Abstract
Thyroid neoplasms are the most frequent neoplasm in the head and neck region. Most thyroid carcinomas are well-differentiated tumors of follicular cell origin. Thyroid papillary carcinoma (TPC) is the most common thyroid malignancy. It constitutes 60% to 90% of all the thyroid carcinomas and cervical lymph node metastases are commonly seen in these patients. Although cervical lymph node metastases are common in this cancer, the management and the prognostic role of lymph nodes in TPC remains controversial. In this paper we reviewed the currently available literature regarding the extent of lateral neck dissection in papillary thyroid carcinoma patients with lateral neck metastasis.
Keywords: Thyroid, cancer, papillary, level IIb, neck dissection
Thyroid neoplasms represent almost 95% of all endocrine tumors, although they are relatively uncommon, accounting for approximately 2% of all human malignancies. Thyroid neoplasms are the most frequent neoplasm in the head and neck region. Most thyroid carcinomas are well-differentiated tumors of follicular cell origin. These lesions are histologically defined as papillary carcinoma, follicular carcinoma, and Hurthle cell carcinoma. Thyroid papillary carcinoma (TPC) is the most common thyroid malignancy. It constitutes 60% to 90% of all the thyroid carcinomas. Cervical lymph node metastases in patients with TPC are commonly seen. The rate of nodal metastases in TPC has been reported to be within the range of 30-90% (1,2). Although cervical lymph node metastases are common in TPC, the management and the prognostic role of lymph nodes in TPC remains controversial. Some studies suggest that the neck metastases have no adverse effect on neither the recurrence nor the survival (3-5). However, some recent articles showed that lymph node metastases were associated with an increase in recurrence rates and have a negative impact on survival (6,7). Neck dissections in the clinically involved lateral cervical lymph nodes are generally well-accepted procedures for TPC. However, the extent of the neck dissection necessary for oncologic management for positive lateral neck nodes is still debated (8,9). Current treatment guidelines of the American Thyroid Association (ATA) recommended, therapeutic central-compartment (level VI) neck dissection for patients with clinically involved central or lateral neck lymph nodes should accompany total thyroidectomy to provide clearance of disease from the central neck. Prophylactic level VI neck dissection may be performed in patients with TPC with clinically uninvolved central neck lymph nodes, especially for advanced primary tumors (T3 or T4). Near-total or total thyroidectomy without prophylactic central neck dissection may be appropriate for small (T1 or T2), noninvasive, clinically node-negative PTC and most follicular cancer. But elective cervical lymph node dissection has not been clearly defined (10).
Unlike squamous cell carcinoma of the head and neck, where regional metastasis have a definite negative prognostic impact, the effect of cervical nodal involvement on survival in differentiated thyroid carcinoma has not been demonstrated clearly. Elective neck dissection is generally recommended if the risk of occult neck metastasis is higher than 20% in head and neck squamous cell carcinomas (3). However, the role of elective neck dissection in TPC has not been clearly defined yet.
In general regional lymph node spread from thyroid cancer can broadly be classified as central neck compartment and lateral neck compartment metastases (1). The lymph node regions of the neck were divided into six levels (levels I-VI). These levels classified as level I is submandibular and submental lymph nodes regions, level II is upper jugular lymph nodes region, level III is middle jugular lymph nodes region, level IV is lower jugular lymph nodes region, level V posterior triangle lymph nodes region and level VI is anterior lymph nodes region (Figure 1) (11). Level II divided into two parts by spinal accessory nerve known as levels IIa and IIb. Level IIb is also known as the submuscular recess or supraspinal recess. It comprises level II nodes that lay superficial to the fascia on the splenius capitis and levator scapulae muscles, superior to the spinal accessory nerve and bordered by the posterior belly of the digastric muscle superiolaterally, the skull base by superiorly and the sternocleidomastoid by muscle posterolaterally (Figure 2) (12-15).
Figure 1.
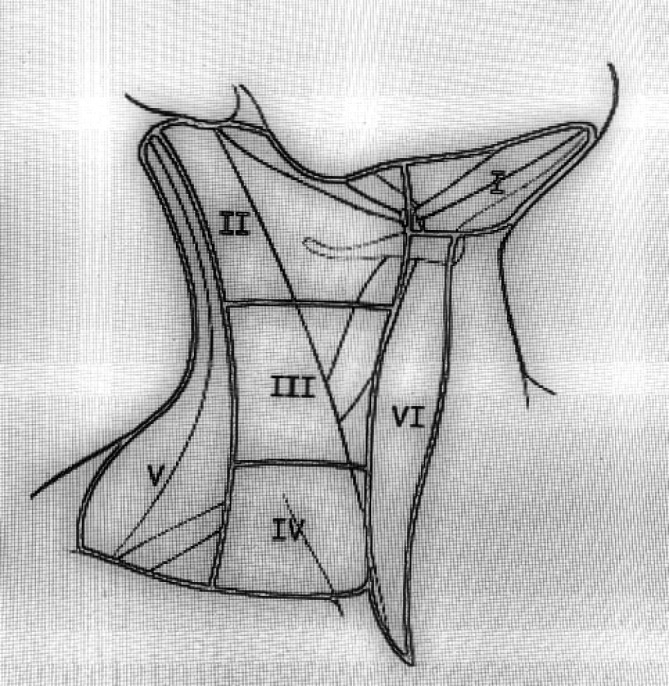
Neck lymph node levels (I-VI).
Figure 2.
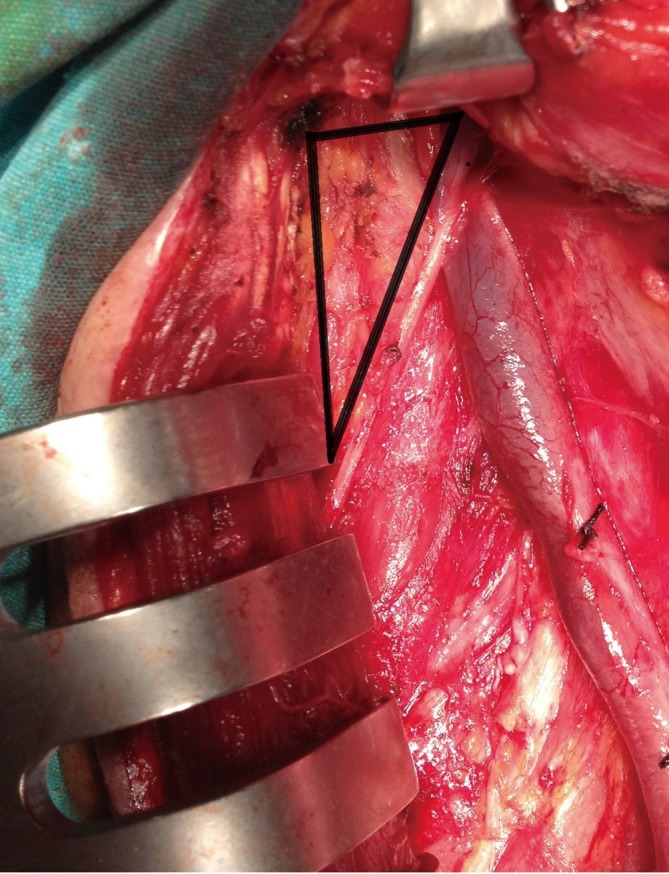
Level IIb seen during surgery.
The extent of neck dissection at the time of initial thyroidectomy has become a topic of contention (16). The cervical lymph nodes metastases initiates in the perithyroidal nodes of the central neck and progresses to the lateral cervical compartments (level I to V) and the superior mediastinum. However, the contralateral lateral and mediastinal compartments are affected more rarely. Skip metastases to the lateral compartment without central neck nodal involvement may occur but it is seen rare (17-20).
The neck dissection indication may be decided by clinically or radiologically lymph node metastasis suspicion. Fine needle aspiration biopsy (FNAB) or intraoperative frozen section usage might be useful reduce the negative neck dissection results. Lymph nodes metastasis assessment has high value to neck dissection decisions in patient with PTC. Various methods are used to assess the extent of lymph node involvement. Physical examination evaluation of the lateral neck for metastatic disease screens for visible or palpable lymph nodes. Because of their anatomic locations, enlarged cervical lymph nodes may not be easily visible or palpable, especially when they are small, located behind the sternocleidomastoid muscles, or located behind a carotid artery or jugular vein and in level VI. Therefore, in order to make treatment decisions regarding neck dissection, it is very important that adequately evaluation of cervical lymph nodes for metastasis. Imaging modalities, such as ultrasonography (US) with or without FNAB, iodine scans, computed tomography (CT), hybrid imaging modalities such as single photon emission CT/CT and positron emission tomography/CT (PET/CT), technetium-99m methoxyisobutylisonitrile scintigraphy (MIBI scan), and magnetic resonance imaging (MRI) can each be important in the assessment of the lateral neck. Also intraoperative frozen section may be helpful for lateral neck lymph node metastasis (21-24). US performed by experienced hands are considered by the ATA, as the screening and surveillance imaging modality of choice for detection of lateral neck metastases. As recommended by the ATA thyroid cancer guidelines surveillance imaging of the lateral neck should include US. In cases when a lateral neck node is enlarged (>1.5 cm in levels I and IIa or >1.0 cm in levels IIb-Vb) or has ultrasonographic features worrisome for disease, an US-guided fine-needle aspiration should be attempted to confirm disease including possibly testing the aspirate for thyroglobuline (Tg) in an aspiration specimen (24). The introduction of these imaging modalities has increased detection of non-palpable cervical lymph node metastases.
The most common morbidities associated with neck dissections are spinal accessory nerve dysfunction and related shoulder disabilities. Shoulder dysfunction is due to traction injury or interruption of blood supply of the spinal accessory nerve during dissection of level IIb. This dysfunction may be avoided by preserving the level IIb lymph nodes during neck dissection in selected patients (25). However, some studies have shown temporary functional deterioration of the spinal accessory nerve even when level IIb is not dissected (26,27). Level IIb is rarely involved in patients with a metastatic neck disease. Metastases at level IIb are not expected in patients with N0 neck. Koybasioğlu et al. (28) showed no metastases at level IIb in dissection specimens of patients with laryngeal cancer. Silverman et al. (26) reported a 1.6% incidence of metastases at level IIb for N0 necks, 1.1% for N1 cases, and total incidence was 4.4% in cases with head and neck cancers. Similarly, Sezen et al. (27) reported that routine level IIb dissection was not necessary in N0 necks. According to them, if the level IIa showed positive metastatic changes, preoperative pathologic examination by frozen section that includes level IIb could be an alternative approach. Recently, similarly reports have been shown for TPC (28-30).
Generally, selective neck dissection, dissecting levels II-V with preservation of the sternocleidomastoid muscle, spinal accessory nerve, and internal jugular vein, is recommended for patients with TPC presenting with clinically palpable cervical lymph nodes or showing pathological appearance seen by imaging studies (31-33). Initial nodal spread from TPC occurs in the central compartment of the ipsilateral neck (level VI) (34-37). However, Gimm et al. (21) and Noguchi et al. (7) reported that some patients had posterolateral lymph node metastasis without involvement of the central compartment. In our previous study (38) all patients with posterolateral lymph node metastases had involvement of central compartment. When there is a metastasis in the lymph nodes of the lateral neck, levels II-V are predictably affected, usually with multiple areas of spread in more than one of these levels. As spread of TPC to the level I lymph nodes is so rare, routine dissection of this area can be omitted (31,37). In addition, levels I and V lymph nodes were never found to be involved without lymph node involvement at other lymph nodes level such as levels II, III or IV (37,38). In our previous study (39), the majority of patients with lateral cervical lymph node metastasis had multiple involved lymph node levels. The lateral cervical lymph nodes (levels II, III, and IV) are at the greatest risk for metastasis in TPC patients. It has been reported that lateral nodes were commonly involved, and level III nodes were the most common sites for metastasis (28,30,37). Although Roh et al. (9) and Vayisoglu et al. (39) reported that level IV nodes were the most common sites for metastasis.
Although level IIb lymph nodes dissection has been routinely included in lateral neck dissection performed for metastatic neck disease with TPC, the incidence of nodal metastasis in this area has not been well established. Recently, some studies investigated the frequency and pattern of level IIb lymph node metastasis for TPC. Table 1 summarizes the studies of incidence of metastatic lymph nodes at level IIa and IIb of PTC patients with lateral neck metastasis. Pingpank et al. reported that level IIb lymph node metastases were common. Therefore, they suggested that neck dissection should include all lymph nodes of levels I-V (40). Kim (43) reported that lymph node metastasis involving level IIb is not rare (20%), and careful level IIb lymph node dissection should be considered. Yanir and Doweck recommended that elective dissection of levels II-VI in patients with clinically positive lateral neck lymph node metastasis in spite of a low incidence of level IIb metastases, due to the possibility of skip metastasis at level IIb (33). Also King et al. reported that the high incidence of multilevel cervical metastasis associated with PTC and suggest the importance of including level IIb when performing a neck dissection (42). In contrast, some studies suggested that level IIb dissection would probably not be necessary in the absence of level IIa involvement because the incidence of metastasis to level IIb has been low if level IIa has not been involved (43-45). In our previous study (39), level IIb metastases were found in only one patient (2.1%), and this patient also had metastasis at levels IIa, III, IV, and V (Figure 3). In five specimens, metastasis was detected at level IIa without level IIb involvement. Koo et al. (31) reported that 11.8% (9 of 76) of the patients had level IIb lymph nodes metastases in TPC patients. Only one of these patients was positive for level IIa. They all had primary tumors of >1 cm, as well as lymphovascular invasion, capsular invasion, or multilevel involvement of metastatic nodes. They suggested that level IIb lymph node dissection might not be necessary in TPC patients with positive lymph nodes in the absence of multilevel involvement (30).Lee et al. (29) also suggested that level IIb dissection has not been necessary in the absence of level IIa involvement. In their study, the metastasis in levels IIa and IIb were 55.5% and 6.8%, respectively. Also all level IIb lymph node metastasis was accompanied by level IIa metastasis. In another study, it has been reported that the metastasis in level II was 60% (33 specimens) among 55 specimens. The incidence of lymph node metastasis at level IIb was 22% (12 specimens). Of 12 specimens with metastasis at level IIb, 11 specimens also had metastasis at level IIa. Therefore, they suggested that level IIb dissection is probably unnecessary when level IIa lymph nodes are uninvolved (30).
Table 1. Results of the studies showing the incidences of lymph nodes metastasis at level IIa and IIb in patients with TPC.
| Authors [years] | Total patients [ND] | Metastasis rates (%) |
Comment | |
|---|---|---|---|---|
| IIa | IIb | |||
| Pingpank et al. (40) [2002] | 44 [51] | 43.1 | 20.6 | Level IIb metastasis is common |
| Lee et al. (30) [2007] | 46 [55] | 58.2 | 21.8 | Level IIb dissection should be performed if level IIa LNM is found |
| Roh et al. (9) [2008] | 52 [57] | 72.7 | 16.7 | Level IIb metastasis always accompanied by level IIa metastasis |
| Lee et al. (29) [2008] | 167 [191] | 55.5 | 6.8 | Level IIb dissection is not necessary if level IIa LNM is negative |
| Yanir et al. (34) [2008] | 27 [28] | 50.0 | 7.1 | Level II-VI dissection should be performed in clinically +N |
| Farrag et al. (41) [2009] | 53 [60] | 56.0 | 8.5 | Level IIb dissection should be performed if level IIa LNM is found |
| Koo et al. (31) [2009] | 76 [76] | 51.3 | 11.8 | Level IIb dissection may be omitted in multilevel involvement including level IIa (-) |
| Vayisoglu et al. (39) [2010] | 33 [47] | 12.7 | 2.1 | Level IIb dissection may be required if multilevel metastasis or intraoperatively (+) LN |
| King et al. (42) [2011] | 32 [39] | 48.7 | 61.5 | Level IIb metastasis is common |
| Kim et al. (43) [2012] | 18 [18] | 46.7 | 20.0 | Level IIb dissection should be considered |
Abbrevation: TPC, thyroid papillary carcinoma; LMN, lymph node metastasis.
Figure 3.
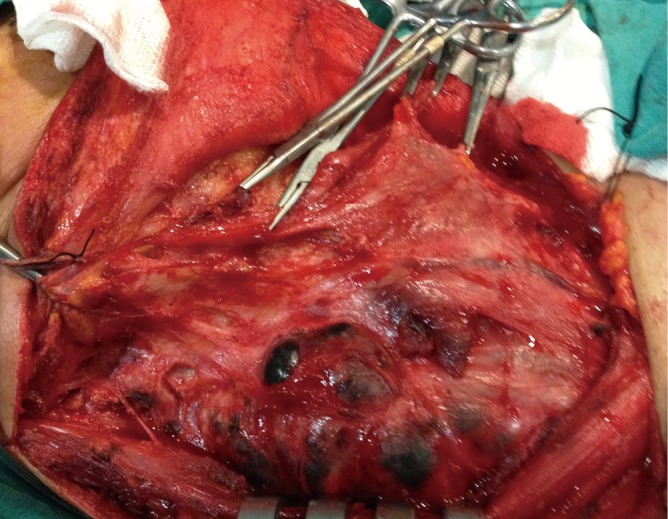
Aggressive multilevel involvement in patient with TPC. Abbreviation: TPC, thyroid papillary carcinoma.
Conclusions
The balance between surgical morbidity and oncological safety should be considered if the lateral neck dissection in PTC patients with lateral cervical metastasis is being decided. In most studies, level IIa lymph nodes metastasis and multilevel lymph nodes metastasis were predictive factors of level IIb metastasis in PTC. Therefore when there is no suspicious lymph node metastasis at levels II or there is not multilevel aggressive neck metastasis, dissection of level IIb may not be necessary in PTC patients with lateral neck metastasis. Consideration of the individualized surgical extent of lateral neck dissection is important in the treatment of PTC patients with lateral cervical metastasis.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Davidson HC, Park BJ, Johnson JT. Papillary thyroid cancer: controversies in the management of neck metastasis. Laryngoscope 2008;118:2161-5 [DOI] [PubMed] [Google Scholar]
- 2.Shaha AR. Implications of prognostic factors and risk groups in the management of differentiated thyroid cancer. Laryngoscope 2004;114:393-402 [DOI] [PubMed] [Google Scholar]
- 3.Andersen PE, Saffold S. Management of cervical metastasis. In: Shah JP. eds. Cancer of the head and neck. Hamilton, London: BC Decker editors CITA, 2001:274-87. [Google Scholar]
- 4.Hughes CJ, Shaha AR, Shah JP, et al. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck 1996;18:127-32 [DOI] [PubMed] [Google Scholar]
- 5.Shah JP, Loree TR, Dharker D, et al. Prognostic factors in differentiated carcinoma of the thyroid gland. Am J Surg 1992;164:658-61 [DOI] [PubMed] [Google Scholar]
- 6.Beasley NJ, Lee J, Eski S, et al. Impact of nodal metastases on prognosis in patients with well-differentiated thyroid cancer. Arch Otolaryngol Head Neck Surg 2002;128:825-8 [DOI] [PubMed] [Google Scholar]
- 7.Noguchi S, Murakami N, Yamashita H, et al. Papillary thyroid carcinoma: modified radical neck dissection improves prognosis. Arch Surg 1998;133:276-80 [DOI] [PubMed] [Google Scholar]
- 8.Rotstein L.The role of lymphadenectomy in the management of papillary carcinoma of the thyroid. J Surg Oncol 2009;99:186-8 [DOI] [PubMed] [Google Scholar]
- 9.Roh JL, Kim JM, Park CI. Lateral cervical lymph node metastases from papillary thyroid carcinoma: pattern of nodal metastases and optimal strategy for neck dissection. Ann Surg Oncol 2008;15:1177-82 [DOI] [PubMed] [Google Scholar]
- 10.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer , Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214 [DOI] [PubMed] [Google Scholar]
- 11.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid Cancer. Thyroid 2006;16:109-42 [DOI] [PubMed] [Google Scholar]
- 12.Robbins KT, Clayman G, Levine PA, et al. Neck dissection classification update: revisions proposed by the American Head and Neck Society and the American Academy of Otolaryngology-Head and Neck Surgery. Arch Otolaryngol Head Neck Surg 2002;128:751-8 [DOI] [PubMed] [Google Scholar]
- 13.Talmi YP, Hoffman HT, Horowitz Z, et al. Patterns of metastases to the upper jugular lymph nodes (the “submuscular recess”). Head Neck 1998;20:682-6 [DOI] [PubMed] [Google Scholar]
- 14.Cappiello J, Piazza C, Nicolai P.The spinal accessory nerve in head and neck surgery. Curr Opin Otolaryngol Head Neck Surg 2007;15:107-11 [DOI] [PubMed] [Google Scholar]
- 15.Ferlito A, Silver CE, Suárez C, et al. Preliminary multi-institutional prospective pathologic and molecular studies support preservation of sublevel IIB and level IV for laryngeal squamous carcinoma with clinically negative neck. Eur Arch Otorhinolaryngol 2007;264:111-4; discussion 109 [DOI] [PubMed] [Google Scholar]
- 16.Koybasioğlu A, Uslu S, Yilmaz M, et al. Lymphatic metastasis to the supraretrospinal recess in laryngeal squamous cell carcinoma. Ann Otol Rhinol Laryngol 2002;111:96-9 [DOI] [PubMed] [Google Scholar]
- 17.Park JY, Koo BS. Individualized optimal surgical extent of the lateral neck in papillary thyroid cancer with lateral cervical metastasis. Eur Arch Otorhinolaryngol. 2013 doi: 10.1007/s00405-013-2630-x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Machens A, Hinze R, Thomusch O, et al. Pattern of nodal metastasis for primary and reoperative thyroid Cancer. World J Surg 2002;26:22-8 [DOI] [PubMed] [Google Scholar]
- 19.Machens A, Holzhausen HJ, Dralle H. Skip metastases in thyroid Cancer leaping the central lymph node compartment. Arch Surg 2004;139:43-5 [DOI] [PubMed] [Google Scholar]
- 20.Chung YS, Kim JY, Bae JS, et al. Lateral lymph node metastasis in papillary thyroid carcinoma: results of therapeutic lymph node dissection. Thyroid 2009;19:241-6 [DOI] [PubMed] [Google Scholar]
- 21.Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg 1998;85:252-4 [DOI] [PubMed] [Google Scholar]
- 22.Watkinson JC, Franklyn JA, Olliff JF. Detection and surgical treatment of cervical lymph nodes in differentiated thyroid cancer. Thyroid 2006;16:187-94 [DOI] [PubMed] [Google Scholar]
- 23.Nixon IJ, Shaha AR. Management of regional nodes in thyroid Cancer. Oral Oncol 2013;49:671-5 [DOI] [PubMed] [Google Scholar]
- 24.Lee DW, Ji YB, Sung ES, et al. Roles of ultrasonography and computed tomography in the surgical management of cervical lymph node metastases in papillary thyroid carcinoma. Eur J Surg Oncol 2013;39:191-6 [DOI] [PubMed] [Google Scholar]
- 25.Stack BC, Jr, Ferris RL, Goldenberg D, et al. American Thyroid Association consensus review and statement regarding the anatomy, terminology, and rationale for lateral neck dissection in differentiated thyroid cancer. Thyroid 2012;22:501-8 [DOI] [PubMed] [Google Scholar]
- 26.Silverman DA, El-Hajj M, Strome S, et al. Prevalence of nodal metastases in the submuscular recess (level IIb) during selective neck dissection. Arch Otolaryngol Head Neck Surg 2003;129:724-8 [DOI] [PubMed] [Google Scholar]
- 27.Sezen OS, Kubilay U, Haytoglu S, et al. Frequency of metastases at the area of the supraretrospinal (level IIB) lymph node in laryngeal Cancer. Head Neck 2007;29:1111-4 [DOI] [PubMed] [Google Scholar]
- 28.Koybaşioğlu A, Bora Tokçaer A, Inal E, et al. Accessory nerve function in lateral selective neck dissection with undissected level IIb. ORL J Otorhinolaryngol Relat Spec 2006;68:88-92 [DOI] [PubMed] [Google Scholar]
- 29.Lee J, Sung TY, Nam KH, et al. Is level IIb lymph node dissection always necessary in N1b papillary thyroid carcinoma patients? World J Surg 2008;32:716-21 [DOI] [PubMed] [Google Scholar]
- 30.Lee BJ, Wang SG, Lee JC, et al. Level IIb lymph node metastasis in neck dissection for papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2007;133:1028-30 [DOI] [PubMed] [Google Scholar]
- 31.Koo BS, Yoon YH, Kim JM, et al. Predictive factors of level IIb lymph node metastasis in patients with papillary thyroid carcinoma. Ann Surg Oncol 2009;16:1344-7 [DOI] [PubMed] [Google Scholar]
- 32.Shah MD, Hall FT, Eski SJ, et al. Clinical course of thyroid carcinoma after neck dissection. Laryngoscope 2003;113:2102-7 [DOI] [PubMed] [Google Scholar]
- 33.Palazzo FF, Gosnell J, Savio R, et al. Lymphadenectomy for papillary thyroid cancer: changes in practice over four decades. Eur J Surg Oncol 2006;32:340-4 [DOI] [PubMed] [Google Scholar]
- 34.Yanir Y, Doweck I.Regional metastases in well-differentiated thyroid carcinoma: pattern of spread. Laryngoscope 2008;118:433-6 [DOI] [PubMed] [Google Scholar]
- 35.Goropoulos A, Karamoshos K, Christodoulou A, et al. Value of the cervical compartments in the surgical treatment of papillary thyroid carcinoma. World J Surg 2004;28:1275-81 [DOI] [PubMed] [Google Scholar]
- 36.Koo BS, Choi EC, Yoon YH, et al. Predictive factors for ipsilateral or contralateral central lymph node metastasis in unilateral papillary thyroid carcinoma. Ann Surg 2009;249:840-4 [DOI] [PubMed] [Google Scholar]
- 37.Kupferman ME, Patterson M, Mandel SJ, et al. Patterns of lateral neck metastasis in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2004;130:857-60 [DOI] [PubMed] [Google Scholar]
- 38.Sivanandan R, Soo KC. Pattern of cervical lymph node metastases from papillary carcinoma of the thyroid. Br J Surg 2001;88:1241-4 [DOI] [PubMed] [Google Scholar]
- 39.Vayisoglu Y, Ozcan C, Turkmenoglu O, et al. Level IIb lymph node metastasis in thyroid papillary carcinoma. Eur Arch Otorhinolaryngol 2010;267:1117-21 [DOI] [PubMed] [Google Scholar]
- 40.Pingpank JF, Jr, Sasson AR, Hanlon AL, et al. Tumor above the spinal accessory nerve in papillary thyroid cancer that involves lateral neck nodes: a common occurrence. Arch Otolaryngol Head Neck Surg 2002;128:1275-8 [DOI] [PubMed] [Google Scholar]
- 41.Farrag T, Lin F, Brownlee N, et al. Is routine dissection of level II-B and V-A necessary in patients with papillary thyroid cancer undergoing lateral neck dissection for FNA-confirmed metastases in other levels. World J Surg 2009;33:1680-3 [DOI] [PubMed] [Google Scholar]
- 42.King JM, Corbitt C, Miller FR. Management of lateral cervical metastases in papillary thyroid cancer: patterns of lymph node distribution. Ear Nose Throat J 2011;90:386-9 [DOI] [PubMed] [Google Scholar]
- 43.Kim YS. Patterns ans predictive factors of lateral lymph node metastasis in papillary thyroid microcarcinoma. Otolaryngol Head Neck Surg 2012;147:15-9 [DOI] [PubMed] [Google Scholar]
- 44.Eskander A, Merdad M, Freeman JL, et al. Pattern of spread to the lateral neck in metastatic well-differentiated thyroid cancer: a systematic review and meta-analysis. Thyroid 2013;23:583-92 [DOI] [PubMed] [Google Scholar]
- 45.Merdad M, Eskander A, Kroeker T, et al. Metastatic papillary thyroid cancer with lateral neck disease: Pattern of spread by level. Head Neck 2013;35:1439-42 [DOI] [PubMed] [Google Scholar]


