “invasive carcinoma”. The confirmation of pure ductal carcinoma in situ should be based on the the results of surgical biopsy.
4.1.2. Treatment of invasive breast cancers
Breast-conserving surgery plus radiotherapy.
Modified radical mastectomy, and breast reconstruction if necessary.
Mastectomy plus sentinel node biopsy, and breast reconstruction if necessary.
Breast cancer in the elderly: extended local lumpectomy plus mastectomy; receptor positive patients should also undergo endocrine therapy, and sentinel node biopsy can be performed if necessary.
4.2. Surgical treatment
4.2.1. Principles
The surgery for breast cancer involves both the breast and the axillary lymph nodes. Breast operations include extended lumpectomy and mastectomy. Sentinel lymph node biopsy or axillary lymph node dissection is required to identify any metastasis to the axillary lymph nodes, except for carcinoma in situ. The surgical approach should be properly determined taking into account the clinical staging and physical condition of the patient.
4.2.2. Breast surgery
Mastectomy This operation is indicated for patients with TNM stages 0, I and II, as well as some with TNM stage III who have no surgical contraindications. Modified radical mastectomy has become the mainstream procedure. The traditional Halsted radical mastectomy has been gradually abandoned in most institutions, as it is not associated with increased survival compared with the modified approach in clinical trials.
Breast-conserving surgery Indications for breast-conserving surgery should be strictly observed. To perform the breast-conserving operation, a medical institution should have the necessary equipment and technology for histological examination of surgical margins to ensure a margin-negative resection, and for implementing postoperative radiotherapy as well. The cosmetic evaluation criteria for breast-conserving surgery are attached in Annex 8.
For patients with the willingness, breast-conserving surgery can be done to achieve complete resection with negative margins, while maintaining favorable cosmetic results. A younger age does not necessarily contraindicate breast-conserving procedures, although patients at the age of 35 or below may be at a higher risk of relapse and recurrence of breast cancer. Hence, the patients should be fully informed of the potential risks when considering such surgical procedures.
Absolute contraindications for breast-conserving surgery include a history of breast or chest wall irradiation; radiation therapy required for pregnant patients; extensive lesions where complete resection is impossible; and a final positive margin. Relative contraindications include lesions with a diameter greater than 5 cm and active connective tissue diseases involving the skin, especially scleroderma and lupus erythematosus.
4.2.3. Axillary lymph node surgery
As a part of the standard surgical management for invasive breast cancer, the operation on axillary lymph nodes is mostly used to identify the status of those nodes for staging and determination of the optimal treatment option.
Sentinel lymph node biopsy for breast cancer
The sentinel lymph node (earliest site of lymphatic drainage and metastasis) is resected and subject to histopathological examination for identifying the status of axillary lymph nodes, thus minimizing upper extremity lymphedema as a result of axillary lymph node dissection. Tracers used for sentinel lymph node biopsy include radioactive colloid and blue dye. The biopsy can be considered as an alternative to axillary lymph node dissection for patients without clinical evidence of axillary lymph node metastases. Axillary lymph node dissection is justified by a positive sentinel lymph node biopsy, but may not be necessary when the biopsy is negative.
Axillary lymph node dissection
The dissection will involve all lymph nodes from the leading edge of the latissimus dorsi to the lateral border of the pectoralis minor (Level I), and from lateral border to medial border of the pectoralis minor (Level II). At least ten axillary lymph nodes must be removed to ensure true representation of the metastatic status. Each of the lymph nodes found from the resected specimens, as many as possible, is then histologically examined. Fine and skilled operation is vital to a successful breast-conserving surgery in view of the extensive involvement through a small incision.
4.2.4. One-stage immediate breast repair and reconstruction surgery
The principle of standardized multidisciplinary management for oncological diseases is strictly observed in the treatment of breast cancer. Upon thorough communication with the patients and their families, one-stage immediate breast repair and reconstruction or two-stage delayed reconstruction may be performed in capable institutions when needed.
Patient selection
Most patients with stage I or II breast cancer, whose lesions are resectable upon preoperative evaluation, are eligible for surgical treatment. The patients should be fully informed of any potential complications.
Surgical options
Breast repair and reconstruction should rely on an extensive consideration of the patient’s physical condition, breast cancer staging and injury after radical surgeries, as well as the conditions of the contralateral breast.
For patients undergoing local tumor resection, reconstruction can be done with the transfer of partial breast tissues or latissimus dorsi muscle flap in view of the relatively small tissue defects. In the case of a large or drooping contralateral breast, breast reduction or lift can be conducted on that side as well;
For young patients undergoing mastectomy alone without breast skin defects or with only small defects, who do not need postoperative radiotherapy, a prosthesis can be directly placed under the pectoralis major;
For serious defects following radical operations, autologous muscular flap may be used for breast reconstruction, such as the TRAM flap, the inferior epigastric artery perforator flap, and the latissimus dorsi myocutaneous flap;
-
When postoperative irradiation is planned before surgery, reconstruction with autologous tissues is preferred to any prosthesis. When the need for radiotherapy is undetermined before surgery and prosthetic breast reconstruction is planned, a tissue expander can be immediately placed beneath the pectoralis major for patients with a skin defect smaller than 4 cm who need irradiation, which could be replaced with a permanent prosthesis upon the end of radiotherapy.
Postoperative care
Care after breast reconstruction is essential for smooth, timely implementation of subsequent treatments. For patients with prosthetic reconstruction or expander placement, a patent drainage and absence of dead space under the flap should be guaranteed in addition to the routine care as given for patients following breast augmentation. When autologous tissues are used for reconstruction, the flap blood supply should be closely monitored, and a favorable body position and immobilization should be maintained for those with abdominal flaps.
Multidisciplinary management and regular follow-up
Immediate breast repair and reconstruction surgery does not affect the tumor treatment or regular follow-up visits. Chemotherapy, radiotherapy, endocrine therapy and targeted therapy can be properly scheduled based on the pathological results in two to three weeks after surgery. The follow-up purpose and intervals are determined in accordance with the oncology treatment guidelines. Since silicone prostheses are opaque to radiation, color Doppler ultrasound, MRI or other imaging studies may be used in the follow-up of patients with prostheses.
4.3. Radiation therapy
4.3.1. Early postoperative radiotherapy following breast-conserving surgery
In principle, postoperative radiation is required for all of the patients undergoing breast-conserving surgery, using either conventional or intensity modulated therapy. Endocrine therapy alone may be considered for hormone receptor-positive patients with TNM stage over 70 years of age.
-
Radiation target region
The ipsilateral breast is the target when either axillary lymph node dissection or sentinel lymph node biopsy is negative, or one to three nodes of metastasis are found but dissection is complete (with ten lymph nodes detected), and the patient is not at a high risk for recurrence;
The ipsilateral breast and supraclavicular/subclavicular lymphatic drainage area are included when four or more axillary lymph nodes are involved;
The ipsilateral breast and/or the supraclavicular/subclavicular lymphatic drainage area are targeted when only one to three metastatic nodes are detected but there are other risk factors of recurrence, including an age of 40 or below, hormone receptor-negative status, insufficient number of lymph nodes from dissection or metastasis found in more than 20% nodes, and Her-2/neu overexpression;
The ipsilateral breast, axilla, and supraclavicular/subclavicular lymphatic drainage area are irradiated when the axillary nodes are not surgically explored or not dissected despite positive sentinel findings.
-
Radiation therapy target planning and measurement
Field for conventional radiation treatment of the breast/chest wall: medial and lateral tangential fields of the whole breast are irradiated.
Upper bound: lower edge of the clavicular head, i.e. lower edge of the first rib.
Lower bound: 1-2 cm inferior to the breast skin folds
Medial margin: body midline
Lateral margin: middle axillary line or posterior axillary line
Radiation dose: 6MV X-ray, whole breast DT 50 Gy/ 5 weeks/25 times, without filler or tissue compensator. An additional boost dose may be needed for the primary tumor bed.
Boost dose for the primary tumor bed: electronic or X-ray tangential field with the appropriate energy is applied to the area 2-3 cm around the intraoperative silver marker or surgical scar in the simulator.
Total amount of boost dose: DT 10-16 Gy/1-1.5 weeks/5-8 times. The additional boost dose may be delivered using high dose rate brachytherapy.
Field for conventional radiation treatment of the supraclavicular/axillary area:
Upper bound: level of the cricothyroid membrane.
Lower bound: joining with the upper bound of the breast/chest wall field, i.e. lower edge of the first rib level.
Medial margin: from body midline to medial edge of the sternocleidomastoid muscle at the level of the sternal notch.
Lateral margin: medial side of the humeral head.
Radiation dose: DT 50 Gy/5 weeks/25 times. Mixed irradiation with electronic and X-rays is possible to minimize the exposure dose of the pulmonary apex and for ease of convergence with the breast tangential field.
Intensity-modulated conformal radiation therapy: each layer of the target region and organs at risk should be outlined on the CT image to reduce the gradient of the radiation doses and improve dose uniformity for better cosmetic results. The short and long term side effects can thus be reduced by minimizing the exposure dose of normal tissues such as the lung, cardiovascular system and the contralateral breast. Either forward or inverse planning of intensity-modulated radiation treatment can be adopted (with the focus on medial and lateral tangential fields). Younger patients with larger breasts may have greater benefit from the procedure. The whole breast and surgical scars should be marked with lead wire prior to CT scan for determining the target area for whole breast irradiation and the tumor bed.
4.3.2. Radiation therapy following modified radical mastectomy
-
Indications Postoperative radiotherapy is required for patients undergoing postoperative systemic therapy, including chemotherapy and/or endocrine therapy, who have one of the following risk factors:
diameter of the primary tumor greater than or equal to 5 cm, or tumor invasion to breast skin and chest wall;
presence of four or more metastatic axillary lymph nodes.
postoperative radiation may be considered for patients with T1, T2, one to three metastatic lymph nodes and one of the risk factors for recurrence (age of 40 or below, hormone receptor-negative status, insufficient number of lymph nodes from dissection or metastasis found in more than 20% nodes, and Her-2/neu overexpression).
-
Radiation therapy target and dose
Supraclavicular/subclavicular fields.
Upper bound: level of the cricothyroid membrane.
Lower bound: joining with the upper bound of the chest wall field, i.e. lower edge of the first rib level.
Medial margin: from body midline to medial edge of the sternocleidomastoid muscle at the level of the sternal notch.
Lateral margin: medial side of the humeral head.
Radiation dose: DT 50 Gy/5 weeks/25 times. Mixed irradiation with electronic and X-rays is possible to minimize the exposure dose of the pulmonary apex.
Chest wall field
Upper bound: lower edge of the clavicular head, i.e. lower edge of the first rib.
Lower bound: 1-2 cm inferior to the contralateral breast skin folds
Medial margin: body midline
Lateral margin: middle axillary line or posterior axillary line
Radiation dose: DT 50 Gy/5 weeks/25 times of electronic or X-ray for the whole chest wall.
A whole chest wall pad compensator is routinely used for electronic radiation at DT 20 Gy/2 weeks/10 times to increase the surface dose. The chest wall thickness is routinely determined by ultrasound. The thickness of the filler (tissue compensator) is then adjusted accordingly, and the energy of the electronic ray is decided based on the thickness to reduce the exposure dose of the lung and big vessels of the heart, so as to minimize radiation-induced lung injury. The chest wall compensator is required to increase the skin exposure dose when delivering X-ray tangential field irradiation.
-
Axillary radiation field Axillary irradiation is warranted when a patient has not undergone axillary lymph node dissection or the dissection is deemed incomplete.
Supraclavicular/axillary field
Radiation scope: supraclavicular and axillary areas, converging at the chest wall field.
Radiation dose: 6 MV X-ray, DT 50 Gy/5 weeks/25 times for the supraclavicular area. The depth of the supraclavicular area is calculated as 3 cm subcutaneously. The axillary depth is calculated based on actual measurements. A boost dose is added to make up the dose to DT 50 Gy, if needed.
Posterior axillary field
Upper bound: inferior edge of the clavicle.
Lower bound: inferior margin of the axilla.
Medial margin: medial edge along the thorax.
Lateral margin: medial side of the humeral head.
Radiation dose: 6 MV X-ray, boost to DT 50 Gy.
For patients with primary tumors located in the medial quadrant and axillary lymph node metastases, internal mammary irradiation may be considered, though such administration is controversial. In conventional positioning, the internal mammary field should include the first to the third intercostal spaces, with the upper bound converging with the supraclavicular field and the lower bound exceeding 0.5-1 cm over the body midline. The width is generally 5 cm, and electronic ray is used in principle when a dose of 2/3 and above is required to reduce the heart exposure.
Compared to the two-dimensional treatment, the three-dimensional treatment planning based on CT localization can significantly improve the target dose uniformity, thus reducing unnecessary exposure of normal tissues. Field convergence is beneficial to patients with irregular anatomical characteristics. During conventional positioning, it is also recommended to optimize the dose reference point in the three-dimensional treatment planning system, identify the angle of the wedge filter and assess the volume and dose of normal tissues for the complete coverage of the target radiation and minimized radiation injury.
4.3.3. Radiation therapy following neoadjuvant chemotherapy or modified radical mastectomy
The indications are similar to those for patients who do not receive neoadjuvant chemotherapy. The initial staging prior to neoadjuvant chemotherapy is used as the basis for planning. The irradiation techniques and doses are similar to those in the radiotherapy following the modified radical operation before neoadjuvant chemotherapy.
When adjuvant chemotherapy is indicated, the postoperative radiation therapy should be carried out upon completion of adjuvant chemotherapy; otherwise, it is prescribed in 8 years after surgery as long as the wound healing is satisfying. The additional Herceptin therapy can be performed concurrently with postoperative radiotherapy. Before the start of radiation therapy, it should be ensured that the left ventricular ejection fraction (LVEF) is higher than 50%. Internal mammary field radiation should be avoided and the dose to the minimized, especially when the lesion is located on the left side.
4.3.4. Radiation therapy for local recurrence following radical or modified radical mastectomy
Recurrence is most commonly seen in the chest wall and the supraclavicular lymph drainage area following radical mastectomy or modified radical mastectomy. For a single recurrence of the chest wall, radiation therapy is generally performed after surgical resection; for any unresectable lesions, radiation therapy should be carried out first. For patients with no history of radiotherapy, the radiation should cover the chest wall and the supraclavicular/subclavicular area. In the case of supraclavicular recurrence, the whole ipsilateral chest wall should be included for patients with no prior postoperative radiation. Prophylactic irradiation of the axillary and internal mammary area is not required if there is no axillary or internal mammary lymph node recurrence. The radiation dose for prophylactic sites is DT 50 Gy/5 weeks/25 times; with a boost dose to DT 60-66 Gy/6-6.5 weeks/30-33 times for the reduced field of recurrence sites. A small local radiation field can be established for patients with recurrence despite previous radiotherapy, when necessary.
A cytological or histological diagnosis of the tumor lesion is required for locoregional recurrence.
4.4. Chemotherapy
4.4.1. Chemotherapy for advanced breast cancer
Therapies for advanced breast cancer are not intended to cure but to improve the quality of life and survival. These include mainly chemotherapy and endocrine therapy, and surgery or radiation therapy may also be considered if necessary. An individualized, multidisciplinary regimen in the appropriate time frame is used according to the characteristics of the primary lesion, treatment history, disease-free survival, metastases, progression rate and physical conditions.
-
Chemotherapy is preferred for patients who meet one of the following:
younger than 35 years;
presenting with rapid disease progression and the need for rapid alleviation of related symptoms;
ER/PR-negative;
having symptomatic visceral metastases.
-
Chemotherapy drugs and programs:
A number of drugs are effective against breast cancer, including anthracyclines, taxanes, vinorelbine, capecitabine, gemcitabine, and platinum drugs;
An individualized prescription should be developed based on the patient’s characteristics and therapeutic purposes;
Sequential single-agent chemotherapy is suitable for patients with fewer metastatic sites and slower progression whose vital organs are not involved. In this case, care should be given to the patient’s tolerance and quality of life;
Combination chemotherapy is used for those with extensive disease and evident symptoms for whom rapid reduction of the lesion is needed;
A chemotherapy agent should be avoided if applied previously. An anthracycline-based program or one using anthracycline taxane drugs is used for the initial chemotherapy, while regimens containing taxane are preferred in whom anthracycline treatment has failed. When both anthracycline and taxane therapies fail, a single-agent or combination chemotherapy containing vinorelbine, capecitabine, gemcitabine or platinum can be considered.
4.4.2. Adjuvant chemotherapy for operable breast cancer
An extensive analysis incorporating the general conditions (age, menopausal status, blood routine results, vital organ functions, and presence of other diseases), tumor characteristics (pathologic type, differentiation degree, lymph node status, HER-2 and hormone receptor status, presence of vascular invasion and tumor thrombosis) and treatment (chemotherapy, endocrine therapy, and targeted drug therapy) will be needed to weigh the risks and benefits of postoperative adjuvant chemotherapy.
-
Indications:
Positive findings in axillary lymph node dissection;
Endocrine therapy alone may be considered for postmenopausal patients with a few lymph node metastases (one to three), receptor-positive and HER2-negative status, a stage I lesion of smaller size and other indicators for better prognosis, or those who can not tolerate or are ineligible for chemotherapy;
For lymph node-negative breast cancer, adjuvant chemotherapy is only applicable to patients with risk factors for recurrence (age <35, tumor diameter ≥2 cm, stage II-III, vascular tumor thrombus, HER2-positive, ER/PR negative, etc.).
-
Regimen and precautions
a combination chemotherapy containing anthracycline is preferred; commonly used agents include: CA (E) F, AC (C: cyclophosphamide, A: adriamycin, E: epirubicin, F: fluorouracil pyrimidine);
anthracycline-taxane combination chemotherapy, such as TAC (T: taxotere);
anthracycline and taxane sequential programs, such as AC→T/P (P: paclitaxel) or FEC→T;
non-anthracycline combination chemotherapy regimens are used for older patients with lower risks, contraindications or intolerability for anthracycline; commonly used agents include: CMF (C: cyclophosphamide, M: methotrexate, F: fluorouracil) or TC (T: docetaxel, C: cyclophosphamide);
there are four to eight cycles generally but can vary in different chemotherapy regimens. It is not recommended to reduce the number of cycles or dose amount unless specifically indicated. Individualized addition of adjuvant chemotherapy is possible in patients over 70;
adjuvant chemotherapy should not be concurrently administered with tamoxifen or postoperative radiation therapy;
women of childbearing age may undergo pregnancy test to ensure that no chemotherapy is implemented during their pregnancy. Contraception should be taken during the chemotherapy period;
all patients to be treated with chemotherapy should provide informed consent before treatment.
4.4.3. Neoadjuvant chemotherapy
Used prior to surgery or regional radiotherapy during surgery, neoadjuvant chemotherapy is a systemic procedure for reducing the clinical stage and increasing the rate of successful resection and breast conservation.
-
Indications:
clinical stage of IIIA (excluding T3, N1, M0), IIIB, IIIC;
clinical stage of IIA, IIB, IIIA (limited to T3, N1, M0), with other indications for breast-conserving surgery in addition to the tumor size.
Chemotherapy regimen
All of the postoperative adjuvant regimens can also be used for neoadjuvant chemotherapy. Anthracycline and/or taxane-based combination programs are recommended. Commonly used chemotherapy regimens include:
anthracycline program: CAF, FAC, AC, CEF, FEC (C: cyclophosphamide, A: adriamycin; E: epirubicin, F: fluorouracil pyrimidine);
anthracycline and taxane programs: A (E) T, TAC (T: docetaxel);
anthracycline and taxane sequential programs: AC→T/P (T: docetaxel, P: paclitaxel);
other potentially effective programs;
-
trastuzumab monoclonal antibody therapy may be added for HER-2 positive patients.
-
Precautions
Core needle biopsy on the primary lesion is required for definite histological diagnosis and immunohistochemical examination prior to chemotherapy. Cytological diagnosis may be used for regional lymph node metastasis;
Neoadjuvant chemotherapy is implemented upon a definite histopathological diagnosis;
The neoadjuvant procedure is not recommended for stage I patients;
Four to eight cycles are needed in general;
The therapeutic effect on the primary tumor and axillary lymph node metastasis should be evaluated both physically and radiologically in accordance with the Response Evaluation Criteria in Solid Tumors or the WHO criteria;
The chemotherapy must be terminated if unresponsive, and surgery, radiation therapy or other systemic treatment should follow (using alternative chemotherapy program or neoadjuvant endocrine therapy);
Radical mastectomy, modified radical mastectomy or breast-conserving surgery is used after neoadjuvant chemotherapy according to the individual conditions;
The postoperative adjuvant chemotherapy should be based on the cycles and efficacy of neoadjuvant chemotherapy and postoperative pathological findings.
-
4.5. Endocrine therapy
4.5.1. Endocrine treatment for advanced breast cancer
-
Indications for endocrine therapy as the treatment of choice
older than 35 years;
disease-free survival of greater than 2 years;
only bone and soft tissue metastases are present;
presence of asymptomatic visceral metastases;
ER and/or PR positive status.
-
Drug selection and precautions
Appropriate endocrine agents should be determined according to the menstrual status of patients. Tamoxifen is generally preferred in pre-menopausal patients, followed by combined drugs or surgical castration. The third-generation aromatase inhibitors are preferred in postmenopausal patients, while aromatase inhibitors can also be chosen for those with a menopausal status through medication or surgery.
When tamoxifen and aromatase inhibitors fail, chemotherapy or other endocrine agents may be considered as an alternative, such as progesterone or toremifene.
4.5.2. Adjuvant endocrine therapy
Indications: hormone receptor (ER and/or PR) positive, early breast cancer.
-
Drug selection and precautions:
tamoxifen is the first choice in adjuvant endocrine treatment for premenopausal patients;
pre-menopausal high-risk patients may receive ovarian suppression/removal as well;
during tamoxifen treatment, if the patient has menopause, an aromatase inhibitor may be used instead;
the third-generation aromatase inhibitors are preferred in post-menopausal patients, and use as the initial agent is recommended;
postmenopausal patients who can not tolerate aromatase inhibitors may still use tamoxifen;
postoperative adjuvant endocrine therapy may last up to a maximum of 5 years;
the treatment may prolong for patients at a high risk of relapse, while the medication should be limited to the third-generation aromatase inhibitors. An individualized treatment plan will be required;
ER and PR negative patients are not eligible for adjuvant endocrine therapy.
4.6. Targeted therapy
Targeted therapy with trastuzumab monoclonal antibody may be used for HER-2 positive patients.
4.6.1. Definition of the HER-2 positive status
HER-2 gene overexpression: immunohistochemical staining 3+, FISH-positive or positive chromogenic in situ hybridization (CISH);
For HER-2 immunohistochemical staining (2+) patients, further FISH or CISH detection is required to identify the HER-2 gene amplification.
4.6.2. Precautions
Pathological evidence of a HER-2 positive status should be obtained before treatment;
Trastuzumab monoclonal antibody is administered at 6 mg/kg (with an initial dose of 8 mg/kg) every three weeks or 2 mg/kg (initial dose of 4 mg/kg) weekly;
The patient is observed 4-8 hours after the first dosage;
The medication is generally not used concurrently with doxorubicin chemotherapy, though sequential use is possible;
Concurrent administration with non-anthracycline chemotherapy, endocrine therapy and radiation therapy is allowed;
-
LVEF should be measured before starting the use of trastuzumab monoclonal antibody and monitored once every three months. In any of the following cases, trastuzumab monoclonal antibody therapy should be suspended for at least four weeks, and LVEF measured once every four weeks:
Absolute decrease of ≥16% in LVEF compared with before treatment;
Absolute decrease of ≥10% and LVEF below the lower limit of the testing center;
Trastuzumab monoclonal antibody may be continued when LVEF returns to normal in 4-8 weeks or absolute decrease ≤15%;
The therapy should be permanently terminated in the case of consecutive decrease of LVEF for more than eight weeks, or suspension due to cardiomyopathy for more than three times.
4.6.3. Targeted therapy for advanced Her-2 positive breast cancer
-
Trastuzumab monoclonal antibody combined with chemotherapy
paclitaxel (weekly program);
docetaxel;
vinorelbine;
capecitabine;
other medicines or combined program may also be considered.
-
Precautions
combined chemotherapy using the monoclonal antibody trastuzumab is recommended for patients with advanced diseases;
for ER and/or PR-positive patients, trastuzumab may be administered concurrently with endocrine therapy.
4.6.4. Postoperative adjuvant targeted therapy for Her-2-positive breast cancer
-
Indications:
HER-2 gene amplification or overexpression detected in the invading lesion;
the longest diameter of the invading mass greater than 1 cm or positive axillary lymph nodes found;
absence of contraindications for trastuzumab.
-
Precautions
The therapy should not be concurrently delivered with anthracycline agents, though concurrent use with taxane is allowed. Trastuzumab monoclonal antibodies are sued during or after taxane-based adjuvant chemotherapy;
Trastuzumab monoclonal antibody adjuvant therapy should last up to a maximum of one year;
During the therapy, adjuvant radiotherapy and adjuvant endocrine therapy may be implemented.
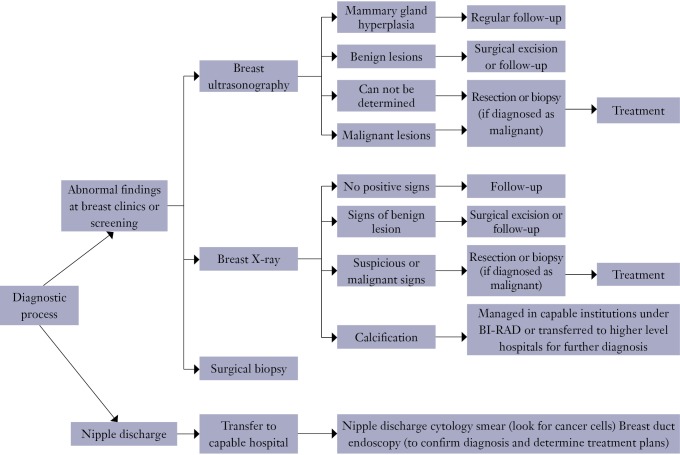
5. Diagnosis and treatment process
5.1. Diagnostic process (Figure 1).
Figure 1.
Diagnostic process.
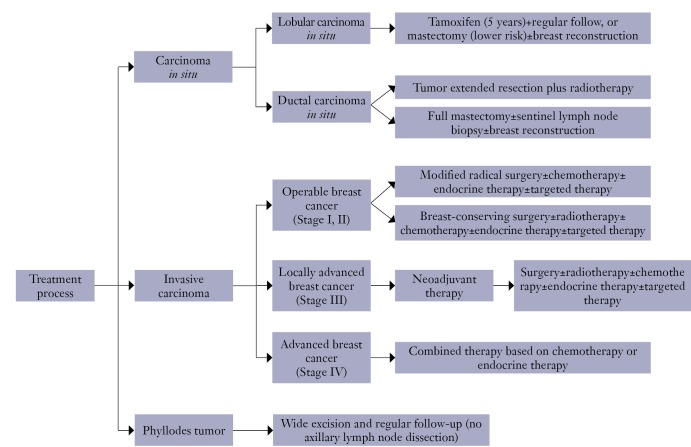
5.2. Treatment process (Figure 2).
Figure 2.
Treatment process.
| Stage 0 | TisN0M0 |
| Stage I | T1N0M0 |
| StageIIA | T0N1M0 |
| T1N1M0 | |
| T2N0M0 | |
| StageIIB | T2N1M0 |
| T3N0M0 | |
| StageIIIA | T0N2M0 |
| T1N2M0 | |
| T2N2M0 | |
| T3N1, 2M0 | |
| StageIIIB | T4N0M0, T4N1M0, T4N2M0 |
| StageIIIC | Any T, N3M0 |
| StageIV | Any T, AnyN, M1 |
6. Follow-up
6.1. Clinical examination: once every 4-6 months in the first two years, once 6 months in the following three; years, and once every year in the following five years;
6.2. Breast ultrasound: once every six months;
6.3. Mammography: once every year;
6.4. Chest X-ray: once every year;
6.5. Abdominal ultrasound: once every six months, and once every year after three years;
6.6. Patients with four or more metastatic axillary lymph nodes should receive baseline bone scan, whole body bone scan once every year and every two years after five years;
6.7. Blood, blood biochemistry and breast cancer markers once every six months, and once every year after three years;
6.8. Annually pelvic examination for patients on tamoxifen.
Annex
Annex 1 Definition of menopause
Menopause is a term used to describe the permanent physiological, or natural, cessation of menstrual cycles. In patients with breast cancer, treatment can also cause the permanent loss of the ovarian function of estrogen synthesis. The criteria for menopause are as follows:
Following the bilateral oophorectomy;
age≥60 years;
age<60 years, has became menopausal for over 1 year without receiving chemotherapy, tamoxifen, toremifene, or ovarian ablation, and meanwhile the blood follicle-stimulating hormone (FSH) and estradiol levels meet their criteria for postmenopausal status; for post-menopausal women who are under 60 years and receiving treatment with tamoxifen and/or toremifene, blood FSH and estradiol levels should be continuously detected to ensure that they are within the normal thresholds after menopause.
Furthermore, the following points should also be emphasized:
It is impossible to judge whether a woman who is treated with an LH-RH agonist or antagonist has reached menopause.
For women who have not reached menopausal before the initiation of adjuvant chemotherapy, the cessation of menstrual cycles can not be used as an evidence of menopause, because most female patients will stop ovulation or become amenorrheac during themotherapy. Nevertheless, these women still have (or, have the potential to regain) the normal ovarian functions.
For women who are in menopause after chemotherapy, if an aromatase inhibitor is considered for endocrine therapy, effective ovarian suppression should be considered (by bilateral ovariectomy or medical ablation); or, the blood FSH and/or estradiol levels should be continuously and frequently monitored to make sure that the patients have reached menopause.
Annex 2 Basic requirements for breast cancer imaging report
1. Mammography
The report should cover the following five aspects:
1.1. Clinical disease history and examination indications
Asymptomatic screening or description of clinical symptoms.
1.2. Mammographic breast composition
The mammographic breast composition is divided into four types based on the glandular density: predominantly fatty tissue, scattered glandular tissue, heterogeneously dense, and extremely dense tissue.
1.3. Findings of mammography
The lesion(s) should be described using the terminology of mammography.
Mass: Size, morphology, margin, and density;
Calcification: morphology and distribution;
Architectural distortion;
Special signs.
Specify the most accurate location using O’clock and/or quadrant.
1.4. Retrieval of prior films
1.5. Assessment Categories
1.5.1 Assessment Is Complete
Category 0: Further imaging evaluation (e.g., additional views including spot compression and magnification or ultrasound) or retrieval of prior films is required.
1.5.2 Assessment Is Complete - Final Categories
Category 1: Negative; no significant abnormality is detected by mammography.
Category 2: Benign findings; definite benign lesion(s), with out malignant signs, is found. It includes calcified fibroadenoma, multiple secretory calcification, lipid-rich lesions (fatty cysts, lipoma, lactiferous duct cyst, and hamartoma of the mixed density), intramammary node, vascular calcifications, implants, and architectural distortion related to prior surgery.
Category 3: Probably Benign Finding-Initial Short-Interval Follow-Up Suggested It is expected that the lesion may become stable or shrunk over the short-term follow-up (less than one year, usually 6 months), but the radiologist would prefer to establish its stability. A finding placed in this category should have less than a 2% risk of malignancy. Three specific findings are described as being probably benign (the noncalcified circumscribed solid mass, the focal asymmetry and the cluster of round (punctate)calcifications; It is recommended that this category will be managed with an initial short-term follow-up (6 months) examination with mammography on the sick side, followed by additional examinations in the 12 months and 24 months by mammography on the contralateral breast. If the lesion(s) keeps stable, mammography-based follow-up may continue; however, biopsy may be considered if the disease progresses.
Category 4: Suspicious Abnormality (but without classic appearance of malignancy) - Biopsy should be considered This category is reserved for findings that do not have the classic appearance of malignancy but have a wide range of probability of malignancy that requires clinical intervention. By subdividing Category 4 into 4A, 4B and 4C, clinicians and their patients can make an informed decision on the ultimate course of action.
4A: Low suspicion of malignancy. If biopsy or cytology shows benign results (which usually is reliable), the patient can be routinely followed or receive a follow-up half year later. Palpable solid mass with clear margin, fibroadenoma, and palpable complex cyst, and palpable abscess can be classified as category 4A.
4B: intermediate suspicion of malignancy A consensus reached between pathologists and radiologists is important for the reliability of biopsy results of this category. If a partially infiltrative masses with clear margin is diagnosed as fibroadenoma or fat necrosis, the diagnosis is acceptable, and the patient should be followed up. It the biopsy shows “papilloma”, a further biopsy should be performed to verify the diagnosis.
4C: moderate concern, but not classic for malignancy Poorly circumscribed opacities with ill-defined and irregular contours and newly onset clusters of microcalcifications can be classified as category 4C.
Category 5: Highly Suggestive of Malignancy, with typical imaging findings of breast cancer and a high probability (≥95%) of being malignancy - Appropriate Action Should Be Taken (Almost certainly malignant) High-density mass with irregular contours and spiculated margin, microcalcifications with a segmental or galactophorous distribution, poorly circumscribed opacities with ill-defined and irregular contours associated with irugular and pleomorphic calcification can be classified as category 5.
Category 6: Known Biopsy-Proven Malignancy - Appropriate Action Should Be Taken. This category is reserved for lesions identified on the imaging study with biopsy proof of malignancy prior to definitive therapy. This category was added for assessing the imaing changes after biopsy or for monitoring the imaging changes in patients treated with neo-adjuvant chemotherapy before surgery.
2. Ultrasound
The ultrasound characteristics include:
2.1. Clinical disease history and examination indications.
2.2. Availability of prior ultrasound results.
2.3. Scope and technology of sonography.
2.4. Description of lesions.
Specify the type of mammary tissue that has been scanned.
Measure the size of lesion (at least two diameters). Small simple cysts need no complete measurement.
Locate the lesion (require O’clock; the distance from nipple should also be noted).
Briefly describe the lesion using ultrasound terminology.
2.5. Combined physical examination, mammography, MRI, and other imaging studies
2.6. Classification and recommendation for further imaging/investigation
2.6.1 Assessment Is Complete
Category 0: The Assessment is not completed yet, and further imaging evaluation or retrieval of prior films is required.
2.6.2 Assessment Is Complete - Final Categories
Category 1: Negative; no significant abnormality.
Category 2: a definitely benign finding, which often includes: simple cyst; intramammary node; implants; stable post-operative changes; and stable fibroadenoma during follow-up.
Category 3: Probably Benign Finding-Initial Short-Interval Follow-Up Suggested Circumscribed oval/round solid masses with transverse diameter larger than height are highly possible to be fibroadenoma; non-palpable complex cyst and clustered microcyst also belong to this category.
Category 4: Suspicious or Indeterminate abnormality, and a biopsy should be recommended. This catergory includes ultrasound-detected solid masses, which have no typical features of fibroadenoma and other benign lesions.
Category 5: Highly suggestive of malignancy - Appropriate Action Should Be Taken (Almost certainly malignant). Ultrasound shows typical abnormalities, with a risk of malignancy higher than 95%. The definitive treatment should be initiated. Before the initiation of sentinel lymph node imaging and neoadjuvant chemotherapy, ultrasound-guided core needle biopsy should be conducted for histological diagnosis.
Category 6: Known Biopsy-Proven Malignancy - Appropriate Action Should Be Taken. This category is reserved for lesions identified on the imaging study with biopsy proof of malignancy prior to definitive therapy. This category was added for assessing the imaing changes after biopsy or for monitoring the imaging changes in patients treated with neo-adjuvant chemotherapy before surgery.
Annex 3 Histologic classification of breast cancer
1. Non-invasive breast cancer
1.1. Ductal carcinoma in situ (DCIS)
Ductal carcinoma in-situ (DCIS) consists of malignant tumor cells that are confined within the mammary ducts and have not invaded beyond the basement membrane boundary into surrounding tissue. The histologic subtypes of DCIS include solid, cribriform, papillary, micropapillary, and comedo. Depending on the degree of nuclear atypia and presence or absence of intraductal necrosis, karyokinesis, and calcification, DCIS can be stratified into three grades. When DCIS of different grades co-exists, or when different DCIS structure is present in the same biopsy tissue or the same duct, the proportions of DCIS of different grades can be recognized.
1.2. Lobular carcinoma in situ (LCIS)
LCIS is located at the terminal duct lobular unit (TDLU), and 75% of LCIS cases has pagetoid extension in the terminal ducts. lobular structure can be found under low powered microscope. The proliferation of cells causes the dilation, although at different levels, of the acini in one or more lobules. The common (conventional) cells that proliferate usually are uniform and small in size; they have round nucleus, uniform size, unclear nucleolus, evenly distributed chromatin, scarce cytoplasm, and unclear cellular contour; the cells usually is loosely lined, with relatively less necrosis, calcification, and karyokinesis. The cell variants include Large acini, polymorphic cell, signet ring cell, apocrine cells, acne-type.
1.3. Paget’s disease of the nipple
Malignant glandular epithelial cells occur in the squamous epithelium of nipple and areola, under which there is often intraductal carcinoma. When remarked invasive carcinoma exists, the disease should be classifies according to the histological classification of invasive carcinoma, with a note of “Paget’s disease of the nipple”.
2. Precursor lesions
2.1. Lobular neoplasia
A small number of cancer cells in part of the ducts break through the basement membrane and pullulate and invade the stroma; the infiltrative cancer cells do not break away from the duct walls.
2.2. Lobular neoplasia
The cancer cells of lobular carcinoma in situ break through the basement membrane of terminal ducts or acini, and then invade the intralobular stroma; nevertheless, the cancer cells are still confined within the lobules, and no interlobular stroma was invaded.
2.3. Microinvasive carcinoma
Microinvasive carcinoma refers to the one or more microscopically identified microinvasive lesions in the interlobular stroma in women with breast carcinoma in situ. If invasion can not be confirmed, the microinvasive lesions should be diagnosed as carcinoma in situ.
2.4. Invasive breast cancer
2.4.1 Invasive ductal carcinoma
(1) “Not otherwise specified”
Invasive ductal carcinoma not otherwise specified (NOS) refers to the largest group of invasive breast cancer that lacks typical features and therefore can not be separately classified as a histological type like lobular carcinoma or ductal carcinoma. If an invasive ductal carcinoma is accompanied with extensive ductal carcinoma in situ components (that is, the ductal carcinoma in situ components account for over 80% of the whole cancer components), the proportion of ductal carcinoma should be noted when a diagnosis of “Invasive ductal carcinoma NOS” is made.
(2) Mixed type carcinoma
A diagnosis of “invasive ductal carcinoma NOS” is made if over 50% of the tumor region of the sampled section shows NOS morphology. Otherwise, the tumor should be classified as mixed type carcinoma; meanwhile, it is recommended to lable the subtypes and proportions of the accompanied NOS.
(3) Pleomorphic carcinoma
Pleomorphic carcinoma, a rare variant of high-grade invasive ductal carcinoma NOS, is characterized by variably sized and shaped cells (often including multinucleate giant cells), with atypical nuclei. They account for 50% of the lesion volume, often with adenocarcinoma or adenocarcinoma with fusiform or scalelike differentiation as their background.
(4) Carcinomas with osteoclast giant cells
Osteoclast-like giant cells, together with inflammatory cell infiltration, fibroblast proliferation, and angiogenesis, can be found in the stroma of these carcinomas. Meanwhile, the extravasation of red blood cells, lymphocytes, and monocytes can also be observed. These cells are lined together with tissue cells, some of which contain hemosiderin. The giant cells of different sizes are located around the epithelial components or in the cavities formed by the cancer cells, containing varying numbers of nuclei. The cancerous tissues in some of these carcinoma are often highly or moderately differentiated invasive ductal carcinoma, whereas many other cancer types, particularly invasive cribriform carcinoma, tubular carcinoma, mucinous carcinoma, papillary carcinoma, lobular carcinoma, squamous cell carcinoma, and metaplastic carcinoma, can also be found.
(5) Carcinoma with choriocarcinoma features
Plasma beta-human chorionic gonadotropin (β-HCG) can rise in patients with invasive ductal carcinoma NOS, and β-HCG-positive cells can be found in 60% of the cases. Very few cases can be accompanied with carcinoma with choriocarcinoma features. Up to now few cases have been reported, all in women aged 50 to 70 years.
(6) Carcinoma with melanotic features
Carcinoma with melanotic features refers to some rare tumors in the breast stroma, featured by the co-existence of ductal carcinoma and malignant melanoma; occasionally, a transition from one cell type to another is visible.
2.4.2 Invasive lobular carcinoma
The histomorphology of invasive lobular carcinoma can be divided into classic and variant. The classic, or pure, variety of invasive lobular carcinoma is characterized by scattered caner cells, which invade the fibrous stroma outside the lobules of mammary glands or show linear arrangements; these cancer cells can also be around the mammary ducts, showing an arrangement of concentric rings. These cancer cells are relatively small and uniform, having less adhesion between each other. The nuclei are round or irregularly oval, and mitotic figures are rare. The cytoplasm has small volume and is concentrated at the cell edge; mucus is occasionally visible inside the cells. The architecture of these tumors is often deranged, whereas the host reaction is relatively mild. Most classic invasive lobular carcinomas are accompanied with lobular carcinoma in situ components. Three more variants include solid, alveolar, and pleomorphic types.
2.4.3 Tubular carcinoma
Tubular carcinoma is a special type of breast cancer, with good prognosis. It is characterized with well-differentiated tubules, which are formed by single epithelial cells.
2.4.4 Invasive cribriform carcinoma
Invasive cribriform carcinoma is an invasive carcinoma with good prognosis. Its morphology is similar to that of the cribriform ductal carcinoma and can be mixed with a small proportion of (less than 50%) of tubular carcinoma components.
2.4.5 Medullary carcinoma
Medullary carcinoma is a special type of breast cancer. It is characterized microscopically by: the tumor has clear boundary; the cancer cells are arranged in syncytoid masses, with obvious heteromorphosis; they distribute in large patches, lack adenoid structures, have few stromal compoments, and are accompanied by a large number of lymphocyte infiltration.
2.4.6 Mucinous carcinoma and other tumors with abundant mucin
They refer to breast cancer types that are able to produce abundant intracellular and/or extracellular mucin. It include mucinous carcinoma,cystadenocarcinoma, columnar cell mucinous carcinoma, andSignet ring cell carcinoma.
2.4.7 Primary neuroendocrine tumors
They refers to a group of tumors that have the same morphological characteristics as neuroendocrine tumors in the gastrointestinal tract and lungs. More than 50% of cancer cells in these tumors can express neuroendocrine markers. Howevery, they do not include invasive ductal carinoma NOS that show positive results during immunohistochemical staining for neuroendocrine markers (either scattered or partial).
2.4.8 Invasive papillary carcinoma
Most invasive papillary carcinoma cases occur after menopause. Microscopy shows invasive papillary carcinoma has an expansive growth pattern, with clear tumor boundary and thin or blunt convex papilate. The cytoplasm of cancer cells is typically double-stained, with marked apical protrusions. The cancer cells show moderate nuclear atypia, and the tumor stroma is not rich.
2.4.9 Invasive micropapillary carcinoma
The invasive micropapillary carcinoma clinically manifests itself as a solid mass, and axillary lymph node metastasis has already occurred in 72% to 77% of cases when a breast mass is discovered. Microscopy shows the tumor cells is arranged in small clusters, forming micro- nipple or micro-glandular tube, which are located in the interstitial fissures similar to the vasculature. The pure type of invasive micropapillary carcinoma is rare, mostly mixed form. The special growth pattern of invasive micropapillary carcinoma is associated with its vascular invasion and lymph node metastasis; particularly, the risk of lymph node metastasis is remarkably higher than that of invasive ductal carcinoma NOS, with poor prognosis. Therefore, a diagnosis of invasive micropapillary carcinoma can be immediately made once its components are found under microscope; meanwhile, the proportion of each component should be noted.
2.4.10 Apocrine carcinoma
A diagnosis of apocrine carcinoma can be made when more than 90% of the tumor cells show the cytological and immunohistochemical features of apocrine cells.
2.4.11 Metaplastic carcinomas
Metaplastic carcinomas represent a morphologically heterogeneous group of invasive breast cancers that consist of various combinations of spindle cell differentiation, squamous metaplasia, and/or mesenchymal differentiation (cancer with osseous metaplasia, cancer with cartilage metaplasia, cancer producing stroma, and carcinosarcoma). The metaplastic spindle cell carcinoma and squamous cell carcinoma can separately exist independent of adenocarcinoma components. Based on tumor compositions, the metaplastic carcinoma is divided into many subtypes.
2.4.12 Lipid-rich carcinoma
In this type of breast cancer, about 90% of its neoplastic cells contain abundant cytoplasmic neutral lipids.
2.4.13 Secretory carcinoma
Secretory carcinoma is a rare low-grade malignant tumor, with solid, micro-cystic, and tubular structures. The tumor cells can produce abundant intracellular and extracellular PAS-positive substances that are not susceptible to amylase digestion.
2.4.14 Oncocytic carcinoma
Oncocytic carcinoma is a type of breast cancer composed of 70% of acidophils.
2.4.15 Adenoid cystic carcinoma
A tumor with of low malignant potential with histological features similar to the same kind of tumor in salivary gland.
2.4.16 Acinic cell carcinoma
Acinic cell carcinoma is a group of tumors with cinar cell (serous) differentiation.
2.4.17 Glycogen-rich clear cell carcinoma
Glycogen-rich clear cell carcinoma is a special breast cancer. Morphologically, over 90% of the cancer cells have clear cytoplasm, which is rich in glycogen.
2.4.18 Sebaceous carcinoma
Sebaceous carcinoma is a primary breast cancer showing the morphological features of sebaceous glands differentiation. However, currently no evidence has demonstrated that the disease arise from the sebaceous glands in the breast skin.
2.4.19 Inflammatory carcinoma
Inflammatory carcinoma, caused by the blockage of lymphatic drainage due to the invasion of cancer cells into the local lymphatic ducts, is a special breast cancer with specific clinical manifestations. The skin lymph tubes can be remarkably affected in most patients. The inflammatory carcinoma, with a clinical stage of T4d, belongs to advanced breast cancer. Patients with cancerous embolus in dermal lymphatic channels but without clinical manifestations should not be diagnosed as inflammatory carcinoma.
Annex 4 TNM staging of breast cancer
B.1 Primary tumor (T)
The clinical and pathological definitions for the stages of primary tumors are same. If the size of a tumor is assessed during physical examination, it can be presented as T1, T2, or T3. When other measurements (such as mammography or pathology) are applied, the four subgroups of T1 can be used. The size of tumor is accurate to 0.1 cm.
TX means that the tumor size cannot be assessed.
T0: No evidence of primary tumor.
Tis: Carcinoma in situ.
Tis ductal carcinoma in situ
Tis lobular carcinoma in situ
Tis Paget’s disease of the nipple with no associated tumor.
Note: Paget’s disease associated with a tumor is classified according to the size of the tumor.
T1: Tumor 2.0 cm or less in greatest dimension
T1mic: Microinvasion 0.1 cm or less in greatest dimension;
T1a: Tumor more than 0.1 cm but not more than 0.5 cm in greatest dimension;
T1b: Tumor more than 0.5 cm but not more than 1.0 cm in greatest dimension;
T1c: Tumor more than 1.0 cm but not more than 2.0 cm in greatest dimension;
T2: Tumor more than 2.0 cm but not more than 5.0 cm in greatest dimension;
T3: Tumor more than 5.0 cm in greatest dimension;
T4: Tumor of any size with direct extension to (a) chest wall or (b) skin.
T4a: Extension to chest wall;
T4b: Edema (including peau d’orange) or ulceration of the skin of the breast or satellite skin nodules confined to the same breast;
T4c: Both of the above (T4a and T4b);
T4d: Inflammatory carcinoma.
B.2 Regional lymph nodes (N)
Clinical
NX: Regional lymph nodes cannot be assessed (e.g., previously removed);
N0: No regional lymph node metastasis;
N1: Metastasis to movable ipsilateral axillary lymph node(s);
N2: Metastases in ipsilateral axillary lymph nodes that are clinically fixed or matted; or,metastases in clinically detected ipsilateral internal mammary nodes in the absence of clinically evident* axillary lymph node metastases.
N2a: Metastases in ipsilateral axillary lymph nodes fixed to one another (matted) or to other structures.
N2b: Metastases only in clinically detected ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastases.
N3: Metastases in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement; or, metastases in clinically detected ipsilateral internal mammary lymph node(s) with clinically evident* axillary lymph node metastases; or, metastases in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement.
N3a: Metastases in ipsilateral infraclavicular lymph node(s).
N3b: Metastases in ipsilateral internal mammary lymph node(s) and axillary lymph node(s).
N3c: Metastases in ipsilateral supraclavicular lymph node(s).
B.3 Distant Metastases (M)
MX: Distant metastasis can not be assessed.
M0: No distant metastasis
M1: Distant metastasis.
B.4 AJCC stage groupings
*“Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination, or by naked-eye observation.
Annex 5 Pathologic Tumor, Nodes, and Metastases (pTNM) staging system of breast cancer
1. pT - Primary tumor
The pTNM staging should be based on the pathologic findings of the primary cancer, with the margin of the excised specimen free of tumor under gross (naked-eye) examination. pT can be staged if tumor tissues on the cutting margin can only be observed under microscope. For the purpose of pathologic staging, the tumor size should be based on the measurements of the infiltrative lesions If relatively large carcinoma in situ (e.g., 4 cm) and small infiltrative lesion (e.g., 0.5 cm) exist, the tumor can be staged as pT1a.
pTX: primary tumor cannot be assessed (e.g., have been resected).
pT0 primary tumor not detected
pTis carcinoma in situ
pTis (DCIS) ductal carcinoma in situ
pTis (LCIS) lobular carcinoma in situ
pTis: Paget’s disease of the nipple with no associated tumor (Paget’s disease associated with a tumor is classified according to the size of the tumor).
pT1: Tumor 2.0 cm or less in greatest dimension.
pT1mic: Microinvasion1 0.1 cm or less in greatest dimensiona.
pT1a: Tumor more than 0.1 cm but not more than 0.5 cm in greatest dimension.
pT1b: Tumor more than 0.5 cm but not more than 1.0 cm in greatest dimension.
pT1c: Tumor more than 1 cm but not more than 2.0 cm in greatest dimension.
pT2: Tumor more than 2 cm but not more than 5.0 cm in greatest dimension.
pT3: Tumor more than 5.0 cm in greatest dimension.
pT4: Tumor of any size with direct extension to chest wall (including ribs, intercostal muscles, and serratus anterior muscle but not pectoral muscle) or skin.
pT4a: Extension to chest wall.
pT4b: Edema (including peau d’orange) or ulceration of the skin of thebreast or satellite skin nodules confined to the same breast.
pT4c: Both of the above (T4a and T4b).
pT4d: Inflammatory carcinoma2.
Note:
(1) Microinvasion refers that the tumor cells break through basilar membrane and invade the neighboring tissues, forming ocal lesions no larger than 0.1 cm. When multiple local lesions exist, the tumor will be classified based on the lesion with the largest diameter. For multifocal microinvasions, it is important to detect the existence of any accompanying large invasive carcinoma.
(2) Inflammatory carcinoma is a clinicopathologic entity characterizedby diffuse brawny induration of the skin of the breast with an erysipeloid edge, usually without an underlying palpable mass. If the result of skin biopsy is negative and no localized measurable primary cancer is found, the T category is pTX when pathologically staging a clinical inflammatory carcinoma (e.g., T4d). Dimpling of the skin, nipple retraction, or other skin changes, except those considered as T4b and 4d, may occur in T1, T2, or T3 cases without affecting the classification.
2. pN - Regional lymph nodes
pNX: Regional lymph nodes cannot be assessed (e.g., previously removed or not included in current surgery).
pN0: No regional lymph node metastasis.
pN1mi: Micrometastases (>0.2 mm and/or >200 cells but none >2.0 mm).
pN1: Metastases in 1-3 axillary lymph nodes AND/OR metastases in internal mammary nodes with metastases detected by sentinel lymph node biopsy but not clinically detected.**
pN1a: Metastases in 1-3 axillary lymph nodes, at least one metastasis >2.0 mm.
pN1b: Metastases in internal mammary nodes detected by sentinel lymph node biopsy but not clinically detected.**
pN1c: Metastases in 1-3 axillary lymph nodes and in internal mammary lymph nodes detected by sentinel lymph node biopsy but not clinically detected.**
pN2: Metastases in 4-9 axillary lymph nodes; or, metastases in clinically detected* internal mammary lymph nodes in the absence of axillary lymph node metastases.
pN2a: Metastases in 4-9 axillary lymph nodes (at least 1 tumor deposit >2 mm).
pN2b: Metastases in clinically detected* internal mammary lymph nodes in the absence of axillary lymph node metastases.
pN3: Metastases in ≥10 axillary lymph nodes; or, metastases in infraclavicular lymph nodes; or metastases in clinically detectedc ipsilateral internal mammary lymph nodes in the presence of one or more positive axillary lymph nodes; or, metastases in >3 axillary lymph nodes and in internal mammary lymph nodes by microscopy but not clinically detected; or,metastases in supraclavicular lymph nodes.
pN3a: Metastases in ≥10 axillary lymph nodes (at least 1 tumor deposit >2.0 mm); or, metastases to the infraclavicular nodes
pN3b: Metastases in clinically detected* ipsilateral internal mammary lymph nodes in the presence of one or more positive axillary lymph nodes; or, metastases in >3 axillary lymph nodes and in internal mammary lymph nodes with micrometastases detected by sentinel lymph node biopsy but not clinically detected.**
pN3c: Metastases in supraclavicular lymph nodes.
Note:
1. “Clinically detected” is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination. * “Not clinically detected” is defined as not detected by imaging studies (excluding lymphoscintigraphy) or not detected by clinical examination.
2. In regional lymph nodes, only isolated tumor cells (ITC) belong to pN0. ITCs are defined as single tumor cells or small clusters of cells ≤0.2 mm. ITCs may be detected by routine immunohistochemical methods or molecular biological methods, but can also be confirmed by HE staining. ITCs usually do no present typical tumor metastasis activities such as proliferation and stromal reaction.
3. “Not clinical evident” refers to the abnormalities that can not be detected by physical examination or imaging studies (excluding lymphscintigraphy).
4. “Clinical evident” refers to the abnormalities that can be detected by physical examination, imaging studies (excluding lymphscintigraphy), or naked-eye observation.
pM - Distant metastasis
pM staging is same as the M staging
pMX: Presence of distant metastasis cannot be assessed
pM0: No distant metastasis
pM1: Distant metastasis present
Annex 6 Format of the breast cancer HER2/neu FISH detection report
Pathological number: ______
Medical record number: ______
FISH detection number: ______
Name ______ Sex ______ Age ______ Nationality ______
Ward ______ Submitting physician ______ Specimen1: Date of receipt ______
Pathological diagnosis: ______
Test results with photos: ______
HE staining ______ immunohistochemical staining
FISH ______ FISH control ______
Total number of tumor cells ( );
Total HER2 gene copies ( ), average number of copies in each nucleus ( );
Total CEP17 gene copies ( ), average number of copies in each nucleus ( );
HER2/CEP17 ratio = ( )
FISH results: Tick in the corresponding brackets as below
Ratio < 1.8 (no amplification) ______ negative______ ( )
Ratio = 1.8 ~ 2.2 (threshold) ______ threshold______ ( )
Ratio> 2.2 (amplification) ______ positive______ ( )
Unable to determine ( )
CEP17 aneuploidy: No □ Yes □ ______ Multiploidy □ ( )
HER2 genetic heterogeneity: No □ Yes □ ( %)
Cell distribution of HER2 gene amplification:
cluster2 □ (%) scattered □ (%)
Note:
1. For needle suction specimens, a FISH detection or repeat detection on the surgical specimen is recommended.
2. For clustered amplification, the regional HER2/CEP17 ratio should be marked.
Annex 7 Content and format of the breast cancer pathology report
Pathological number ______
Medical record number ______
Name ______ Sex ______ Age ______ Nationality ______
Ward ______ Submitting physician ______ Date of receipt ______
Clinical diagnosis: ______
Specimen type: needle aspiration □ endoscope □ dissection □ breast-conserving □ radical mastectomy □ modified radical mastectomy □ A/B
□ Other ( )
Size: ______ × ______× ______ cm
Skin: ______ × ______ cm
Tumor/lesion site: left □ right □
A B C D E C'
a b c d e c'
Tumor/lesion size: ______
Naked eye observation: ______ × ______ × ______ cm;
Microscope: ______ × ______ × ______ cm
Nipple involvement: No □ Yes □: Gross/endoscope (invasive carcinoma/carcinoma in situ/Paget’s disease)
Involving other tissues:No □ Yes □:
Gross/endoscope ______ skin, fat, chest wall, pectoralis Other ( )
Carcinoma in situ: No □ Yes □ ( %)
Type of carcinoma in situ:ductal ______
lobular ______ Paget’s disease ______
DCIS: nuclear grade: I II III; necrosis: No □ Yes □
Subtypes: acne, cribriform, solid, papillary, micro-papillary, flat, other ( )
Lobular carcinoma in situ: subtype: classic, acne, acinar, signet ring, other ( )
Paget’s disease: intraductal component: No □ Yes □ ( %)
Invasive carcinoma component: No □ Yes □ ( %)
Maximum diameter under microscope: ______ Histological type: ______
Histological grade: ______ Response to chemotherapy: ______
Margin: negative: minimal distance from tumor to margin ______ cm
Positive1: component: (invasive cancer, carcinoma in situ, untypical hyperplasia2)
Scope ( )
Lymphatic vessel invasion: (–) (±) (+) (++) (+++) unevaluable
Vascular invasion: (–) (±) (+) (++) (+++) unevaluable
Neural invasion: (–) (±) (+) (++) (+++) unevaluable
Lymph nodes: Sentinel: ______/______; Axillary apex: ______/______; Subclavicular: ______/______; Posterior group: ______/______; Intermuscular: ______/______; Lateral group______/______; Other: ______/______
Maximum diameter of lymph node metastasis: ______ cm
Microcalcifications: No □ Yes □ (localized______extensive)
Located in: carcinoma in situ invasive carcinoma non-tumor tissue ( )
Other lesions: ____________
Pathological diagnosis: ____________
pTNM: ____________
Remarks: ____________
Pathologist: ____________
Date:
Note:
1. A cancer-positive margin in the intraoperative frozen section means that cancer cells are detected on the surgically dissected margin. The distance from the tumor tissues to the margin should be marked in the postoperative paraffin specimen.
2. Due to difficulty differentiating low-grade carcinoma in situ and atypical hyperplasia, as well as the confounding coagulated tissues, in the intraoperative frozen section, it is advisable to mark any atypical hyperplasia found on the margin of breast-conserving operation as cancer-positive and perform additional dissection until no such tissue is found.
Annex 8 Evaluation criteria for the cosmetic results after breast-conserving surgery
Excellent: Same shape of both ipsilateral and contralateral breasts;
Good: Slightly different shape between ipsilateral and contralateral breasts, though not noticeable;
Fair: Noticeable shape difference with the contralateral breast, but no severe deformity;
Poor: Severe deformities in the ipsilateral breast.