Abstract
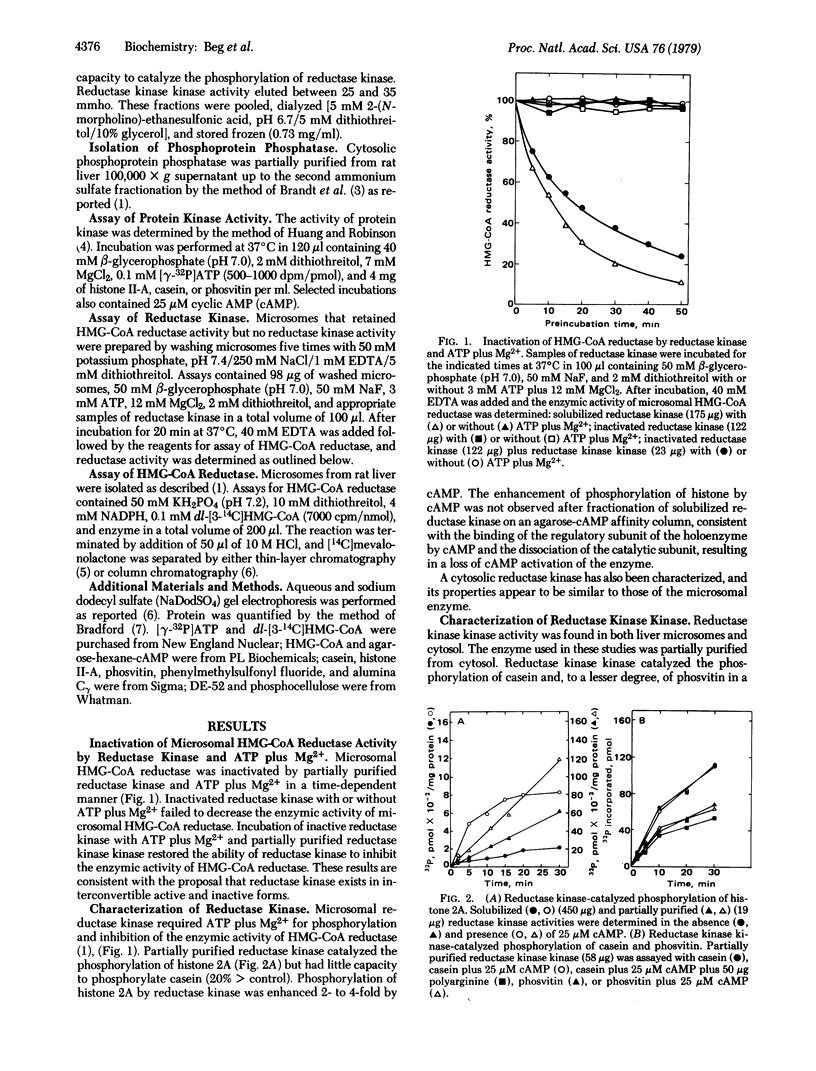
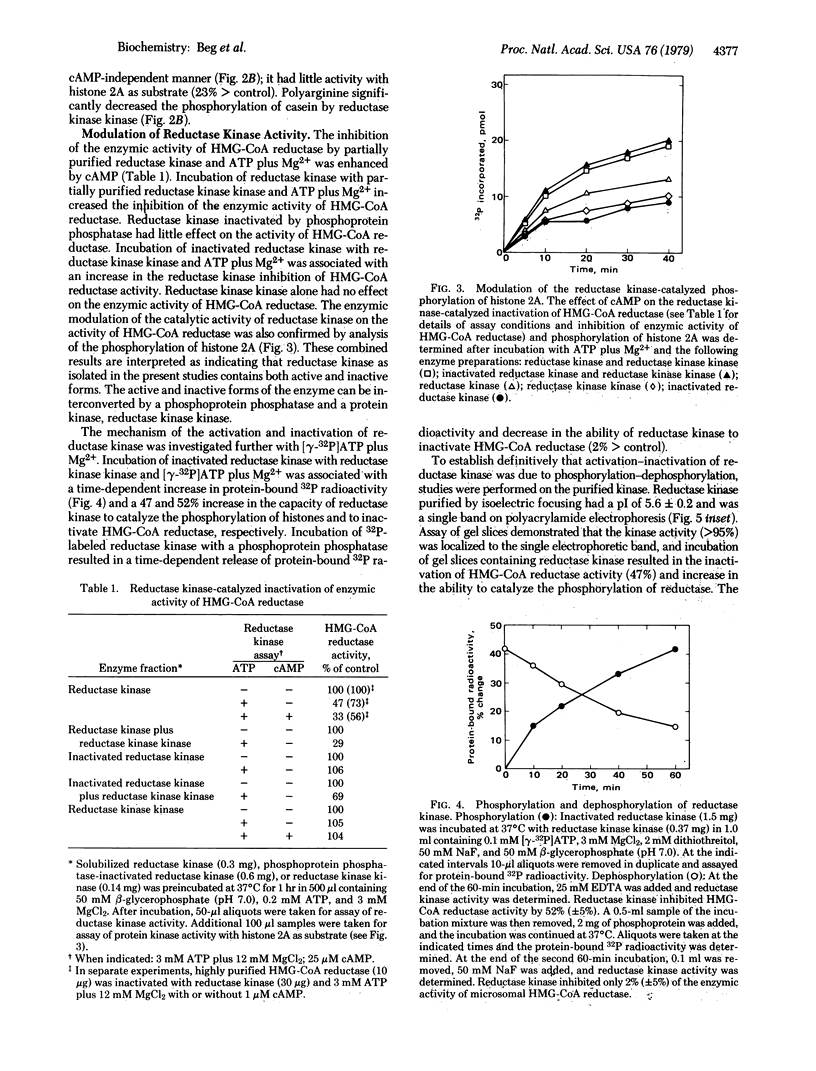
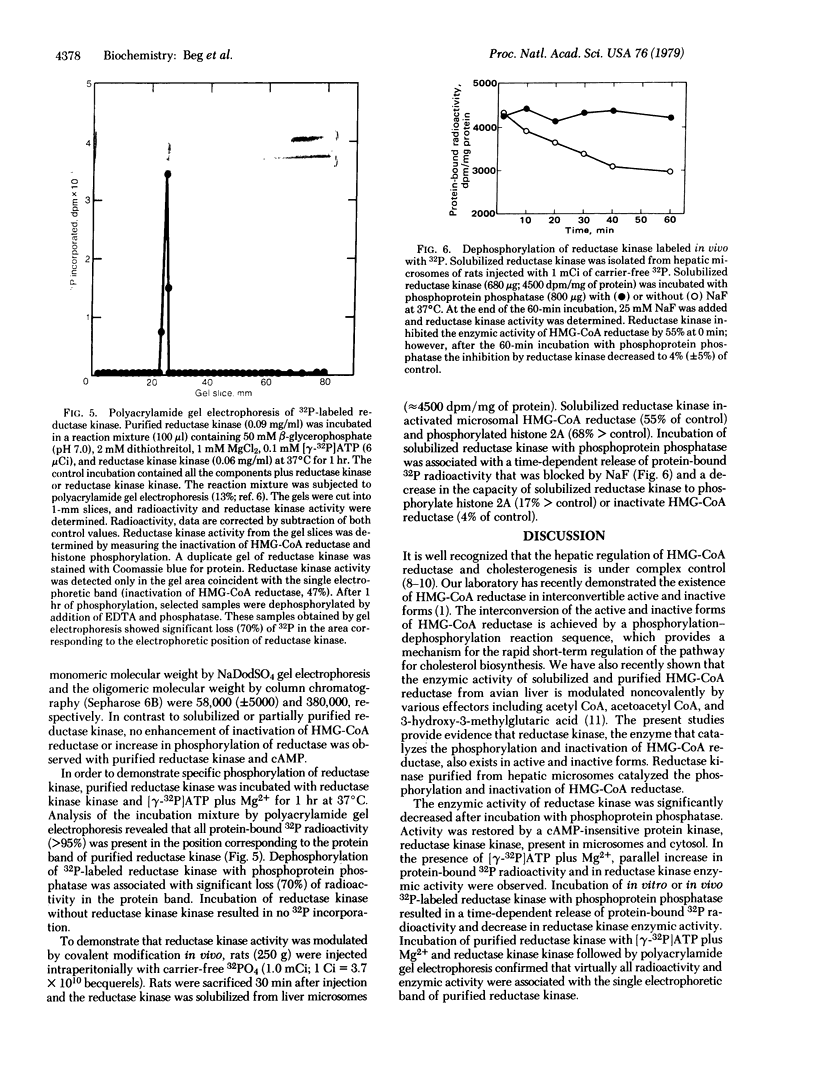
The activity of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase [HMG-CoA reductase; mevalonate:NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34] can be modulated in vitro by a phosphorylation-dephosphorylation reaction sequence. A microsomal reductase kinase catalyzes the phosphorylation of HMG-CoA reductase and histones. Histone phosphorylation was enhanced 2- to 3-fold by cyclic AMP. Reductase kinase exists in interconvertible active and inactive forms. Incubation of reductase kinase with phosphoprotein phosphatase resulted in a time-dependent decrease in the ability of reductase kinase to catalyze the phosphorylation of histones and to inactivate HMG-CoA reductase. Incubation of phosphoprotein phosphatase-inactivated reductase kinase with [γ-32P]ATP plus Mg2+ and a partially purified protein kinase designated reductase kinase kinase resulted in parallel increases in protein-bound 32P radioactivity and ability to inactivate HMG-CoA reductase. Incubation of 32P-labeled reductase kinase with phosphoprotein phosphatase resulted in a time-dependent loss of protein-bound 32P radioactivity and a decrease in the ability to inactivate HMG-CoA reductase. Polyacrylamide gel electrophoresis of purified reductase kinase incubated with reductase kinase kinase and [γ-32P]ATP plus Mg2+ revealed that the 32P radioactivity and reductase kinase enzymic activity were located in a single electrophoretic position. Dephosphorylation of 32P-labeled purified reductase kinase with phosphoprotein phosphatase was associated with significant loss of radioactivity and enzymic activity in the protein band ascribed to reductase kinase. These results provide evidence that the activity of reductase kinase, like HMG-CoA reductase, is modulated by a reversible phosphorylation-dephosphorylation reaction sequence.
Keywords: phosphorylation, covalent modification, cholesterol, cyclic AMP, enzyme regulation
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beg Z. H., Allmann D. W., Gibson D. M. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1362–1369. doi: 10.1016/0006-291x(73)91137-6. [DOI] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase from avian liver. Catalytic properties. Biochim Biophys Acta. 1979 Jan 29;572(1):83–94. doi: 10.1016/0005-2760(79)90202-9. [DOI] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3678–3682. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Purification and characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase from chicken liver. FEBS Lett. 1977 Aug 1;80(1):123–129. doi: 10.1016/0014-5793(77)80421-3. [DOI] [PubMed] [Google Scholar]
- Bishop J. S. Inability of insulin to activate liver glycogen transferase D phosphatase in the diabetic pancreatectomized dog. Biochim Biophys Acta. 1970 May 12;208(2):208–218. doi: 10.1016/0304-4165(70)90239-4. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brandt H., Capulong Z. L., Lee E. Y. Purification and properties of rabbit liver phosphorylase phosphatase. J Biol Chem. 1975 Oct 25;250(20):8038–8044. [PubMed] [Google Scholar]
- Gibson D. M., Ingebritsen T. S. Reversible modulation of liver hydroxymethylglutaryl CoA reductase. Life Sci. 1978 Dec 31;23(27-28):2649–2664. doi: 10.1016/0024-3205(78)90644-6. [DOI] [PubMed] [Google Scholar]
- Gold A. H. The effect of diabetes and insulin on liver glycogen synthetase activation. J Biol Chem. 1970 Feb 25;245(4):903–905. [PubMed] [Google Scholar]
- Goris J., Defreyn G., Vandenheede J. R., Merlevede W. Protein inhibitors of dog-liver phosphorylase phosphatase dependent on and independent of protein kinase. Eur J Biochem. 1978 Nov 15;91(2):457–464. doi: 10.1111/j.1432-1033.1978.tb12698.x. [DOI] [PubMed] [Google Scholar]
- Huang F. L., Glinsmann W. H. Inactivation of rabbit muscle phosphorylase phosphatase by cyclic AMP-dependent kinas. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3004–3008. doi: 10.1073/pnas.72.8.3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C. A rapid and sensitive assay method for protein kinase. Anal Biochem. 1976 May 7;72:593–599. doi: 10.1016/0003-2697(76)90571-6. [DOI] [PubMed] [Google Scholar]
- Ingebritsen T. S., Lee H. S., Parker R. A., Gibson D. M. Reversible modulation of the activities of both liver microsomal hydroxymethylglutaryl coenzyme A reductase and its inactivating enzyme. Evidence for regulation by phosphorylation-dephosphorylation. Biochem Biophys Res Commun. 1978 Apr 28;81(4):1268–1277. doi: 10.1016/0006-291x(78)91273-1. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Zinman S. M., Zebrowski E. J. The effect of streptozotocin-induced diabetes and of insulin supplementation on glycogen metabolism in rat liver. Biochem J. 1977 Dec 15;168(3):541–548. doi: 10.1042/bj1680541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmanan M. R., Nepokroeff C. M., Ness G. C., Dugan R. E., Porter J. W. Stimulation by insulin of rat liver -hydroxy- -methylglutaryl coenzyme A reductase and cholesterol-synthesizing activities. Biochem Biophys Res Commun. 1973 Feb 5;50(3):704–710. doi: 10.1016/0006-291x(73)91301-6. [DOI] [PubMed] [Google Scholar]
- Nichols W. K., Goldberg N. D. The relationship between insulin and apparent glucocorticoid-promoted activation of hepatic glycogen synthetase. Biochim Biophys Acta. 1972 Sep 15;279(2):245–259. doi: 10.1016/0304-4165(72)90140-7. [DOI] [PubMed] [Google Scholar]




