Abstract
Large-conductance, calcium-activated potassium (BKCa) channels are regulated by voltage and near-membrane calcium concentrations and are determinants of membrane potential and excitability in airway smooth muscle cells. Since the T helper–2 (Th2) cytokine, interleukin (IL)-4, is an important mediator of airway inflammation, we investigated whether IL-4 rapidly regulated BKCa activity in normal airway smooth muscle cells. On-cell voltage clamp recordings were made on subconfluent, cultured human bronchial smooth muscle cells (HBSMC). Interleukin-4 (50 ng ml−1), IL-13 (50 ng ml−1) or histamine (10 μm) was added to the bath during the recordings. Immunofluorescence studies with selective antibodies against the α and β1 subunits of BKCa were also performed. Both approaches demonstrated that HBSMC membranes contained large-conductance channels (>200 pS) with both calcium and voltage sensitivity, all of which is characteristic of the BKCa channel. Histamine caused a rapid increase in channel activity, as expected. A new finding was that perfusion with IL-4 stimulated rapid, large increases in BKCa channel activity (77.2 ± 63.3-fold increase, P < 0.05, n = 18). This large potentiation depended on the presence of external calcium. In contrast, IL-13 (50 ng ml−1) had little effect on BKCa channel activity, but inhibited the effect of IL-4. Thus, HBSMC contain functional BKCa channels whose activity is rapidly potentiated by the cytokine, IL-4, but not by IL-13.These findings are consistent with a model in which IL-4 rapidly increases near-membrane calcium concentrations to regulate BKCa activity.
Contraction of airway smooth muscle cells contributes to airway obstruction in the inflammatory disease, asthma. Large-conductance, calcium-activated potassium (BKCa) channels are abundant in normal airway smooth muscle cells and modulate membrane potential, electromechanical coupling and contraction (Savaria et al. 1992; Snetkov et al. 1996; Wang et al. 1997; Semenov et al. 2006). Activation of BKCa channels allows an efflux of potassium ions from the cell which hyperpolarizes the plasma membrane and favours smooth muscle cell relaxation (Kotlikoff & Kamm, 1996). A fundamental property of BKCa channels is their high sensitivity to and rapid activation by increases in calcium. These channels are also regulated by protein phosphorylations, G–proteins and membrane voltage (Kume et al. 1989; Wang et al. 1997; Vergara et al. 1998). Owing to their importance in regulating contraction and relaxation, there is interest in understanding how inflammatory mediators regulate BKCa channel activity in airway smooth muscle cells (Savaria et al. 1992; Kotlikoff & Kamm, 1996).
Interleukin (IL)-4 and IL-13 are inflammatory mediators that clearly have important roles in the pathogenesis of allergic inflammation (Grunig et al. 1998; Wills-Karp et al. 1998; Barnes, 2001). However, even though it is clear that IL-4 promotes the asthma phenotype, there is some evidence that IL-4 has unexpected inhibitory effects on calcium signalling in airway smooth muscle (Madison & Ethier, 2001). Specifically, studies from bovine trachealis cells have shown that IL-4, but not IL-13, inhibits the calcium signalling of contractile agonists by depleting sarcoplasmic reticulum (SR) calcium through ryanodine receptors over exposures as short as approximately 20 min (Madison & Ethier, 2001; Ethier et al. 2005; Ethier & Madison, 2006). Since consistent increases in cytosolic calcium were not detected by calcium imaging techniques, those findings suggested the possibility that IL-4 might be causing relatively small, difficult to detect and possibly localized, changes in subplasmalemmal calcium to regulate the function of plasma membrane ion channels. Since BKCa channels are plasma membrane ion channels that are highly sensitive to changes in subplasmalemmal calcium, we hypothesized that IL-4 might be activating BKCa channel activity in airway smooth muscle cells. Therefore, we tested that hypothesis by using on-cell patch clamping of BKCa channels in cultured human bronchial smooth muscle cells (HBSMC), a cell type previously shown to express abundant BKCa channels (Snetkov et al. 1996).
Methods
Cell culture
Human bronchial smooth muscle cells, staining positive for smooth muscle actin and negative for factor VIII, were obtained commercially at passage 3 from Cambrex Bioproducts (Walkersville, MD, USA). Cells were seeded and cultured in growth media (SMGM2, Cambrex Bioproducts) consisting of smooth muscle basal medium (SMBM, Cambrex Bioproducts) supplemented with 5% fetal bovine serum, 5 μg ml−1 insulin, 2 ng ml−1 fibroblast growth factor, 0.5 ng ml−1 epidermal growth factor, 50 μg ml−1 gentamicin and 50 ng ml−1 amphotericin B. Cells were maintained at 37°C in a humidified atmosphere (5% CO2), fed every 48 h, and passaged when 80–90% confluent.
For channel recording, HBSMC (passage 4 or 5) were seeded in 35mm culture dishes (Nunclon, Rochester, NY, USA) and grown to 35% confluence in SMGM2. Cultures were maintained in serum-containing media up to the time of study and were referred to as ‘fed’ cells. Some cultures were ‘starved’ by incubating them for 24–144 h in serum- and growth factor-free medium, as indicated. Immediately prior to experiments, both fed and starved cells were washed and overlayed with modified Krebs–Ringer–Henseleit (KRH) buffer.
Electrophysiology
Cell-attached patch clamp recording
The activity of BKCa channels was recorded in cell-attached patch clamp mode (Hamill et al. 1981). Briefly, patch electrodes were pulled from borosilicate capillary glass (Drumond, Broomall, PA, USA) on a Brown-Flaming puller (P–97, Sutter Instruments, Novato, CA, USA). The glass was then coated near the tip with Sylgard to minimize stray capacitive currents. The tips were fire polished with a microforge (MP–83 Narishige, Tokyo, Japan) shortly before use to a final resistance (when filled) of 5–7 MΩ. The capillaries were first filled with vacuum through the tip and then backfilled with the recording solution. The BKCa channel activity was recorded in cell-attached voltage-clamp mode using an EPC–10 double patch clamp amplifier from HEKA Electronik (Lambrecht/Pfalz, Germany). Gigaohm seals were formed in the usual way and seal resistance was monitored throughout the recording (Hamill et al. 1981). The BKCa channel activity was acquired with Patchmaster software version 2.03 (HEKA Electronik) and analysed with TAC and TACfit, single-channel analysis programs running on an Apple computer (PowerPC G5). The signal was sampled at 5 kHz and filtered at 2 kHz. Potentials were not corrected for liquid junction potentials, but the latter are estimated to be ±4 mV. Signals were automatically reversed by Patchmaster when the amplifier was set to its ‘on-cell’ recording mode (Fig. 1A).
Figure 1. Characterization of BKCa channels.

A, typical ‘on-cell’ recording from a single patch on a HBSMC, illustrating the voltage sensitivity in 5 μm Ca2+. In this and subsequent figures, C represents the closed state and O1, O2, O3 and O4 indicate the number of channels open. B, following these recordings, the patch was pulled from the cell to obtain an ‘inside-out’ recording, and the calcium sensitivity (held at +30 mV) was determined as shown. C, the slope conductance (γ) is computed from data from the recordings shown in A.
Inside-out patch clamp recording
Occasionally the ‘inside-out’ recording configuration was achieved by pulling the on-cell pipette away from the cell after the formation of the on-cell configuration (Ogden, 1994). This allowed us to confirm the presence of BKCa channels in the patch of membrane by exposing the cytosolic side of the patch to known concentrations of calcium (Fig. 1B).
Superfusion and reagent application
For cell-attached recordings, a slow bath application of reagents dissolved in KRH buffer was used at an average flow rate of 1.5 ml min−1. Standard reagent testing was as follows. After seal formation, the membrane potential was held at −50 mV (this is the amplifier voltage command; no attempt was made to determine the resting membrane potential for the cell). In control conditions, BKCa channel activity was evoked by applying a positive voltage step for 20 s. The amplitude of the voltage step was adjusted in order to obtain a low BKCa channel open probability (Po). The amplitude of the voltage steps necessary to evoke a reasonable BKCa channel activity (Po ~ 0.1) was around 140–150 mV for most cells, which gave a potential across the membrane patches of ~100 mV, assuming resting membrane potentials of smooth muscle cells to be around −50 mV. In control conditions, this step was repeated two or three times before single muscle cells were exposed to reagents to ensure that BKCa channel activity was stable (Martin & Siggins, 2002). The reagent concentrations reported here are for the solutions applied to the bath. They do not represent the final bath concentration of the reagent. The order of stimulation with test substances was varied from cell to cell without a noticeable alteration in effect. Each stimulus was superimposed on the same control voltage step to make it possible to detect both positive and negative shifts in activation caused by application of the reagents. Steps were given as quickly as possible, but in most cases the gap between successive traces is estimated to be less than 1 s.
Immunostaining
Human bronchial smooth muscle cells were grown on coverslips as described above (in the Cell culture subsection), fixed in 2% paraformaldehyde and permeabilized/blocked in a buffer containing 0.1% Triton X-100, 10% normal goat serum and 0.05% bovine serum albumin, all in phosphate-buffered saline. Cells were then stained with antibodies against the BKCa channel α subunit (a gift from James Trimmer, UC Davis; 1:10 000 dilution, mouse antibodies; Trimmer & Rhodes, 2004) followed by incubation with the fluorescently tagged secondary antibodies (Alexa 594, Molecular Probes, Eugene, OR, USA; a red fluorophor, conjugated with anti-mouse antibodies) and then with rabbit antibodies against the BKCa β1 subunit (Affinity Bioreagents, Golden, CO, USA; PA1-924, 1:300 dilution). We previously evaluated the specificity of this latter antibody, in a study of HEK 293 cells expressing the α subunit with or without the β1 subunit (Martin et al. 2004). Then the cells were incubated with the secondary antibodies (Alexa 488, a green fluorophor, conjugated with anti-rabbit antibodies). Next, cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (1:5000 dilution; a blue fluorophor) to visualize nuclei and mounted on microslides (SuperFrost Plus, VWR Scientific, San Dimas, CA, USA) in Prolong Antifade medium (Molecular Probes).
Statistics
Data are expressed as means ± s.e.m. Means were compared by paired and unpaired Student’s t test as appropriate. For assessing the effects of different culture media on responses, proportions were compared by Fisher’s exact test. GraphPad Prism Software (San Diego, CA, USA) was used for analyses and P < 0.05 was considered significant.
Materials
Alexa 594 and Alexa 488-conjugated antibodies were obtained from Molecular Probes (Eugene, OR, USA). Insulin and transferrin were obtained from Invitrogen (Carlsbad, CA, USA).Human recombinant IL-4 and IL-13 were obtained from R&D Systems Inc. (Minneapolis, MN, USA). Other reagents were obtained from Sigma (St Louis, MO, USA). The KRH solution contained (mm): 115 NaCl, 5 KCl, 1 KH2PO4, 1 MgSO4, 25Hepes, 15 glucose and 2 CaCl2 (Wang et al. 1997). The recording electrode solution contained (mm): 135 potassium gluconate, 15 Hepes, 2 CaCl2, 2 EGTA, 2 MgCl2 and 10 glucose (pH adjusted to 7.35 with KOH; osmolarity, 305 mosmol l−1).
Results
Human bronchial smooth muscle cells express α and β1 subunits of BKCa channels
The BKCa channels were abundant in HBSMC. As illustrated in Fig. 1, channels displayed voltage sensitivity (Fig. 1A), calcium sensitivity (Fig. 1B), slope conductance (Fig. 1C) and a lack of inactivation (Fig. 1B, top trace, 10 μm Ca2+), all of which are characteristic of potassium channels of the BKCa type (Vergara et al. 1998; Salkoff et al. 2006). Approximately 86% of all the successful gigaohm seals obtained in these experiments contained one or more BKCa channels (n = 123 recordings). Using a specific antibody directed against the core α subunit (red staining), we found that BKCa channels are highly expressed in HBSMC (Fig. 2). These data also reveal the presence of the β1 subunit of the BKCa channel (green staining).
Figure 2. Expression of BKCa channels.
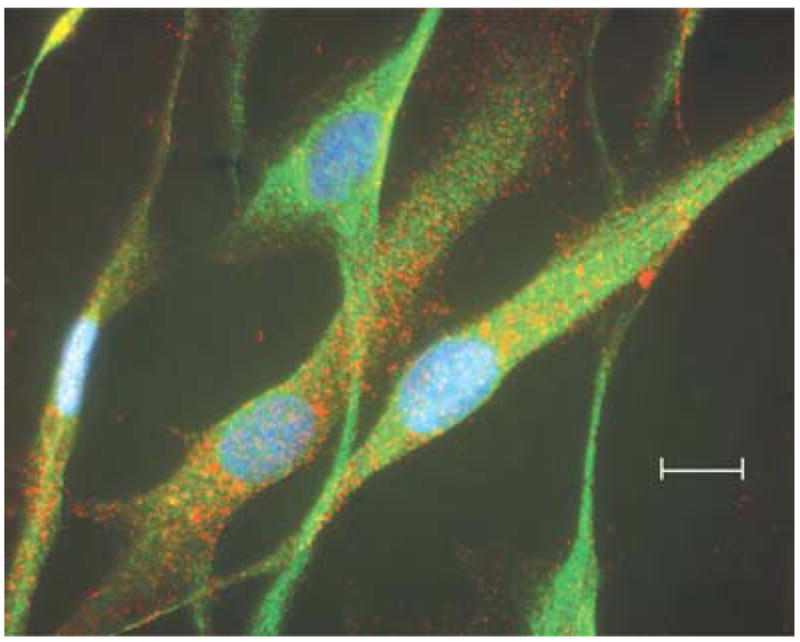
The HBSMC grown on coverslips were fixed and stained with antibodies and fluorophores as described in the Methods. Red fluorophor represents staining against the BKCa channel α subunit. Green fluorophor represents staining against the BKCa channel β1 subunit. Blue fluorophor represents staining with DAPI to visualize cell nuclei. The scale bar represents a length of 5 microns.
As expected from the known calcium sensitivity of BKCa channels, on-cell recordings from four of four cells exposed to histamine (10 μm) showed rapid increases in BKCa channel activity (Fig. 3). In control conditions, one BKCa channel opened sporadically (upward deflection) during the voltage step. Less than 1 min following histamine superfusion into the bath, its activity was increased dramatically during the next step shown. This figure also illustrates the procedure for quantification of the response. Here, the response obtained after histamine application is quantified by computing the open probability (Po) value derived from the all-point histograms (for the entire 20 s step, a portion of which is shown above each graph) for each segment (control versus reagent) of the recording. In control conditions, the channel was open ~7% of the time. Several minutes after the addition of histamine, it was open ~71% of the time. Thus, in this channel, histamine elicited a 10-fold increase in activity. When averaged over four cells, histamine increased BKCa activity an average of 7.7 ± 0.9-fold (P < 0.05). These findings are consistent with a model in which the BKCa channel is responding to the increases in cytosolic calcium normally elicited by histamine. Histamine causes measurable bulk increases in intracellular calcium in airway smooth muscle (Sims et al. 1996).
Figure 3. BKCa channel responses to histamine.

Typical on-cell recordings illustrating a portion of the 20 s control voltage step (top, left) and the same duration step after the bath application of 10 μm histamine (top, right). The bottom panels are the all-point histograms for the 20 s recordings shown above them with an indication of the open probability (Po) derived from the histograms.
Activity of BKCa channels is potentiated by IL-4 but not by IL-13
Bath application of IL-4 (50 ng ml−1) alone elicited an increase in BKCa channel activity. A typical response to IL-4 is shown in Fig. 4. In control conditions, the channel was open about 1% of the time. Following bath application of IL-4, the next three voltage steps (not shown) retained the same open probability. In the next step, however, the channel open probability increased to 23% (right panel of Fig. 4B), and gradually decreased thereafter (not shown). The timing and magnitude of the response varied from patch to patch. For example, in Fig. 5, recordings from another patch show that the control conditions appear to have only one BKCa channel in the membrane during the voltage step. Application of IL-4 to the bath at the onset of the second 20 s voltage step was associated with the near simultaneous opening of at least seven to nine channels in the patch after a delay of ~4 s. For most of these channels, it is clear that they remained in the open state for a substantial portion of the step. Although IL-4 remained in the bath for the remainder of the recording, the BKCa channels in the patch then closed sequentially during the next voltage step, and channel activity gradually returned towards baseline during the next two steps. This phasic response was commonly observed but the amplitude and duration of the response varied from patch to patch. In 13 cells, responses were characterized as moderate, with IL- 4 potentiating BKCa channel activity 4.9 ± 0.6-fold (range=0.96- to 8.3-fold, P < 0.05). In five cells, this effect was much larger and more variable (264.5 ± 220.8- fold, range=12.8 to 1148.0, P < 0.05). For all 18 cells, the mean increase in BKCa channel activity was 77.2 ± 63.3- fold (P < 0.05).
Figure 4. BKCa channel responses to IL-4.

Typical on-cell recordings illustrating a portion of the 20 s control voltage step (A, top trace) and the same step after ~1 min after the bath application of 50 ng ml−1 of IL-4 (A, bottom trace). The panels in B are the all-point histograms for the recordings shown above them with an indication of the average open probability (nPo) derived from the histograms.
Figure 5. On-cell recordings illustrating the typical response to IL-4 stimulation.

In this patch, there are 7 or more channels responding. In the top right panel, the figure has a cropped appearance only because the signal was unexpectedly too large for the scale set by the gain. Two adjacent voltage steps (gap ~1 s) have been joined to show the onset and offset of the response. In the bottom two panels, the slow recovery back to control levels for sequential voltage steps in the continued presence of IL-4 is shown.
In contrast, IL-13 (50 ng ml−1) barely altered BKCa channel activity (Fig. 6). In nine of 10 cells, BKCa channel activity increased by only 1.1 ± 0.1-fold (n.s.). In one cell only, IL-13 potentiated BKCa channel open probability by nearly 2.5-fold. When cells were treated with IL-13 for 3 min and then exposed to IL-4 in the continued presence of IL-13, BKCa channel activity increased, but the increases in activity were small and delayed (Fig. 7). Even when compared with the moderate responses to IL-4 alone (4.9 ± 0.6-fold increases in BKCa channel activity), responses to IL-4 in the presence of IL-13were significantly less (2.0 ± 0.3-fold increase, n = 7, P < 0.01).
Figure 6. Lack of BKCa channel responses to IL-13.

Typical on-cell recording illustrating the lack of response to the application of IL-13 (50 ng ml−1). The sequential voltage steps shown are less than 1 s apart (read from left to right and top to bottom).
Figure 7. BKCa channel responses to IL-4 in the presence of IL-13.
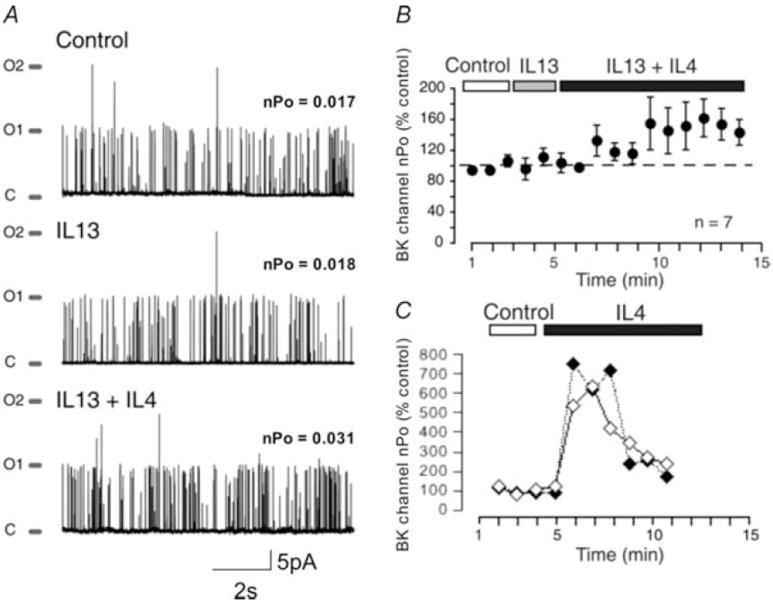
A, on-cell recordings illustrating the response to IL-4 in the presence of IL-13. The sequential voltage steps shown are less than 1 s apart (read from top to bottom). B, BKCa channel activity is expressed as a percentage of control as cells are exposed to IL-13 (50 ng ml−1) and then IL-4 (50 ng ml−1) in the continued presence of IL-13 (mean ± s.e.m., n = 7 cells). C, from the same experiments, BKCa channel activity is expressed as a percentage of control for 2 cells exposed to IL-4 alone (see Figs 4 and 5).
In optimizing culture conditions, serum starvation had significant effects on the pattern of the IL-4 response observed. Since previous studies have reported that serum starvation can induce phenotypic changes in smooth muscle cells in culture (Halayko et al. 1999) and in BKCa channels of airway smooth muscle cells (Snetkov et al. 1996), the effect of serum starvation on IL-4 effects on BKCa channel activity was tested. In Fig. 8, diary plots show the response to IL-4 in four starved (Fig. 8A) and four fed cells (Fig. 8B). The BKCa channel open probability is plotted as a function of time (nPo measured every 2 s of the recording). In fed cells, there were phasic responses to IL-4 in 11 of 11 cells, whereas in starved cells the response was very nearly tonic (5 of 5 cells, P < 0.05). As noted earlier, very large phasic responses were also occasionally seen in fed cells but are not included in this figure for illustration purposes. It is emphasized that the apparent magnitude of responses is confounded in part by the number of channels that happen to occur in each patch.
Figure 8. Effect of serum starvation on BKCa channel responses to IL-4.

Diary plots of activity in recordings from 4 starved cells (A) and 4 fed cells (B) in response to IL-4 (50 ng ml−1). Each point is the average open probability (nPo) for successive 2 s intervals of the recording. These are raw scores. No attempt has been made to normalize or synchronize the observed response to bath application of IL-4. The responses plotted were selected for the duration of the recordings.
The IL-4-mediated potentiation of BKCa channel activity depends on extracellular calcium
If BKCa activation by IL-4 depends on an increase in cytosolic calcium, it is important to determine the source of the calcium. One potential source of calcium is from the exterior of the cell. Unfortunately, it is very difficult to obtain and hold on-cell seals when cells are bathed in solutions without external calcium. To avoid this problem and investigate the need for external calcium, we used two approaches. In one approach, external calcium was replaced with an equivalent amount of barium. Barium permeates calcium channels but does not modulate the activity of BKCa channels at the concentrations employed. A second approach was to form seals in calcium-containing solutions, then perfuse the bath with calcium-free KRH and, finally, apply IL-4 (50 ng ml−1) in calcium-free KRH. For both approaches, there is zero external calcium present when IL-4 is applied.
Responses elicited by IL-4 were not observed in recordings made in solutions that lacked external calcium (Fig. 9). For eight cells, IL-4 increased BKCa channel activity by only 0.14 ± 0.05-fold (n.s.). Collectively, this suggests that BKCa channel activation by IL-4 is dependent on calcium entry from the cell exterior through plasma membrane calcium-permeable channels.
Figure 9. BKCa channel requirement for extracellular calcium.

A, on-cell recordings showing no response to IL-4 in a cell bathed in an extracellular solution (KRH solution) in which calcium has been replaced by an equivalent amount of barium. B, all-point histograms for the recordings shown in A, with an indication of the average open probability (nPo) derived from the histograms. C, on-cell recordings from a different cell, illustrating the lack of response to IL-4 in a cell bathed in an extracellular solution (KRH solution) without calcium.
Discussion
Human bronchial smooth muscle cells have functional BKCa channels as determined by electrophysiology and express both α and β1 subunits as determined by immunocytochemistry. The main conclusion of this study is that IL-4, a cytokine important in the pathophysiology of asthma, rapidly increased BKCa channel activity in normal human airway smooth muscle cells. Notably, the closely related cytokine, IL-13, a central mediator of asthma (Wills-Karp et al. 1998), did not share the effect of IL-4 on BKCa channel activity, but did antagonize the effect of IL-4. These findings support prior observations in bovine trachealis cells showing that IL-4, but not IL-13, inhibits contractile agonist signalling, an effect favouring cell relaxation (Madison & Ethier, 2001; Ethier et al. 2005; Ethier & Madison, 2006).
Cultured HBSMC expressed abundant potassium channels of the BKCa type (Snetkov et al. 1996; Vergara et al. 1998; Salkoff et al. 2006). Depending on cell type, BKCa channels each consist of four α subunits and one of several subtypes of β subunits. In this study, immunocytochemistry was used to demonstrate the expression of both the α and the β1 subunits of the BKCa channel in HBSMC using selective antibodies (Martin et al. 2004). The β subunit comprising BKCa channels has not been subtyped previously in human airway smooth muscle cells, and our finding that these cells express the β1 subunit is new. The finding is consistent with a recent study showing β1 subunits in BKCa channels of mouse tracheal smooth muscle (Semenov et al. 2006). In common with other smooth muscle cell types, we assume that the presence of the β1 subunit increases the calcium sensitivity of the channel (Cox & Aldrich, 2000; Lohn et al. 2001; Morrow et al. 2006).
The major new finding of this study is that IL-4 increased BKCa channel activity when applied to single, cultured HBSMC and, therefore, the first question was whether this new effect of IL-4 occurred at the same concentrations needed to elicit other responses to IL- 4. In the present study, IL-4 (50 ng ml−1) was released onto single cells from a pipette near the cell. Although the tissue concentrations of IL-4 in vivo are not known, the concentration of IL-4 (50 ng ml−1) in the pipette was the same concentration that has been used in other in vitro studies of IL-4 and IL-13 in non-haematopoietic cells, including smooth muscle and non-smooth muscle cell types (Berkman et al. 1996; Laporte et al. 2001;Moore et al. 2002; Nabeshima et al. 2005). Since the IL-4 was released onto single cells from a pipette, the concentration of IL-4 reaching the cells in our study would be expected to be even less than the 50 ng ml−1 typically used in other in vitro studies (Hawker et al. 1998; Kellner et al. 2007). All this suggests that the large and rapid effects of IL-4 on BKCa channel activity occur at concentrations similar to or less than other known effects of IL-4.
Since BKCa channels are so abundant in airway smooth muscle cells, most patches contained multiple BKCa channels. The occurrence of multiple channels in each patch complicated analysis of channel kinetics and, therefore, it was not possible to determine whether IL- 4 stimulates a higher frequency of channel opening or increases the duration of the open state. The finding that IL-4 activates BKCa channels is significant because it is additional evidence suggesting that IL-4 may have effects favouring cell relaxation and it is also evidence that the inhibitory effects of IL-4 on calcium signalling in bovine trachealis cells may extend to human cells as well. Furthermore, if we had observed no effect of IL-4 on BKCa activity, the findings would have argued strongly against IL-4 causing a release of SR calcium to increase subplasmalemmal calcium concentrations.
Prior studies in bovine airway smooth muscle cells showed that IL-4 rapidly inhibits calcium signalling by cholinergic agonists, but those studies could not resolve the time course of the IL-4 effect (Madison & Ethier, 2001). In those earlier studies, the inhibitory effect of IL-4 on SR refilling was detectable within 5–20 min and was due to a gradual depletion of SR calcium stores through ryanodine receptors (Madison & Ethier, 2001; Ethier & Madison, 2006).By recording BKCa channel activity during IL-4 application to single cells, the present study shows that IL-4 has rapid effects on ionic signalling that occur within seconds of IL-4 application. These findings suggest that changes in ionic signalling are among the earliest events stimulated by IL-4. It is acknowledged that the present findings are limited to cultured cells and that there are differences between BKCa channels of cultured versus freshly dispersed cells (Snetkov et al. 1996). However, our earlier studies in freshly dispersed bovine airway smooth muscle cells suggest that rapid effects of IL-4 on ionic signalling are not unique to cultured cells (Ethier & Madison, 2006).
In the present study, we compared the effects of two closely related cytokines, IL-4 and IL-13, and found that IL-13 did not activate BKCa channels in human cells. This finding is significant because an important feature of the inhibitory effects of IL-4 on calcium signalling in bovine trachealis cells was that IL-13 did not share the same effects in those cells either (Madison & Ethier, 2001; Ethier & Madison, 2006); that is, IL-13 did not open ryanodine receptors to deplete SR calcium stores. Moreover, in the present study, IL-13 partly inhibited the BKCa channel response to IL-4. To understand these differences between IL-4 and IL-13 and their interaction at airway smooth muscle cells, it is important to recognize that the cellular effects of both IL-4 and IL- 13 are mediated by type II receptor complexes containing the IL-4 receptor alpha (IL-4Rα) chain (Murata et al. 1998; Nelms et al. 1999; Hirst et al. 2002). That both cytokines act via the same receptor complex but have different effects may seem contradictory; however, both shared and differential effects of these two cytokines have already been well described for this cell type in multiple studies (Hawker et al. 1998; John et al. 1998a, b; Pype et al. 1999; Bamford et al. 2000; Laporte et al. 2001; Madison & Ethier, 2001; Hirst et al. 2002; Moore et al. 2002; Faffe et al. 2006). The molecular basis of these shared and differential effects may relate to differences in binding kinetics, rates of receptor dimerization or differences in the conformational change each cytokine induces in the dimerized receptor complex. For airway smooth muscle, the present study identifies another difference between IL-4 and IL-13 and introduces the new concept that IL-13 can inhibit effects of IL-4. Based on the present study, it is proposed that for certain signal transduction events (i.e. BKCa channel activity), the binding of IL-13 to the type II receptor can antagonize IL-4 signal transduction through the same receptor complex.
The mechanisms linking IL-4 receptor activation to the opening of BKCa channels are not known, but the present study demonstrates dependence on extracellular calcium. When calcium was omitted from the extracellular media or when barium ions were substituted for calcium in the media, IL-4 did not cause increases in BKCa channel activity in HBSMC. This result was unexpected because prior studies had shown that IL-4 caused release of calcium from intracellular stores through ryanodine receptors and, therefore, we expected that IL-4 might increase BKCa channel activity without a requirement for extracellular calcium. Since extracellular calcium was in fact required for IL-4 activation of BKCa channels, we now speculate that a rapid calcium influx induced by IL-4 activates BKCa channels directly and/or is the signal that stimulates the opening of ryanodine receptors by calcium-induced calcium release, and these possibilities will be the focus of future studies. Although the chain of events by which IL-4 induces calcium influx in these cells is not yet clear, we assume that one or more calcium channels will prove to be involved (Triggle, 1999). Such a functional interaction between ryanodine receptors, calcium channels and BKCa channels has been supported by other studies of airway smooth muscle (ZhuGe et al. 1999, 2000).
In performing these studies, we attempted to optimize culture conditions to facilitate our measurement of the responses to IL-4. Cells were serum starved because this culture condition may have an effect on BKCa activity (Snetkov et al. 1996; Halayko et al. 1999). Our results showed that starvation had little effect on the magnitude of the increases in BKCa activity when IL-4 was applied. However, interestingly, BKCa responses to IL-4 were consistently more prolonged in starved cells. Although the physiological mechanism underlying this effect of serum starvation and its significance is not yet known, the observation raises the possibility that the coupling of IL-4 receptors to BKCa activity is closely regulated during the cell cycle or during cell differentiation in culture (Halayko et al. 1999; Cooper, 2003).
The rapid increase in BKCa channel activity induced by IL-4, a Th2 cytokine that promotes the asthma phenotype, is intriguing but seemingly counterintuitive because opening of BKCa channels is associated with relaxation of the muscle cell (Kotlikoff & Kamm, 1996). However, a relaxant effect for IL-4 is supported by a recent preliminary report showing that, in vitro, IL-4 bronchodilated mouse airways preconstricted with methacholine (Robinson et al. 2006), and IL-4 has been previously shown to have other inhibitory effects on airway smooth muscle, such as inhibition of calcium transients, muscle cell growth and secretion of specific inflammatory mediators (Hawker et al. 1998; Pype et al. 1999; Madison & Ethier, 2001).
The implications of our finding that IL-13 inhibited the effects of IL-4 are not known, but the observation raises the possibility that IL-4 regulates BKCa channel activity when IL-13 is low. Abundant evidence indicates that expression and secretion of IL-4 and IL-13 by inflammatory cells are independently regulated (Li et al. 1998; Esnault et al. 1996; Luttmann et al. 1999; Redrup et al. 1998) and, therefore, IL-4 expression with low levels of IL-13 may possibly occur during non-allergic inflammation (Li et al. 1998;Herz et al. 1999; Pynaert et al. 2003) and/or during the early phases of allergic inflammation (Kroegel et al. 1996; Li et al. 1998). In asthma, when both IL-4 and IL-13 are known to be high in airways, our findings predict that IL-4 loses its ability to activate BKCa channels. Interestingly, in one study, antigen-stimulated mononuclear cells from normal subjects expressed much lower levels of IL-13 than atopic subjects (Li et al. 1998). Speculatively, the high level of IL-13 characteristic of atopic responses may inhibit the stimulation of BKCa channels by IL-4, and this might be one factor favouring smooth muscle contraction.
In summary, the Th2 cytokine, IL-4, stimulated increases in BKCa channel activity in HBSMC. This unexpected effect, by a cytokine known to promote the asthma phenotype, was rapid and antagonized by the closely related cytokine, IL-13. These findings constitute further evidence that IL-4 and IL-13 have differential effects on airway smooth muscle cells, adding to the complexity of Th2 cytokine regulation of the airways.
Acknowledgments
This work was supported in part by National Institute on Alcohol Abuse and Alcoholism grant AA12054 to S.N.T. and National Heart, Lung and Blood Institute grant HL-54143 to J.M.M.
References
- Bamford TL, Guida E, Harris ET, Wilson JW, Nash A, Stewart AG. IL-13 activates human cultured airway smooth muscle through the IL-13Ra1 receptor. Am J Respir Crit Care Med. 2000;161:A599. [Google Scholar]
- Barnes PJ. Th2 cytokines and asthma: an introduction. Respir Res. 2001;2:64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman N, Robichaud A, Krishnan VL, Roesems G, Robbins R, Jose PJ, Barnes PJ, Chung KF. Expression of RANTES in human airway epithelial cells: effect of corticosteroids and interleukin–4, −10 and −13. Immunology. 1996;87:599–603. doi: 10.1046/j.1365-2567.1996.477579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Reappraisal of serum starvation, the restriction point, G0, and G1 phase arrest points. FASEB J. 2003;17:333–340. doi: 10.1096/fj.02-0352rev. [DOI] [PubMed] [Google Scholar]
- Cox DH, Aldrich RW. Role of the β1 subunit in large-conductance Ca2+-activated K+ channel gating energetics. Mechanisms of enhanced Ca2+ sensitivity. J Gen Physiol. 2000;116:411–432. doi: 10.1085/jgp.116.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault S, Benbernou N, Lavaud F, Shin HC, Potron G, Guenounou M. Differential spontaneous expression of mRNA for IL-4, IL-10, IL-13, IL-2 and interferon–gamma (IFN–γ) in peripheral blood mononuclear cells (PBMC) from atopic patients. Clin Exp Immunol. 1996;103:111–118. doi: 10.1046/j.1365-2249.1996.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethier MF, Cappelluti E, Madison JM. Mechanisms of interleukin–4 effects on calcium signaling in airway smooth muscle cells. J Pharmacol Exp Ther. 2005;313:127–133. doi: 10.1124/jpet.104.079343. [DOI] [PubMed] [Google Scholar]
- Ethier MF, Madison JM. IL-4 inhibits calcium transients in bovine trachealis cells by a ryanodine receptor dependent mechanism. FASEB J. 2006;20:154–156. doi: 10.1096/fj.05-4031fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faffe DS, Flynt L, Bourgeois K, Panettieri RA, Jr, Shore SA. Interleukin–13 and interleukin–4 induce vascular endothelial growth factor release from airway smooth muscle cells: role of vascular endothelial growth factor genotype. Am J Respir Cell Mol Biol. 2006;34:213–218. doi: 10.1165/rcmb.2005-0147OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, Sheppard D, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13 independently of IL-4 in experimental asthma. Science. 1998;282:2261–2263. doi: 10.1126/science.282.5397.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halayko AJ, Camoretti-Mercado B, Forsythe SM, Vieira JE, Mitchell RW, Wylam ME, Hershenson MB, Solway J. Divergent differentiation paths in airway smooth muscle culture: induction of functionally contractile myocytes. Am J Physiol. 1999;276:L197–L206. doi: 10.1152/ajplung.1999.276.1.L197. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hawker KM, Johnson PR, Hughes JM, Black JL. Interleukin-4 inhibits mitogen-induced proliferation of human airway smooth muscle cells in culture. Am J Physiol Lung Cell Mol Physiol. 1998;275:L469–L477. doi: 10.1152/ajplung.1998.275.3.L469. [DOI] [PubMed] [Google Scholar]
- Herz U, Ruckert R, Wollenhaupt K, Tschernig T, Neuhaus-Steinmetz U, Pabst R, Renz H. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness – a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Hirst SJ, Hallsworth MP, Peng Q, Lee TH. Selective induction of eotaxin release by interleukin–13 or interleukin–4 in human airway smooth muscle cells is synergistic with interleukin–1β and is mediated by the interleukin–4 receptor α–chain. Am J Respir Crit Care Med. 2002;165:1161–1171. doi: 10.1164/ajrccm.165.8.2107158. [DOI] [PubMed] [Google Scholar]
- John M, Au BT, Jose PJ, Lim S, Saunders M, Barnes PJ, Mitchell JA, Belvisi MG, Chung KF. Expression and release of interleukin–8 by human airway smooth muscle cells: inhibition by Th–2 cytokines and corticosteroids. Am J Respir Cell Mol Biol. 1998a;18:84–90. doi: 10.1165/ajrcmb.18.1.2813. [DOI] [PubMed] [Google Scholar]
- John M, Lim S, Seybold J, Jose P, Robichaud A, O’Connor B, Barnes PJ, Chung KF. Inhaled corticosteroids increase interleukin–10 but reduce macrophage inflammatory protein–1α, granulocyte-macrophage colonystimulating factor, and interferon–γ release from alveolar macrophages in asthma. Am J Respir Crit Care Med. 1998b;157:256–262. doi: 10.1164/ajrccm.157.1.9703079. [DOI] [PubMed] [Google Scholar]
- Kellner J, Gamarra F, Welsch U, Jörres RA, Huber RM, Bergner A. L-13Ralpha2 reverses the effects of IL-13 and IL-4 on bronchial reactivity and acetylcholine-induced Ca+ signaling. Int Arch Allergy Immunol. 2007;142:199–210. doi: 10.1159/000097022. [DOI] [PubMed] [Google Scholar]
- Kotlikoff MI, Kamm KE. Molecular mechanisms of β–adrenergic relaxation of airway smooth muscle. Annu Rev Physiol. 1996;58:115–141. doi: 10.1146/annurev.ph.58.030196.000555. [DOI] [PubMed] [Google Scholar]
- Kroegel C, Julius P, Matthys H, Virchow J-C, Luttmann W. Endobronchial secretion of interleukin–4 and eosinophil counts. Eur Respir J. 1996;9:899–904. doi: 10.1183/09031936.96.09050899. [DOI] [PubMed] [Google Scholar]
- Kume H, Takai A, Tokuno H, Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989;341:152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Laporte JC, Moore PE, Baraldo S, Jouvin MH, Church TL, Schwartzman IN, Panettieri RA, Jr, Kinet JP, Shore SA. Direct effects of interleukin–13 on signaling pathways for physiological responses in cultured human airway smooth muscle cells. Am J Respir Crit Care Med. 2001;164:141–148. doi: 10.1164/ajrccm.164.1.2008060. [DOI] [PubMed] [Google Scholar]
- Li Y, Simons ER, HayGlass KT. Environmental antigen-induced IL-13 responses are elevated among subjects with allergic rhinitis, are independent of IL-4, and are inhibited by endogenous IFN–γ synthesis. J Immunol. 1998;161:7007–7014. [PubMed] [Google Scholar]
- Lohn M, Lauterbach B, Haller H, Pongs O, Luft FC, Gollasch M. β1-Subunit of BK channels regulates arterial wall [Ca2+] and diameter in mouse cerebral arteries. J Appl Physiol. 2001;91:1350–1354. doi: 10.1152/jappl.2001.91.3.1350. [DOI] [PubMed] [Google Scholar]
- Luttmann W, Sengler C, Herzog V, Balkow S, Matthys H, Virchow JCJr. Differential modulation of interleukin–4 and interleukin–13 secretion from human peripheral blood mononuclear cells. Immunol Letters. 1999;69:225–231. doi: 10.1016/s0165-2478(99)00063-2. [DOI] [PubMed] [Google Scholar]
- Madison JM, Ethier MF. Interleukin–4 rapidly inhibits calcium transients in response to carbachol in bovine airway smooth muscle cells. Am J Respir Cell Mol Biol. 2001;25:239–244. doi: 10.1165/ajrcmb.25.2.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman S. Somatic localization of a specific large-conductance calcium-activated potassium channel subtype controls compartmentalized ethanol sensitivity in the nucleus accumbens. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Siggins GR. Electrophysiological evidence for expression of glycine receptors in freshly isolated neurons from nucleus accumbens. J Pharmacol Exp Ther. 2002;302:1135–1145. doi: 10.1124/jpet.102.033399. [DOI] [PubMed] [Google Scholar]
- Moore PE, Church TL, Chism DD, Panettieri RA, Jr, Shore SA. IL-13 and IL-4 cause eotaxin release in human airway smooth muscle cells: a role for ERK. Am J Physiol Lung Cell Mol Physiol. 2002;282:L847–L853. doi: 10.1152/ajplung.00245.2001. [DOI] [PubMed] [Google Scholar]
- Morrow JP, Zakharov SI, Liu G, Yang L, Sok AJ, Marx SO. Defining the BK channel domains required for β1-subunit modulation. Proc Natl Acad Sci U S A. 2006;103:5096–5101. doi: 10.1073/pnas.0600907103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata T, Obiri NI, Puri RK. Structure of and signal transduction through interleukin–4 and interleukin–13 receptors. Int J Mol Med. 1998;1:551–557. doi: 10.3892/ijmm.1.3.551. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Hiragun T, Morita E, Mihara S, Kameyoshi Y, Hide M. IL-4 modulates the histamine content of mast cells in a mast cell/fibroblast co-culture through a Stat6 signaling pathway in fibroblasts. FEBS Lett. 2005;579:6653–6658. doi: 10.1016/j.febslet.2005.09.104. [DOI] [PubMed] [Google Scholar]
- Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- Ogden DSP. Patch clamp techniques for single channel and whole-cell recording. In: Ogden D, editor. Microelectrode Techniques The Plymouth Workshop Handbook. The Company of Biologists; Cambridge: 1994. pp. 53–78. [Google Scholar]
- Pynaert G, Rottiers P, Haegeman A, Sehra S, Van Belle T, Korf J, Grooten J. Antigen presentation by local macrophages promotes nonallergic airway responses in sensitized mice. Am J Respir Cell Mol Biol. 2003;29:634–641. doi: 10.1165/rcmb.2003-0014OC. [DOI] [PubMed] [Google Scholar]
- Pype JL, Dupont LJ, Menten P, Van CE, Opdenakker G, Van DJ, Chung KF, Demedts MG, Verleden GM. Expression of monocyte chemotactic protein (MCP)–1, MCP–2, and MCP–3 by human airway smooth-muscle cells. Modulation by corticosteroids and T–helper 2 cytokines. Am J Respir Cell Mol Biol. 1999;21:528–536. doi: 10.1165/ajrcmb.21.4.3660. [DOI] [PubMed] [Google Scholar]
- Redrup AC, Howard BP, MacGlashan DW, Jr, Kagey-Sobotka A, Lichtenstein LM, Schroeder JT. Differential regulation of IL-4 and IL-13 secretion by human basophils: their relationship to histamine release in mixed leukocyte cultures. J Immunol. 1998;160:1957–1964. [PubMed] [Google Scholar]
- Robinson KA, Bai Y, Sanderson MJ, Madison JM. Interleukin (IL)-4 rapidly relaxes airway smooth muscle in mouse lung slices. Proc Am Thoracic Soc. 2006;3:A771. [Google Scholar]
- Salkoff L, Butler A, Gerreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Neuroscience. 2006;5:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- Savaria D, Lanoue C, Cadieux A, Rousseau E. Large conducting potassium channel reconstituted from airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 1992;262:L327–L336. doi: 10.1152/ajplung.1992.262.3.L327. [DOI] [PubMed] [Google Scholar]
- Semenov I, Wang B, Herlihy JT, Brenner R. BK Channel β1 subunit regulation of calcium handling and constriction in tracheal smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L802–L810. doi: 10.1152/ajplung.00104.2006. [DOI] [PubMed] [Google Scholar]
- Sims SM, Jiao Y, Zheng ZG. Intracellular calcium stores in isolated tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 1996;271:L300–L309. doi: 10.1152/ajplung.1996.271.2.L300. [DOI] [PubMed] [Google Scholar]
- Snetkov VA, Hirst SJ, Ward JP. Ion channels in freshly isolated and cultured human bronchial smooth muscle cells. Exp Physiol. 1996;81:791–804. doi: 10.1113/expphysiol.1996.sp003977. [DOI] [PubMed] [Google Scholar]
- Triggle DJ. The pharmacology of ion channels: with particular reference to voltage-gated Ca2+ channels. Eur J Pharmacol. 1999;375:311–325. doi: 10.1016/s0014-2999(99)00329-5. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Wang YX, Fleischmann BK, Kotlikoff MI. Modulation of maxi-K+ channels by voltage-dependent Ca2+ channels and methacholine in single airway myocytes. Am J Physiol Cell Physiol. 1997;272:C1151–C1159. doi: 10.1152/ajpcell.1997.272.4.C1151. [DOI] [PubMed] [Google Scholar]
- Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin–13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- ZhuGe R, Fogarty KE, Tuft RA, Lifshitz LM, Sayar K, Walsh JV., Jr Dynamics of signaling between Ca2+ sparks and Ca2+-activated K+ channels studied with a novel image-based method for direct intracellular measurement of ryanodine receptor Ca2+ current. J Gen Physiol. 2000;116:845–864. doi: 10.1085/jgp.116.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]


