Abstract
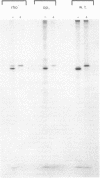
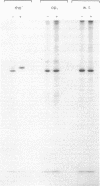
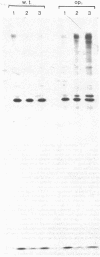
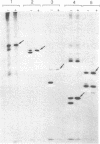
Earlier work has shown that mitochondrial proteins synthesized in the cytosol are initially made as larger precursors which are then transferred into the organelles and processed to their mature size in the absence of protein synthesis. It is now demonstrated that depletion of the mitochondrial matrix ATP in intact yeast spheroplasts by various combinations of inhibitors and mutations prevents the processing of precursors to the three largest subunits of the mitochondrial F1-ATPase and two subunits of the cytochrome bc1 complex. These polypeptides are all synthesized outside the mitochondria and transported to the mitochondrial matrix or inserted into the mitochondrial inner membrane. In contrast, depletion of the matrix ATP does not inhibit processing of the precursor to cytochrome c peroxidase; this enzyme is located in the mitochondrial intermembrane space which is freely accessible to ATP made in the cytosol. The processing of extramitochondrially made precursors or the transfer of these precursors across the mitochondrial inner membrane is thus dependent on ATP.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore A. R., Broadhurst M. K., Gray R. E. Cell-free synthesis of leaf protein: Identification of an apparent precursor of the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Feb;75(2):655–659. doi: 10.1073/pnas.75.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Schmidt G. W. Post-translational transport into intact chloroplasts of a precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6110–6114. doi: 10.1073/pnas.75.12.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Côté C., Solioz M., Schatz G. Biogenesis of the cytochrome bc1 complex of yeast mitochondria. A precursor form of the cytoplasmically made subunit V. J Biol Chem. 1979 Mar 10;254(5):1437–1439. [PubMed] [Google Scholar]
- Djavadi-Ohaniance L., Rudin Y., Schatz G. Identification of enzymically inactive apocytochrome c peroxidase in anaerobically grown Saccharomyces cerevisiae. J Biol Chem. 1978 Jun 25;253(12):4402–4407. [PubMed] [Google Scholar]
- Dobberstein B., Blobel G., Chua N. H. In vitro synthesis and processing of a putative precursor for the small subunit of ribulose-1,5-bisphosphate carboxylase of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1082–1085. doi: 10.1073/pnas.74.3.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etlinger J. D., Goldberg A. L. A soluble ATP-dependent proteolytic system responsible for the degradation of abnormal proteins in reticulocytes. Proc Natl Acad Sci U S A. 1977 Jan;74(1):54–58. doi: 10.1073/pnas.74.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Kolarov J., Klingenberg M. The adenine nucleotide translocator in genetically and physiologically modified yeast mitochondria. FEBS Lett. 1974 Sep 1;45(1):320–323. doi: 10.1016/0014-5793(74)80871-9. [DOI] [PubMed] [Google Scholar]
- Kolarov J., Subík J., Kovac L. Oxidative phosphorylation in yeast. IX. Modification of the mitochondrial adenine nucleotide translocation system in the oxidative phosphorylation-deficient mutant op 1 . Biochim Biophys Acta. 1972 Jun 23;267(3):465–478. doi: 10.1016/0005-2728(72)90174-0. [DOI] [PubMed] [Google Scholar]
- Kovác L., Lachowicz T. M., Slonimski P. P. Biochemical genetics of oxidative phosphorylation. Science. 1967 Dec 22;158(3808):1564–1567. doi: 10.1126/science.158.3808.1564. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyton R. O., Schatz G. Cytochrome c oxidase from bakers' yeast. IV. Immunological evidence for the participation of a mitochondrially synthesized subunit in enzymatic activity. J Biol Chem. 1975 Jan 25;250(2):762–766. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte H. R., Weijers P. J., Wit-Peeters E. M. The localization of mitochondrial creatine kinase, and its use for the determination of the sidedness of submitochondrial particles. Biochim Biophys Acta. 1973 Feb 16;291(3):764–773. doi: 10.1016/0005-2736(73)90479-3. [DOI] [PubMed] [Google Scholar]
- Subík J., Kolarov J., Kovác L. Bongkrekic acid sensitivity of respiration-deficient mutants and of petite-negative species of yeasts. Biochim Biophys Acta. 1974 Sep 20;357(3):453–456. doi: 10.1016/0005-2728(74)90036-x. [DOI] [PubMed] [Google Scholar]
- Williams P. G., Stewart P. R. The intramitochondrial location of cytochrome c peroxidase in wild-type and petite Saccharomyces cerevisiae. Arch Microbiol. 1976 Feb;107(1):63–70. doi: 10.1007/BF00427868. [DOI] [PubMed] [Google Scholar]