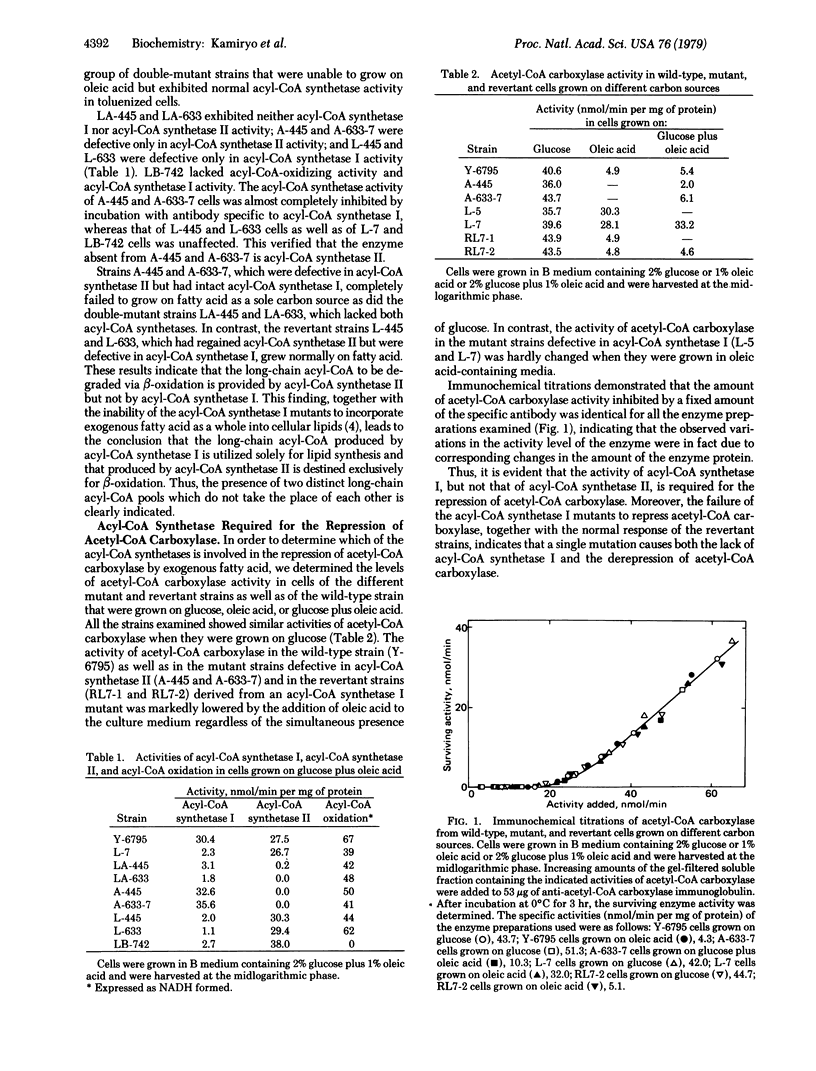
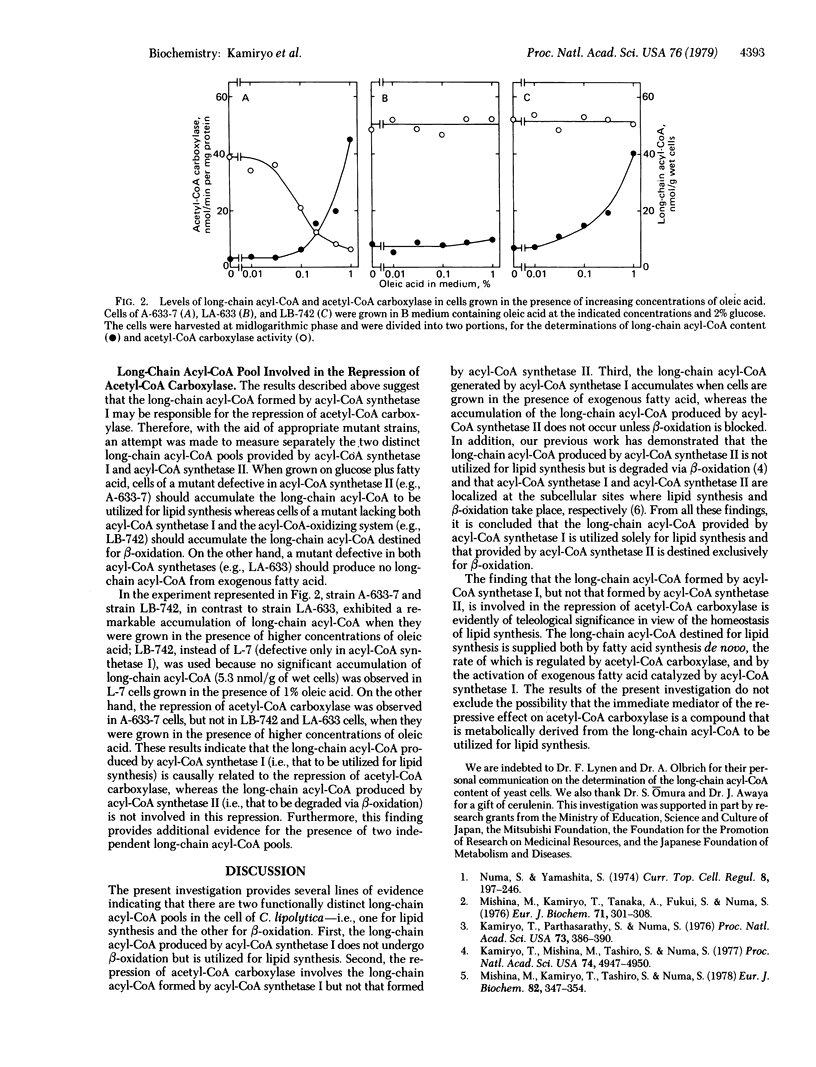
Abstract
Mutant strains of Candida lipolytica defective in acyl-CoA synthetase II [acid:CoA ligase (AMP-forming), EC 6.2.1.3] have been isolated. The mutants fail to grow on fatty acid as a sole carbon source but are capable of incorporating exogenous fatty acid into cellular lipids. This observation, together with our previous finding that mutant strains defective in acyl-CoA synthetase I cannot incorporate exogenous fatty acid into cellular lipids but are able to degrade fatty acid via beta-oxidation, indicates the presence of two functionally distinct long-chain acyl-CoA pools in the cell--i.e., one for lipid synthesis and the other for beta-oxidation. Unlike the wild-type and the revertant strains as well as the mutants lacking acyl-CoA synthetase II, the mutants defective in acyl-CoA synthetase I do not exhibit the repression of acetyl-CoA carboxylase [acetyl-CoA:carbon-dioxide ligase (ADP-forming), EC 6.4.1.2] by exogenous fatty acid. Measurement of the two long-chain acyl-CoA pools with the aid of appropriate mutant strains has indicated that the long-chain acyl-CoA to be utilized for lipid synthesis, but not that to be degraded via beta-oxidation, is involved in the repression of acetyl-CoA carboxylase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hosaka K., Mishina M., Tanaka T., Kamiryo T., Numa S. Acyl-coenzyme-A synthetase I from Candida lipolytica. Purification, properties and immunochemical studies. Eur J Biochem. 1979 Jan 2;93(1):197–203. doi: 10.1111/j.1432-1033.1979.tb12811.x. [DOI] [PubMed] [Google Scholar]
- Kamiryo T., Mishina M., Tashiro S. I., Numa S. Candida lipolytica mutants defective in an acyl-coenzyme A synthetase: isolation and fatty acid metabolism. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4947–4950. doi: 10.1073/pnas.74.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiryo T., Parthasarathy S., Numa S. Evidence that acyl coenzyme A synthetase activity is required for repression of yeast acetyl coenzyme A carboxylase by exogenous fatty acids. Proc Natl Acad Sci U S A. 1976 Feb;73(2):386–390. doi: 10.1073/pnas.73.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tanaka A., Fukui S., Numa S. Acetyl-coenzyme-A carboxylase of Candida lipolytica. 2. Regulation of cellular content and synthesis of the enzyme. Eur J Biochem. 1976 Dec;71(1):301–308. doi: 10.1111/j.1432-1033.1976.tb11116.x. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tashiro S., Hagihara T., Tanaka A., Fukui S., Osumi M., Numa S. Subcellular localization of two long-chain acyl-coenzyme-A synthetases in Candida lipolytica. Eur J Biochem. 1978 Sep 1;89(2):321–328. doi: 10.1111/j.1432-1033.1978.tb12533.x. [DOI] [PubMed] [Google Scholar]
- Mishina M., Kamiryo T., Tashiro S., Numa S. Separation and characterization of two long-chain acyl-CoA synthetases from Candida lipolytica. Eur J Biochem. 1978 Jan 16;82(2):347–354. doi: 10.1111/j.1432-1033.1978.tb12029.x. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Numa S. Purification of rat liver acetyl coenzyme A carboxylase and immunochemical studies on its synthesis and degradation. Eur J Biochem. 1970 Sep;16(1):161–173. doi: 10.1111/j.1432-1033.1970.tb01068.x. [DOI] [PubMed] [Google Scholar]
- Numa S., Yamashita S. Regulation of lipogenesis in animal tissues. Curr Top Cell Regul. 1974;8(0):197–246. doi: 10.1016/b978-0-12-152808-9.50012-2. [DOI] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Gancedo J. M., Gancedo C. Assay of yeast enzymes in situ. A potential tool in regulation studies. Eur J Biochem. 1973 May 2;34(3):479–482. doi: 10.1111/j.1432-1033.1973.tb02783.x. [DOI] [PubMed] [Google Scholar]
- Snow R. An enrichment method for auxotrophic yeast mutants using the antibiotic 'nystatin'. Nature. 1966 Jul 9;211(5045):206–207. doi: 10.1038/211206a0. [DOI] [PubMed] [Google Scholar]



