Abstract
Benign breast disease is common among women, and when symptomatic, definitive surgical management is preferred by both clinicians and patients. Given the nonmalignant nature of these lesions, an important factor in treatment is cosmesis. Novel minimally invasive techniques for breast lesions are rapidly emerging and demonstrate good efficacy, safety and cosmesis. This review will describe minimally invasive techniques of breast lesions via surgical and percutaneous approaches and discuss the outcomes, advantages and limitations for each. Based on promising initial results, the future standard of care for benign breast lesions may focus on one or more of these minimally invasive techniques.
Keywords: Minimally invasive surgical procedures, breast diseases, fibroadenoma
Benign breast disease accounts for 80% of 1.6 million breast biopsies performed every year in the United States (1). The most common benign breast tumor is fibroadenoma, which is discovered in 67-94% of all biopsies in women under the age of 20 and is identified in 10% of all women in their lifetimes (2,3). Surgical excision is the definitive procedure performed for symptomatic benign breast tumors to alleviate anxiety regarding potential for growth or malignancy as well as physical discomfort (4). Furthermore, treatment for giant (or juvenile) fibroadenomas in adolescents is necessary because of distortion of the breast, potential to cause psychological harm, and potential for enlargement that may cause venous congestion, glandular distortion, pressure necrosis and ulceration (5). Therefore, fibroadenomas are commonly surgically treated at a rate of approximately 500,000 per year (3).
Given the nonmalignant nature of fibroadenomas and other benign breast lesions, an important goal in treatment should be cosmesis. Currently, the accepted definitive treatment of surgical excision results in scar formation and potential for keloids, as well as breast volume loss and potential for nipple areolar distortion or displacement (6). However, novel clinical experiences have been published describing new minimally invasive techniques with promising outcomes and cosmesis. These minimally invasive techniques include endoscopic lumpectomy, vacuum-assisted percutaneous excisional biopsy, and percutaneous thermoablation with radiofrequency, laser, or cryotherapy. Minimally invasive techniques are at the forefront of surgical and interventional innovation and are being evaluated in the setting of clinical trials, as they do not represent current standard of care for breast tumors, benign or malignant.
Cochrane et al. have found that cosmesis and patient satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised; the best cosmesis and patient satisfaction occurred when less than 10% of the breast volume is excised (7). When biopsies are performed with endoscopic lumpectomy or vacuum-assisted percutaneous excisional biopsy, there is a subsequent tissue volume defect, as opposed to little to no tissue loss with percutaneous ablation technique. However, the biopsy material from endoscopic lumpectomy and vacuum-assisted percutaneous excisional biopsy provides more material for accurate pathological analysis, which can be an advantage as opposed to percutaneous thermoablation, which leaves the nonviable specimen in situ for subsequent resorption.
Herein, we review novel minimally invasive techniques for the surgical management of benign breast tumors.
Endoscopic breast surgery
Endoscopic techniques were developed in East Asia for the removal of breast tumors, both benign and malignant. The use of endoscopy for breast surgery was first performed by plastic surgeons to evaluate for breast implant rupture or leakage of silicone (8-10). First described in 1995 by Eaves et al., 70 patients were described having undergone endoscopic techniques for breast augmentation with excellent results (11). In 1998, Kitumura reported the first use of endoscopic surgery for removal of benign breast tumors in 6 patients, and in 2001, he reported a larger experience in 36 patients with benign breast lesions (12,13). Operative details include three small incisions (5, 12, 2 mm) in the mid-axillary line, the insertion of a trocar to the tumor, the creation of space with a pre-peritoneal distention balloon and carbon dioxide insufflation of 6 mmHg, and dissection of the tumor and retrieval of the mass with an Endocatch bag (Figure 1A,B). The tumor is pulled through the 12 mm incision if it is less than 3 cm. Tumors greater than 3 cm are cut within the bag and removed piecemeal. The cosmetic results were excellent in all patients with no scars on the breast itself, but rather small scars in the mid-axillary line, which are concealed by the arm (Figure 1C). Transient postoperative complications included subcutaneous emphysema to the neck and a small skin burn.
Figure 1.
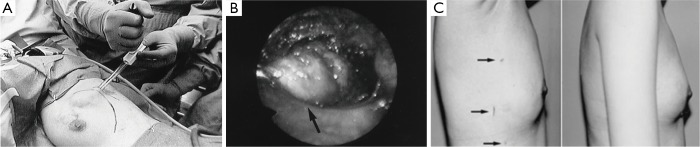
Endoscopic breast surgery using (A) a pre-peritoneal distension balloon to create working space and allows for (B) direct vision of the tumor (arrow). Excellent cosmesis is demonstrated with (C) three small mid-axillary incisions (arrows), which are easily concealed by the arm. Reprinted with permission Kitamura et al. (12,13).
A modified approach to endoscopic breast surgery is via the retromammary space. In 2006, Lee et al. described such a technique for breast cancer (14). This technique differs from Kitumura in that it includes just two incisions (one periareolar and one axillary through which a retromammary space is dissected), subcutaneous tunneling with a VisiportTM to the affected breast quadrant, creation of subcutaneous flaps, and finally lumpectomy. At three months follow-up, 89.5% of patients reported good to excellent cosmetic results. In 2009, Liu et al. described a similar technique for benign breast tumors (15). The operative details include three axillary incisions (10, 5, 5 mm), insertion of a pre-peritoneal dissection balloon into the retromammary space, insufflation with 8 mmHg of carbon dioxide, and dissection of the tumor. The cosmetic results were assessed by the ABNSW scoring system [assessing breast asymmetry (A), breast shape (B), nipple shape (N), skin condition (S), and wound scars (W)], which reported very good cosmesis. Transient complications included mild local subcutaneous emphysema in 5 of 18 patients, and brachial palsy in one case which resolved.
Recently, a minimally invasive surgical technique has been reported in a small case series to excise giant juvenile fibroadenomas (5-10 cm in size) (16). The operative technique involves the use of a single periareolar incision, dissection of the tumor free from the surrounding parenchyma, which is facilitated by the creation of flaps around the tumor, and subsequent retrieval of the specimen with an endoscopic plastic bag through the periareolar incision (Figure 2). The tumor was removed intact for 2 of 3 patients, and morcelated and removed piecemeal for one patient. The remaining periareolar scar is cosmetically inconspicuous, and thus a preferred approach among plastic and breast surgeons (17). The follow-up at one month revealed excellent cosmetic outcome and breast symmetry in all patients.
Figure 2.
Minimally invasive surgery for a giant fibroadenoma by means of (A) a small 1.5 cm periareolar incision; (B) insertion of an endoscopic specimen pouch for (C) retrieval of a dissected free tumor via an endoscopic bag; (D) the 5 cm tumor was intact upon gross examination. Reprinted with permission Cheng et al. (16).
Vacuum-assisted percutaneous excisional biopsy
In 1996, the United States Food and Drug Administration (FDA) approved the use of an image-guided vacuum-assisted core biopsy device named the Mammotome (Ethicon Endosurgery) for percutaneous breast biopsies (18). Moreover, the National Institute for Health and Clinical Excellence in the United Kingdom supports the use of vacuum-assisted core biopsy devices for the biopsy of any breast lesion, and for the removal of benign breast lesions (19). The procedure involves ultrasound or stereotactic-guided percutaneous insertion of an 8-gauge or 11-gauge needle probe device, and the breast tissue is aspirated by vacuum suction. The incision is small (3-4 mm), and the duration of procedure ranges from 13-60 minutes. The efficacy as defined by complete removal of the lesion is largely adequate and ranges between 22% and 98% depending on the quality of imaging technique, gauge of needle and size of lesion removed. As such, benign lesions such as fibroadenomas may recur after this procedure. The most common complication is hemorrhage and hematoma due to serial core biopsies for a goal of complete removal of the tumor. Hematoma formation occurred at a rate of 0-13% while post-procedural pain also occurred (mild in 39% of patients, moderate in 4%).
In 2012, Yao et al. reported the outcomes of percutaneous vacuum-assisted complete biopsies with the Mammotome in 738 patients with presumed benign breast lesions (n=1,766) by mammography (20). Multiple biopsies (mean of 11.6) were performed in order to remove the entire tumor (mean diameter 1.25 cm). There were no reported serious adverse outcomes, and 5% of patients required pressure for bleeding. Eleven breast tumors were upstaged after the procedure (5 atypical ductal hyperplasia, 2 ductal carcinoma in situ, 4 invasive breast cancer). These findings are in line with a study by Luo et al., who reported similar outcomes in 2,167 ultrasound-guided complete excisional biopsies from 1,119 patients with presumed benign breast tumors (21). They concluded that breast tumors up to 30 mm can be safely and efficiently removed.
Wang et al. followed the patients after vacuum-assisted complete excision of benign breast tumor for six months, and noted a 3.4% rate of residual tumor by ultrasound six months post-procedure (22). Incomplete removal was associated with initial tumor size of greater than 20 mm. In terms of patient satisfaction, responses were very positive in terms of breast appearance and healing incision. Furthermore, 97% of patients would recommend the procedure to other patients. In the same year, Thurley et al. corroborated the findings of high patient satisfaction, with 94% of patients preferring vacuum biopsy to surgical excision as well as recommending the procedure to others (23). However, the procedural efficacy was lower with a 30% rate of residual (fibroadenoma) tumor present. In sum, there is high patient satisfaction despite variable procedural efficacy in the form of residual tumor on follow-up.
The presence of residual tumors and positive margins are weaknesses of the vacuum-assisted breast biopsy technique, although these rates are comparable to that of surgical excisional biopsy (24). Furthermore, tumor margins cannot be assessed with the vacuum technique. Although a residual mass may not be critical in benign disease, it is more significant for malignant tumors, as prognosis depends on local control of disease. For these reasons, this procedure is not used or recommended for malignant breast tumors.
For benign breast tumors, additional advantages of image-guided vacuum-assisted breast biopsy include lower costs, as it is an office-based procedure done under local anesthesia as compared to surgical excision in the operating room under general anesthesia (25). The procedure itself is noted to have an easy learning curve (26). Zografos et al. reports breast clinical fellows demonstrate proficiency with vacuum-assisted biopsy at 4 weeks versus hook-wire localization at 7 weeks, and ultrasound-guided core-needle biopsy at 12 weeks. This was attributed to the “standardized procedure of the vacuum-assisted breast biopsy.”
Given the positive efficacy and safety profile, as well as cosmetic outcome, cost-effectiveness and technical ease of the procedure, image-guided vacuum-assisted breast biopsy has gained wide popularity and support, and may become the new standard of care for benign breast tumors.
Percutaneous thermoablation
Thermoablation is a validated treatment strategy for many tumors including liver tumors, renal tumors, adrenal tumors, and pulmonary tumors (24,27). Thermoablation is currently being assessed as a treatment option for breast tumors, both benign and malignant. The American Society of Breast Surgeons cautions on the extent of assessment and has released a position statement regarding the use of ablative treatment in breast cancer as well as percutaneous excision of breast cancer by rotational or vacuum-assisted devices (28). It states that ablative techniques are currently investigational and should not be performed in the United States unless it is studied as part of a clinical research trial. Therefore, these techniques are currently being closely evaluated.
Thermoablation procedures by heat generation include radiofrequency ablation, focused ultrasound ablation and laser ablation (24). The most popular of these is radiofrequency ablation. In this procedure, an alternating high-frequency electric current is used to reach a target temperature of 95 degrees Celsius. This alternating high-frequency current causes excitation, motion and friction of intracellular ions, and this in turn causes thermal heating (29). Thermal damage occurs at tissue temperatures above 41 degrees Celsius, and necrosis occurs above 46 degrees Celsius. When the ablated tissue is evaluated by histology, there is coagulation necrosis and protein denaturation. However, in many case series, there is a specimen with very few but viable cells present, which is concerning for potential of recurrence.
Radiofrequency ablation can target 3-5 cm lesions, hence tumors up to 3 cm can be treated with this technique. It is feasible to target and treat tumors up to 7 cm (29). However, assessment of the extent of tissue necrosis during real-time is difficult because no visible changes occur on ultrasound during treatment. Therefore, estimating the negative margins is difficult and furthermore, the patient may be at risk for thermal injury in the form of muscle or skin burns. Other weaknesses of radiofrequency ablation include patient pain from thermal heating; to address this problem, general anesthesia is sometimes used for analgesia, which can be costly as compared to office-based procedures (29). For these reasons, radiofrequency ablation of the breast has not gained popularity.
Thermoablation procedures by cryotherapy (or freezing) have a better profile than radiofrequency ablation. Since 2001, cryotherapy has been FDA approved for treatment of fibroadenomas without subsequent resection in the United States (30). The cryotherapy procedure consists of local anesthesia, percutaneous placement of the cryoprobe through the fibroadenoma at its long-axis (Figure 3A). The argon gas flows to cool the probe to –160 degrees Celsius; cytotoxic temperatures of –20 degrees Celsius are noted at the periphery of the “iceball” (Figure 3B). The “freeze-thaw-freeze” technique is used to achieve complete ablation (24,27,31). The pathophysiology of cryotherapy involves disruption of the cellular membrane of gland cells both near and far away from the probe, as well as capillaries, resulting in thrombosis and hypoxia of the target lesion. The fibroadenoma tissue is then hyalinized with histology revealing acellular collagenized stroma which then gradually resorbs (31). In addition to good efficacy, safety and cosmesis, other advantages unique to cryotherapy include analgesia properties of freezing, visualization of the freezing phase change by ultrasound, lack of mammographic changes related to the procedure itself and evidence of a favorable immunostimulatory response.
Figure 3.

Ultrasound images of percutaneous cryoablation of a breast fibroadenoma. (A) Longitudinal view of placement of the needle trochar (arrows) through the fibroadenoma (arrowheads); (B) transverse view of an enlarging iceball (straight arrows) and the thermocouple (curved arrow) which monitors and confirms cytotoxic temperatures.
There is promising data regarding the use of cryotherapy for fibroadenoma ablation from Nurko et al., who reported the analysis of 444 fibroadenomas treated with cryotherapy from 55 community practice settings in the United States (32). Follow-up of patients at six months and one year evaluated palpability of residual mass and patient satisfaction. At one year, there was 65% efficacy at converting a palpable mass to a nonpalpable mass and breast tumors originally less than 2 cm with a better likelihood of resorption and nonpalpability. Most patients with a palpable mass at 1 year report less prominence and softening of the mass. Patient satisfaction at 6 months and 1 year was 91% and 88%. The high satisfaction could be due to alleviating patient concerns about an untreated breast lesion, elimination of palpability, and good cosmetic outcome. The authors recommend patient counseling with expectations for length of time of mass resorption, its contingency on initial mass size, as well as turning a previously nonpalpable mass to a palpable one until resorption occurs. Another study of patients with fibroadenomas treated by cryoablation with 1-year follow-up was performed by Kaufman and colleagues who had similar findings (33). Longer follow-up by the same group report that most breast fibroadenomas completely resolve by ultrasound at 2-3 years follow-up (34). Kaufman et al. offer additional insight that the ablative residual lesions did not have a potential for regrowth, which cannot be stated for still-viable residual lesions after vacuum-assisted percutaneous excision.
Currently, basic science research is focused on the immune response induced by cryotherapy (35). In a case series of 80 patients with prostate cancer who were treated with cryoablation, several patients were noted to have regression of distant metastatic tumors after treatment (36). The concept is that freezing primary tumors and leaving them in situ may permit the immune system to detect tumor-specific antigens, which may subsequently control local and distant disease. Sabel, one of the leaders in cryo-immunology research, performed cryotherapy of orthotopic breast cancers in mice that demonstrated longer survival and decreased metastatic disease (37). These findings provide a unique rationale for use of cryotherapy for treatment of breast cancer, both for early stage and metastatic (38-41).
Conclusions
Minimally invasive techniques are rapidly emerging and show great promise for effective, safe and cosmetic treatment of benign breast lesions. Endoscopic breast surgical techniques, utilized mostly in East Asia, demonstrate technique mastery of breast tissue removal with small axillary incisions. Percutaneous treatment of breast tumors, for the most part, is performed in the office setting with local anesthesia and hence, reduces costs incurred by surgical procedures with general anesthesia. Of the percutaneous treatment procedures, image-guided vacuum-assisted breast biopsy and cryoablation show the most promise. Vacuum-assisted breast biopsy, which removes the lesion by multiple core needle biopsies, confers both advantages and disadvantages to the procedure. Cryoablation destroys breast lesions in situ, avoids breast tissue volume defects and potential breast distortion. In addition, cryoablation immune biology is an exciting area of research that may have implications for novel treatment strategies for breast cancer. We look forward to the future in which we expect a reshaping of the standard of care regarding surgical and interventional treatment for benign breast disease.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Grady D. Study of breast biopsies finds surgery used too extensively. February 18, 2011. Available online: http://www.nytimes.com/2011/02/19/health/19cancer.html (Accessed: 1 Feb 2014).
- 2.Laufer MR, Goldstein DP. The breast: examination and lesions. In: Emans SJ, Laufer MR, Goldstein DP. eds. Pediatric & Adolescent Gynecology. 5th ed. Philadelphia: Lippincott Williams & Wilkins, 2005:729-59. [Google Scholar]
- 3.Smith BL. Fibroadenomas. In: Harris JR, Hellman S, Henderson IC, et al. eds. Breast diseases. 2nd ed. Philadelphia: Lippincott, 1991;34-7. [Google Scholar]
- 4.Dixon JM, Dobie V, Lamb J, et al. Assessment of the acceptability of conservative management of fibroadenoma of the breast. Br J Surg 1996;83:264-5 [PubMed] [Google Scholar]
- 5.Park CA, David LR, Argenta LC. Breast asymmetry: presentation of a giant fibroadenoma. Breast J 2006;12:451-61 [DOI] [PubMed] [Google Scholar]
- 6.Anderson BO, Masetti R, Silverstein MJ. Oncoplastic approaches to partial mastectomy: an overview of volume-displacement techniques. Lancet Oncol 2005;6:145-57 [DOI] [PubMed] [Google Scholar]
- 7.Cochrane RA, Valasiadou P, Wilson AR, et al. Cosmesis and satisfaction after breast-conserving surgery correlates with the percentage of breast volume excised. Br J Surg 2003;90:1505-9 [DOI] [PubMed] [Google Scholar]
- 8.Kompatscher P.Endoscopic capsulotomy of capsular contracture after breast augmentation: a very challenging therapeutic approach. Plast Reconstr Surg 1992;90:1125-6 [DOI] [PubMed] [Google Scholar]
- 9.Dowden RV, Anain S. Endoscopic implant evaluation and capsulotomy. Plast Reconstr Surg 1993;91:283-7 [DOI] [PubMed] [Google Scholar]
- 10.Colon GA, D’Amore TF. Mammoscopy: the endoscopic intracapsular evaluation of mammary prostheses. Plast Reconstr Surg 1993;91:382-3 [PubMed] [Google Scholar]
- 11.Eaves FF, 3rd, Bostwick J, 3rd, Nahai F, et al. Endoscopic techniques in aesthetic breast surgery. Augmentation, mastectomy, biopsy, capsulotomy, capsulorrhaphy, reduction, mastopexy, and reconstructive techniques. Clin Plast Surg 1995;22:683-95 [PubMed] [Google Scholar]
- 12.Kitamura K, Hashizume M, Sugimachi K, et al. Early experience of endoscopic extirpation of benign breast tumors via an extra-mammary incision. Am J Surg 1998;176:235-8 [DOI] [PubMed] [Google Scholar]
- 13.Kitamura K, Inoue H, Ishida M, et al. Endoscopic extirpation of benign breast tumors using an extramammary approach. Am J Surg 2001;181:211-4 [DOI] [PubMed] [Google Scholar]
- 14.Lee EK, Kook SH, Park YL, et al. Endoscopy-assisted breast-conserving surgery for early breast cancer. World J Surg 2006;30:957-64 [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Huang CK, Yu PC, et al. Retromammary approach for endoscopic resection of benign breast lesions. World J Surg 2009;33:2572-8 [DOI] [PubMed] [Google Scholar]
- 16.Cheng PJ, Vu LT, Cass DL, et al. Endoscopic specimen pouch technique for removal of giant fibroadenomas of the breast. J Pediatr Surg 2012;47:803-7 [DOI] [PubMed] [Google Scholar]
- 17.Shrotria S.Techniques for improving the cosmetic outcome of breast conservation surgery. Eur J Surg Oncol 2001;27:109-12 [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute. Improving methods for breast cancer detection and diagnosis. Available online: http://www.cancer.gov/images/documents/3a938c62-363a-4161-b676-843dd4fb7bbb/Fs5_14.pdf (Accessed 1 Feb 2014).
- 19.National Institute for Health and Clinical Excellence. Image-guided vacuum-assisted excision biopsy of benign breast lesions. 2006. Available online: http://www.nice.org.uk/nicemedia/pdf/ip/IPG156guidance.pdf (Accessed 1 Feb 2014).
- 20.Yao F, Li J, Wan Y, et al. Sonographically guided vacuum-assisted breast biopsy for complete excision of presumed benign breast lesions. J Ultrasound Med 2012;31:1951-7 [DOI] [PubMed] [Google Scholar]
- 21.Luo HJ, Chen X, Tu G, et al. Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J 2011;17:490-7 [DOI] [PubMed] [Google Scholar]
- 22.Wang WJ, Wang Q, Cai QP, et al. Ultrasonographically guided vacuum-assisted excision for multiple breast masses: non-randomized comparison with conventional open excision. J Surg Oncol 2009;100:675-80 [DOI] [PubMed] [Google Scholar]
- 23.Thurley P, Evans A, Hamilton L, et al. Patient satisfaction and efficacy of vacuum-assisted excision biopsy of fibroadenomas. Clin Radiol 2009;64:381-5 [DOI] [PubMed] [Google Scholar]
- 24.Vlastos G, Verkooijen HM. Minimally invasive approaches for diagnosis and treatment of early-stage breast cancer. Oncologist 2007;12:1-10 [DOI] [PubMed] [Google Scholar]
- 25.Alonso-Bartolomé P, Vega-Bolívar A, Torres-Tabanera M, et al. Sonographically guided 11-G directional vacuum-assisted breast biopsy as an alternative to surgical excision: utility and cost study in probably benign lesions. Acta Radiol 2004;45:390-6 [DOI] [PubMed] [Google Scholar]
- 26.Zografos GC, Zagouri F, Sergentanis TN. Vacuum-assisted breast biopsy: an easy-to-learn procedure? Am J Surg 2008;196:798. [DOI] [PubMed] [Google Scholar]
- 27.Tatli S, Acar M, Tuncali K, et al. Percutaneous cryoablation techniques and clinical applications. Diagn Interv Radiol 2010;16:90-5 [DOI] [PubMed] [Google Scholar]
- 28.The American Society of Breast Surgeons. Position statement on ablative and percutaneous treatment of breast cancer. Available online: https://www.breastsurgeons.org/statements/PDF_Statements/Ablative_Percutaneous.pdf (Accessed 1 Feb 2014).
- 29.Jeffrey SS, Birdwell RL, Ikeda DM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg 1999;134:1064-8 [DOI] [PubMed] [Google Scholar]
- 30.Simmons RM. Ablative techniques in the treatment of benign and malignant breast disease. J Am Coll Surg 2003;197:334-8 [DOI] [PubMed] [Google Scholar]
- 31.Littrup PJ, Freeman-Gibb L, Andea A, et al. Cryotherapy for breast fibroadenomas. Radiology 2005;234:63-72 [DOI] [PubMed] [Google Scholar]
- 32.Nurko J, Mabry CD, Whitworth P, et al. Interim results from the FibroAdenoma Cryoablation Treatment Registry. Am J Surg 2005;190:647-51; discussion 651-2 [DOI] [PubMed] [Google Scholar]
- 33.Kaufman CS, Bachman B, Littrup PJ, et al. Cryoablation treatment of benign breast lesions with 12-month follow-up. Am J Surg 2004;188:340-8 [DOI] [PubMed] [Google Scholar]
- 34.Kaufman CS, Littrup PJ, Freeman-Gibb LA, et al. Office-based cryoablation of breast fibroadenomas with long-term follow-up. Breast J 2005;11:344-50 [DOI] [PubMed] [Google Scholar]
- 35.Sabel MS. Cryo-immunology: a review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009;58:1-11 [DOI] [PubMed] [Google Scholar]
- 36.Alblin RJ, Soanes WA, Gonder MJ. Prospects for cryo-immunotherapy in cases of metastasizing carcinoma of the prostate. Cryobiology 1971;8:271-9 [DOI] [PubMed] [Google Scholar]
- 37.Sabel MS, Su G, Griffith KA, et al. Rate of freeze alters the immunologic response after cryoablation of breast cancer. Ann Surg Oncol 2010;17:1187-93 [DOI] [PubMed] [Google Scholar]
- 38.Manenti G, Perretta T, Gaspari E, et al. Percutaneous local ablation of unifocal subclinical breast cancer: clinical experience and preliminary results of cryotherapy. Eur Radiol 2011;21:2344-53 [DOI] [PubMed] [Google Scholar]
- 39.Niu L, Mu F, Zhang C, et al. Cryotherapy protocols for metastatic breast cancer after failure of radical surgery. Cryobiology 2013;67:17-22 [DOI] [PubMed] [Google Scholar]
- 40.White RL., Jr Cryoablative therapy in breast cancer: no. J Surg Oncol 2008;97:483-4 [DOI] [PubMed] [Google Scholar]
- 41.Sabel MS. Cryoablation for breast cancer: no need to turn a cold shoulder. J Surg Oncol 2008;97:485-6 [DOI] [PubMed] [Google Scholar]



