Abstract
Rationale and Objectives
To evaluate the potential of dynamic CT enhanced by Iohexol or a novel macromolecular contrast agent, PEG12000-Gen4-triiodo, to monitor microvascular changes in tumors treated with the angiogenesis inhibitor bevacizumab.
Materials and Methods
Ten female nude rats with MDA-MB 435 xenograft tumors were treated with 1 mg intraperitoneal bevacizumab when tumors reached 1 cm diameter and, for 4 rats, treated again 7 days later. Just prior to and 24 hours after the first injection of anti-VEGF antibody, the tumors were imaged by dynamic CT scans enhanced with PEG12000-Gen4-triiodo [n=3 rats] or Iohexol [n=3 rats]. The other 4 rats underwent dynamic CT scans enhanced with PEG12000-Gen4-triiodo just prior to and 24 hours after the second injection of anti-VEGF antibody. Microvascular leakiness (KPS) was calculated for the tumors using a two-compartment tissue model.
Results
PEG12000-Gen4-triiodo-enhanced CT scans showed progressive reductions in KPS from Day 1 to 2 to 9 (from 2.55 to 1.27 to 0.69 μl min−1 cm−3, respectively, p < 0.005 for each comparison of Day 1 to 2, and Day 2 to 9).
No significant difference was seen in the KPS estimates derived from Iohexol-enhanced CT scans obtained before or after treatment (276 versus 223.8 μl min−1cm−3, respectively, p = 0.54). The microvascular leak (KPS) was significantly larger for Iohexol than for PEG12000-Gen4-triiodo-enhanced CT, p<0.05.
Conclusion
Dynamic macromolecular contrast-enhanced CT can be used to monitor serial decreases in tumor microvessel leakiness induced by repeated doses of an angiogenesis inhibitor drug
Keywords: Dynamic computed tomography (CT), contrast media, breast neoplasms, angiogenesis, animal study
Introduction
The inhibition of angiogenesis is an intense focus of interest for the development of oncologic therapy (1-6). Angiogenesis inhibitor drugs are now clinically available for the treatment of a variety of malignancies, including gastrointestinal stromal tumors, colon cancer, and non-small cell lung cancer and are in clinical trials for the treatment of other tumors including breast cancer and renal cell carcinoma (7, 8). In parallel with the development of angiogenesis inhibitors, there is a growing demand for useful biomarkers to determine effective drug doses, select appropriate patients, and monitor treatment effectiveness of these drugs. Determination of optimal drug dosage is a challenge because several angiogenesis inhibitors have few side effects, hence dosage cannot be selected based on the maximum tolerated dose. Also, timely identification of patients with tumors that are particularly susceptible to a given drug or, conversely, require a higher dose, would allow more individualized treatment. Therefore, new, well-tested, noninvasive, and reproducible methods are needed to monitor angiogenesis inhibitor drug therapy.
The microvessels of tumors differ greatly from those of normal tissues, and show tortuosity of the lumen with endothelial cell irregularity, loss of normal pericyte binding, derangements of the basement membrane, and a universal and well documented hyperpermeability to macromolecules (9). A promising method for monitoring tumor response to angiogenesis inhibitors takes advantage of the leakiness of tumor microvessels to macromolecules, a property not seen in most normal tissues (10). Unlike most conventional CT and MRI contrast materials, which are less than 1 kilodalton in molecular weight and leak nonspecifically from both normal and malignant microvessels, macromolecular contrast material (similar in size or larger than albumin) does not leak out of microvessels of normal tissues, but does leak out of tumor microvessels. Dynamic macromolecular contrast-enhanced MRI has been validated in several animal studies as a reproducible method of quantifying tumor angiogenesis since tumor microvessel leakiness (KPS) correlates well with histological tumor grade and microvessel density (11-14). Furthermore, dynamic macromolecular contrast-enhanced MRI can quantify decreases in tumor microvessel leakiness as soon as 1 day after treatment with an angiogenesis inhibitor (15).
However, prior studies have not yet addressed whether or not incremental reductions in KPS can be measured with these methods following repeated doses of angiogenesis inhibitors. In addition, only one prior study has described the use of dynamic CT enhanced with an iodinated macromolecular contrast agent to measure KPS in tumor microvessels (16). While this latter study was promising, further in vivo assessment of whether dynamic macromolecular contrast-enhanced CT can monitor changes in KPS is clearly warranted. As such, the purpose of our study is to test the hypothesis that CT enhanced with macromolecular contrast material can monitor incremental decreases in KPS after repeated doses of an angiogenesis inhibitor in a human cancer xenograft rat model.
Materials and Methods
The study was performed in accordance with the guidelines of the National Institutes of Health for the care and use of laboratory animals. Approval from the Institutional Animal Care and Use Committee was obtained.
Tumor model
Twelve homozygous, female, four-week-old nude rats (Harlan Inc, Indianapolis, IN) were each injected with five million human breast cancer cells, MDA MB-435 (ATCC, Manassas, VA), representing a poorly-differentiated adenocarcinoma (17), suspended in a total volume of 0.5 ml, 1:1 mixture of phosphate-buffered saline and Matrigel™ (BD Biosciences, Bedford, MA) placed into the right mammary fat pad. Animals had free access to food and water. Animals were checked visually every other day for tumor development. The animals weighed between 120 to 170 grams.
When tumors reached approximately 1 cm in diameter, the twelve rats were divided into 3 groups of 4 rats: In group 1, on day 1, the rats were imaged by macromolecular contrast-enhanced CT then treated with 1 mg intraperitoneal injection of bevacizumab, an angiogenesis inhibitor derived from an antibody to vascular endothelial growth factor (VEGF). The rats were then imaged again by macromolecular contrast-enhanced CT on Day 2; In group 2, the animals were treated by 1 mg intraperitoneal injection of bevacizumab on Days 1 and 8. Just prior to and again 24 hours after the Day 8 bevacizumab treatment, these rats were imaged by macromolecular contrast-enhanced CT; In group 3, the animals were imaged by iohexol-enhanced CT then treated with 1 mg intraperitoneal injection of bevacizumab, then imaged again by iohexol-enhanced CT on Day 2.
Contrast agents
A) PEG12000-Gen4-triiodo
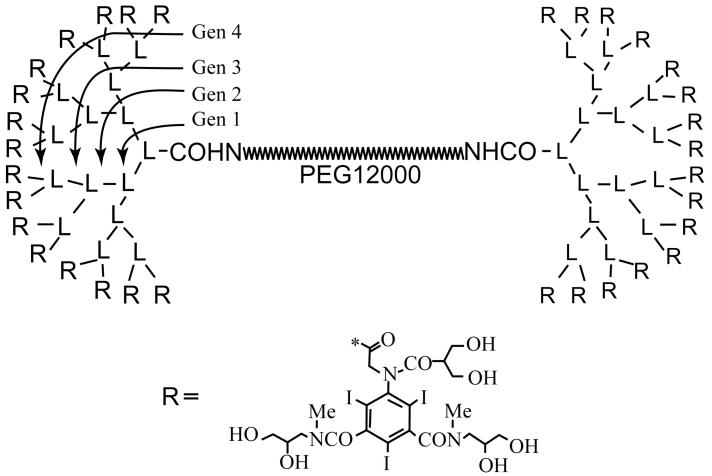
We used a novel 42.5 kDa CT contrast agent composed of a 12 kDa polyethyleneglycol(PEG)-based backbone and poly-L-lysines in a dendrimer arrangement with the 32 terminal amino groups conjugated to 2,4,6-triiodo-5-acylamino-isophthalamide (“triiodo”) (Figure 1). The pharmacokinetics of this contrast agent were previously described, and relevant values are summarized in Table 1. In particular, although this molecule has a molecular weight slightly smaller than that of albumin (66 kDa), it has a much larger apparent molecular size of 142.7 kDa due to the linear nature of the polyethyleneglycol core. The blood half-life of more than 75 minutes and the volume of distribution indicate the excellent blood-pool distribution properties of this macromolecular contrast material. A more detailed chemical description of this substance, its synthesis and characteristics, was published by Fu et al. (18). The contrast agent was administered intravenously at a dose of 300 mg iodine per kg body weight.
Figure 1.
Chemical structure of PEG12000-Gen4-triiodo conjugate
Table 1. Pharmacokinetics of PEG12000-Gen4-triiodo conjugate in rats.
| α-t½ [min] | 4.8 |
| β-t½ [min] | 71.9 |
| Distribution volume (Vc) [l/kg] | 0.05 |
| Total clearance [ml min−1 kg−1] | 0.45 |
B) Iohexol
Iohexol (Omnipaque, GE Healthcare Inc, Princeton, NJ) is a widely used, small molecular weight iodinated CT contrast agent with a molecular weight of 821.14 Da. The contrast agent was administered intravenously at a dose of 300 mg iodine per kg body weight.
CT imaging
The rats were anesthetized by intraperitoneal injection of 35 mg/kg pentobarbital (Nembutal, Abbott Laboratories, North Chicago, IL, USA) combined with 0.025 mg/kg buprenorphin (Buprenex, Reckitt Benckiser Pharmaceuticals, Richmond, VA, USA). Animals were placed supine on a heating pad to maintain a physiologic body temperature. CT scans were performed on a clinical multidetector-row CT scanner (GE Light Speed QX/I, GE Healthcare, Milwaukee, WI) at a tube voltage of 80 kV, tube current of 130 mAs, and field of view of 13 cm. Four contiguous slices with a slice thickness of 2.5 mm were repeatedly acquired through the tumor. After acquisition of three sets of precontrast images, the contrast agent PEG12000-Gen4-triiodo (n=8) or Iohexol (n=4), warmed to body temperature, was hand-injected as a bolus into a lateral tail vein, followed by 0.2 ml of saline flush followed immediately by dynamic CT imaging through the same area. For rats injected with PEG12000-Gen4-triiodo, CT scans were acquired at 60 for a total post-contrast imaging time of 35 minutes. For rats injected with Iohexol, CT scans were acquired at 0.5 second intervals for 1 minute, which is the maximum rate possible on this CT scanner, then at 15 second intervals for 4 minutes, for a total post-contrast imaging time of 5 minutes. The shorter inter-scan time intervals for iohexol than for PEG12000-Gen4-triiodo was chosen to accommodate the faster distribution and clearance kinetics of iohexol compared to those of macromolecular contrast agents. Immediately after completion of the CT scan, the angiogenesis inhibitor bevacizumab (Avastin™, Genentech Inc, South San Francisco, CA) was injected intraperitoneally at a dose of 1 mg per animal. Twenty-four hours later, the dynamic contrast-enhanced CT scan was repeated in each animal using the same contrast agent and the same imaging parameters.
Image analysis
The CT images were transferred to a desktop computer (Dell Dimension 4700, Round Rock, TX) for data analysis using a commercially available image analysis software program (MIStar; Apollo Medical Imaging, North Melbourne, Australia). Two authors (---., ---.) reviewed all CT images and manually placed circular or polygonal regions of interest on the inferior vena cava and the mid-portion of each tumor for each time point to measure CT attenuation and generate time-attenuation curves. The CT attenuation from the three pre-contrast acquisitions were averaged for the tumor and inferior vena cava and subtracted from the post-contrast values to determine the change in CT attenuation (ΔHU). A well-described two-compartment model (11, 19, 20), implemented in the SAAM II software (SAAM Institute, University of Washington, Seattle, WA), was then used to calculate endothelial leakiness (coefficient of permeability surface area product, KPS [μl min−1 cm−3]) and fractional plasma volume (fPV [%]).
Statistical analysis
Statistical analysis was performed using the Stata software package version 8.0 (Stata Corporation, College Station, TX). The KPS and fPV were compared between groups using analysis of variance, and within groups prior to and after angiogenesis inhibition treatment using a paired Student’s t-test. An unpaired Student’s t-test was used for comparison of the microvascular parameters between the two different contrast agents. For all tests, p-values < 0.05 were considered to be statistically significant.
Results
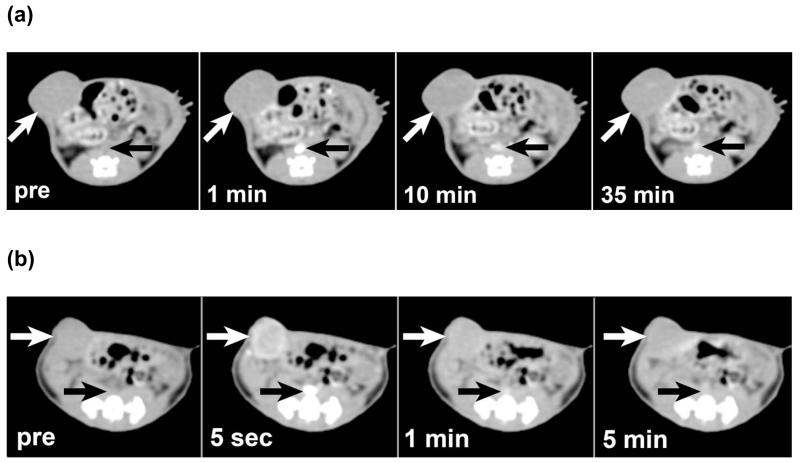
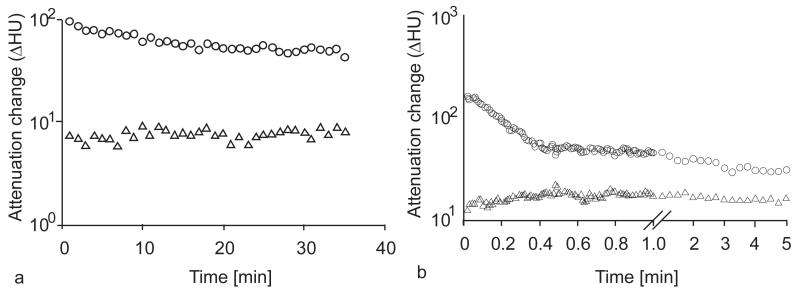
In groups 1 and 3, one rat died during anesthesia induction prior to contrast administration for the CT experiment, leaving three rats imaged with PEG12000-Gen4-triiodo on days 1 and 2 (group 1), four rats imaged with PEG12000-Gen4-triiodo on days 8 and 9 (group 2), and three rats imaged with Iohexol on days 1 and 2 (group 3). Both the PEG12000-Gen4-triiodo and Iohexol were well tolerated in all rats without obvious adverse events. Images from dynamic contrast-enhanced CT scans for each contrast agent are shown in Figure 2 and representative graphs showing the ΔHU over time for the two contrast agents are shown in Figure 3.
Figure 2.
Dynamic CT derived from MDA-MB 435 tumor-bearing nude rats. (a) For CT scans enhanced by PEG12000-Gen4-triiodo, gradual enhancement of the tumor (white arrow) is seen over a period of 35 minutes as the contrast in the inferior vena cava (black arrows) is seen to diminish slowly over time. (b) However, for CT scans enhanced by iohexol, the tumor (white arrows) and inferior vena cava (black arrows) both enhance quickly and show rapid de-enhancement.
Figure 3.
Representative semilog plots for blood and tumor enhancement (Hounsfield units, HU) in the inferior vena cava (○) and tumor (△) for (a) the macromolecular PEG12000-Gen4-triiodo contrast agent and (b) the low-molecular-weight contrast agent Iohexol.
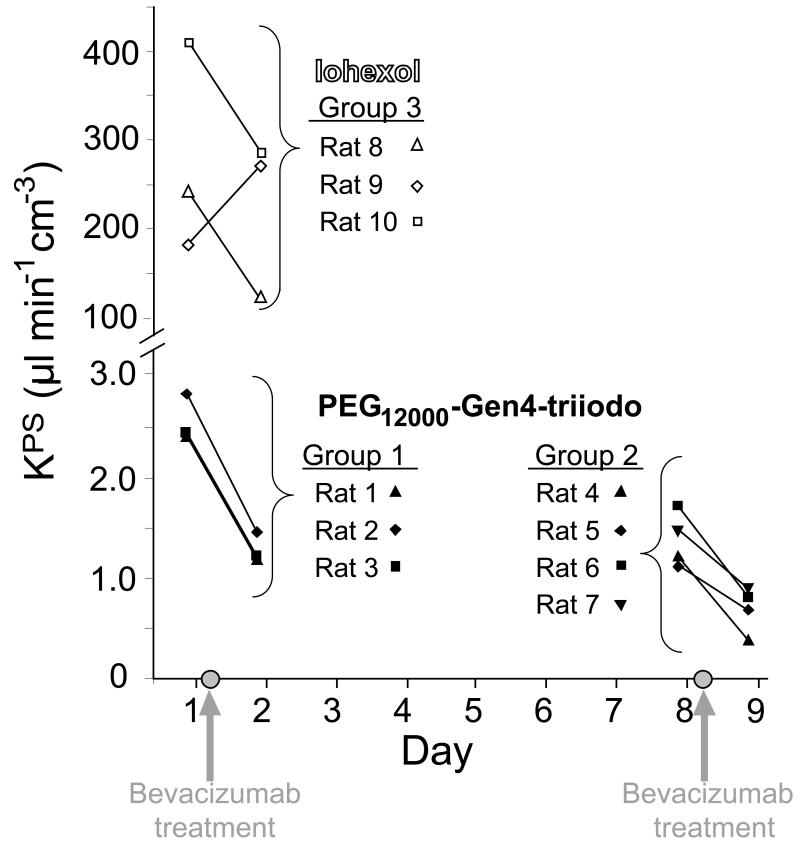
Vascular leakiness (KPS) (Table 2, Figure 4)
Table 2.
CT-derived estimates of microvascular leakiness (KPS) and fractional plasma volume (fPV) in MDA-MB 435 breast cancers in rats measured by PEG12000-Gen4-triiodo and Iohexol-enhanced dynamic CT. Intraperitoneal angiogenesis inhibitor drug (bevacizumab, 1 mg) was given immediately after the CT scans on days 1 and 8. SD = standard deviation.
| Contrast agent |
Group | Rat # | KPS [μl min−1 cm−3] | fPV [%] | ||
|---|---|---|---|---|---|---|
| PEG12000-Gen4-triiodo | Group 1 | Day 1 | Day 2 | Day 1 | Day 2 | |
| 1 | 2.40 | 1.18 | 6.6 | 7.2 | ||
| 2 | 2.82 | 1.45 | 8.0 | 16.8 | ||
| 3 | 2.43 | 1.20 | 9.3 | 5.0 | ||
|
| ||||||
| mean | 2.55 | 1.27 | 8.0 | 9.7 | ||
| SD | 0.23 | 0.15 | 1.4 | 6.3 | ||
|
| ||||||
| Group 2 | Day 8 | Day 9 | Day 8 | Day 9 | ||
| 4 | 1.21 | 0.38 | 3.3 | 3.2 | ||
| 5 | 1.18 | 0.67 | 2.2 | 3.9 | ||
| 6 | 1.71 | 0.81 | 3.4 | 4.5 | ||
| 7 | 1.48 | 0.89 | 11.3 | 8.7 | ||
|
| ||||||
| mean | 1.40 | 0.69 | 5.1 | 5.1 | ||
| SD | 0.25 | 0.22 | 4.2 | 2.5 | ||
|
| ||||||
| Iohexol | Group 3 | Day 1 | Day 2 | Day 1 | Day 2 | |
| 8 | 239.6 | 118.1 | 3.8 | 1.2 | ||
| 9 | 180.5 | 270.4 | 4.6 | 4.5 | ||
| 10 | 407.7 | 282.8 | 5.2 | 10.0 | ||
|
| ||||||
| mean | 276.0* | 223.8* | 4.5 | 5.2 | ||
| SD | 117.9 | 91.7 | 0.7 | 4.4 | ||
Figure 4.
Graph of KPS as determined by dynamic contrast-enhanced CT scans for the macromolecular PEG12000-Gen4-triiodo contrast agent and the low-molecular-weight contrast agent Iohexol. Gray circles on the x-axis indicate the time of intraperitoneal injection of bevacizumab, an antiangiogenesis drug, which was given immediately following the day 1 and day 8 CT scans. A significant reduction in KPS was seen between the pre- and post-treatment CT scans for the macromolecular contrast agent for both the first dose (Group 1, mean, 2.56 versus 1.28 μl min−1 cm−3 on day 1 versus 2, respectively, p < 0.005) and second dose (Group 2, mean, 1.40 versus 0. 69 μl min−1 cm−3 on day 8 versus 9, respectively, p < 0.005). Similarly, the KPS remained significantly lower on day 8 than prior to the first dose of angiogenesis inhibitor drug (p<0.005), and was not significantly different between day 2 and day 8 (p = 0.50). No significant difference was seen for the KPS between the pre- and post-treatment CT scans for iohexol (Group 3, mean, 276.0 versus 223.8 μl min−1 cm−3 on day 1 versus 2, respectively, p=0.54)
The measured vascular leakiness (KPS) differed significantly between the three rat groups as determined by analysis of variance (p<0.001). For the PEG12000-Gen4-triiodo-enhanced CT scans, a significant reduction in the implanted tumor KPS was seen after each dose of angiogenesis inhibitor drug. The mean KPS decreased 48% from 2.55 ± 0.23 to 1.27 ± 0.15 μl min−1 cm−3 from day 1 to 2, respectively (p < 0.005) and then 59% from 1.40 ± 0.25 to 0.69 ± 0.22 μl min−1 cm−3 from day 8 to 9, respectively (p < 0.005), In contrast, for CT scans obtained with the small molecular weight contrast material Iohexol, the mean KPS did not change significantly after angiogenesis inhibitor drug treatment (mean, 276.0 ± 117.9 versus 223.8 ± 91.7 μl min−1 cm−3 on day 1 versus 2, respectively, p=0.54) Notably, the mean Iohexol-derived KPS at baseline was significantly larger (approximately 110 times higher) than the PEG12000-Gen4-triiodo-derived leak (p < 0.001).
Fractional plasma volume – fPV (Table 2)
The mean tumor fractional plasma volumes were not significantly different between the three rat groups as determined by analysis of variance (p=0.11). In particular, the mean fractional plasma volume values derived from PEG12000-Gen4-triiodo –enhanced CT did not change significantly after treatment with a single dose or two doses of angiogenesis inhibitor drug: The mean tumor fractional plasma volumes were 8.0 ± 1.4% for day 1 versus 9.7 ± 6.3% for day 2, p = 0.70 and 5.1 ± 4.2% for day 8 versus 5.1 ± 2.5%, for day 9, p = 0.98. Similarly, the mean fractional plasma volume derived from iohexol-enhanced CT was unchanged before and after treatment with the angiogenesis inhibitor (4.5 ± 0.7% for day 1 versus 5.2 ± 4.4% for day 2, p = 0.91).
Discussion
Our results show that a dynamic macromolecular contrast-enhanced CT can be used to quantify a reduction in tumor microvascular leakiness (KPS) just 24 hours after a single dose of angiogenesis inhibitor drug. A similar reduction in mean KPS was not observed using a conventional small molecular weight iodinated contrast material. More importantly, our results show that dynamic macromolecular contrast-enhanced CT can measure serial reductions in tumor KPS with repeated administration of an angiogenesis inhibitor drug. While numerous previously published studies have shown, predominately with MRI, that macromolecular contrast material can be used to measure changes in KPS induced by an angiogenesis inhibitor (15, 21-23), our finding that serial reductions in KPS can be monitored strengthens the notion that such imaging methods may be valuable to non-invasively monitor the effects of angiogenesis inhibitors on tumors and potentially determine dosing guidelines.
To date, only one prior report has described the use of macromolecular CT contrast material for the purpose of measuring KPS (16). However, that report only assessed whether CT could differentiate the measured KPS in tumor and normal muscle and did not assess whether CT could monitor changes in KPS induced by systemic angiogenesis inhibition treatment. The paucity of literature describing the use of macromolecular contrast material at CT, as opposed to MRI or other modalities, for KPS quantification is likely due to several reasons. One reason is the concern that relatively large doses of intravenous contrast material are needed for CT compared to MRI to vividly enhance the vasculature and internal organs. The larger doses of intravenous contrast material required for CT may predispose patients to nephrotoxicity (24-26). However, macromolecular CT contrast agents such as PEG12000-Gen4-triiodo have 32 times as many iodine atoms per molecule and theoretically should be less nephrotoxic than a similar iodine dose of conventional iodinated contrast material due to a marked reduction in the required number of molecules of contrast material that need to be given. Also, the smaller volume of distribution of macromolecular contrast agents, which remain largely in the blood pool, should result in a smaller required dose than for conventional contrast material which readily disperses into the large extravascular interstitial space. In addition, recent concerns of nephrogenic systemic fibrosis may limit the use of gadolinium-containing contrast material for certain patients with renal injury (27, 28). A potential concern regarding the as PEG12000-Gen4-triiodo compound is the polymeric structure, which could theoretically predispose to allergic reactions. However, polyethylene glycol is commonly incorporated into drug development due to its high hydrophilicity which is known to shield bound structures from the immune system, and no reactions were observed in our experiments.
Furthermore, CT has many potential clinical advantages over other modalities for the quantification of vascular parameters. Firstly, quantification of vascular and tissue contrast concentrations is straightforward for CT because X-ray attenuation increases linearly with contrast material (iodine) concentration and, unlike for scintigraphy and MRI, signal does not vary with the distance from the detector. Secondly, CT scanners are widely available and scans are less costly than for MRI or PET; CT for assessment of tumor vascularity could potentially be adopted very quickly into routine clinical practice without the need to populate hospitals with additional equipment. In particular, a potantial benefit of macromolecular over conventional small molecular weight contrast material is that distribution kinetics are much slower than for conventional agents and so slower CT scanners should give similar results as for faster scanners. Thirdly, CT scans generally require less acquisition time than MRI, and such rapid imaging is more tolerable in debilitated patients or patients who have difficulty holding their breath. Since CT is the backbone of modern oncologic imaging. It is a natural extension to explore the potential capabilities of CT in preclinical trials. But, as with most arguments, it is not entirely one-sided: MRI does not expose patients to ionizing radiation and MRI is more sensitive to detecting gadolinium ions than CT is at detecting iodine atoms.
Our finding that changes in mean tumor microvessel leakiness was not observed using dynamic CT enhanced with a clinically-approved small-molecular contrast agent is limited by small sample size, but is similar to previously published reports that compare macromolecular and small molecular weight MRI contrast materials (11, 29-32). Both normal and tumor microvessels are highly permeable to conventional contrast agents due to the small molecular size of these agents, and our results reaffirm that the KPS measured by a small molecular weight contrast agent is over 100 times as high as for KPS measured with a macromolecular agent. In contrast, unlike the microvessels of normal tissues, those of tumors show pathological hyperpermeability of their endothelium to macromolecules (33).
Our study has several limitations. In particular, the number of animals studied was small, due in part to the limited availability of the macromolecular contrast material. However the results were significant and we are bound by our Institutional Animal Care and Use Committee to use the minimum number of animals necessary for preclinical studies. Our initial findings suggest that further study of dynamic macromolecular contrast material-enhanced CT is warranted, including correlation with other biomarkers of treatment response. A second limitation is that the macromolecular contrast agent we tested is not approved for clinical use and will require additional elimination and toxicology studies prior to clinical trials. Nevertheless, no obvious immediate side effects were observed in the rats. Thirdly, only a xenograft tumor model was examined, and so further study will be needed to determine the generalizability of our findings. Fourthly, since we studied animals with small molecular weight contrast material only at days 1 and 2, we cannot draw conclusions as to whether changes in KPS could be determined using small molecular weight contrast material at days 8 and 9. Fifth, the CT protocol we used for this preclinical study employed a large number of CT scan acquisitions as a means to improve accuracy. For clinical studies, the CT scan parameters will need to be optimized to reduce the radiation dose. Nonwithstanding these limitations, we found that dynamic macromolecular contrast-enhanced CT can be used to monitor incremental changes in KPS in tumors treated by an angiogenesis inhibitor. Conventional small molecular weight iodinated contrast material was not reliable for assessing changes in KPS.
Acknowledgments
This work was supported in part by NIH Grants R01 CA122257, R01 CA082923, and R01 CA103850
References
- 1.Tabernero J. The role of VEGF and EGFR inhibition: implications for combining anti-VEGF and anti-EGFR agents. Mol Cancer Res. 2007;5:203–220. doi: 10.1158/1541-7786.MCR-06-0404. [DOI] [PubMed] [Google Scholar]
- 2.Zalatnai A. Novel therapeutic approaches in the treatment of advanced pancreatic carcinoma. Cancer Treat Rev. 2007;33:289–298. doi: 10.1016/j.ctrv.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Zelnak AB, O’Regan RM. Targeting angiogenesis in advanced breast cancer. BioDrugs. 2007;21:209–214. doi: 10.2165/00063030-200721040-00001. [DOI] [PubMed] [Google Scholar]
- 4.Lavisse S, Lejeune P, Rouffiac V, et al. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100–111. doi: 10.1097/RLI.0b013e3181577cfc. [DOI] [PubMed] [Google Scholar]
- 5.Persigehl T, Matuszewski L, Kessler T, et al. Prediction of antiangiogenic treatment efficacy by iron oxide enhanced parametric magnetic resonance imaging. Invest Radiol. 2007;42:791–796. doi: 10.1097/RLI.0b013e3180d5cbd9. [DOI] [PubMed] [Google Scholar]
- 6.Palmowski M, Morgenstern B, Hauff P, et al. Pharmacodynamics of streptavidin-coated cyanoacrylate microbubbles designed for molecular ultrasound imaging. Invest Radiol. 2008;43:162–169. doi: 10.1097/RLI.0b013e31815a251b. [DOI] [PubMed] [Google Scholar]
- 7.Ellis LM, Rosen L, Gordon MS. Overview of anti-VEGF therapy and angiogenesis. Part 1: Angiogenesis inhibition in solid tumor malignancies. Clin Adv Hematol Oncol. 2006;4(suppl 1-10) quz 11-12. [PubMed] [Google Scholar]
- 8.Diaz-Rubio Vascular endothelial growth factor inhibitors in colon cancer. Adv Exp Med Biol. 2006;587:251–275. doi: 10.1007/978-1-4020-5133-3_20. [DOI] [PubMed] [Google Scholar]
- 9.Raatschen HJ, Simon GH, Fu Y, et al. Vascular permeability during antiangiogenesis treatment: MR imaging assay results as biomarker for subsequent tumor growth in rats. Radiology. 2008;247:391–399. doi: 10.1148/radiol.2472070363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak HF, Nagy JA, Dvorak JT, Dvorak AM. Identification and characterization of the blood vessels of solid tumors that are leaky to circulating macromolecules. Am J Pathol. 1988;133:95–109. [PMC free article] [PubMed] [Google Scholar]
- 11.Daldrup H, Shames DM, Wendland M, et al. Correlation of dynamic contrast-enhanced MR imaging with histologic tumor grade: comparison of macromolecular and small-molecular contrast media. AJR Am J Roentgenol. 1998;171:941–949. doi: 10.2214/ajr.171.4.9762973. [DOI] [PubMed] [Google Scholar]
- 12.Brasch RC, Daldrup H, Shames D, Wendland M, Okuhata Y, Rosenau W. Macromolecular contrast media-enhanced MRI estimates of microvascular permeability correlate with histopathologic tumor grade. Acad Radiol. 1998;5(Suppl 1):S2–5. doi: 10.1016/s1076-6332(98)80043-3. [DOI] [PubMed] [Google Scholar]
- 13.Daldrup HE, Shames DM, Husseini W, Wendland MF, Okuhata Y, Brasch RC. Quantification of the extraction fraction for gadopentetate across breast cancer capillaries. Magn Reson Med. 1998;40:537–543. doi: 10.1002/mrm.1910400406. [DOI] [PubMed] [Google Scholar]
- 14.Turetschek K, Huber S, Floyd E, et al. MR imaging characterization of microvessels in experimental breast tumors by using a particulate contrast agent with histopathologic correlation. Radiology. 2001;218:562–569. doi: 10.1148/radiology.218.2.r01fe37562. [DOI] [PubMed] [Google Scholar]
- 15.Gossmann A, Helbich TH, Kuriyama N, et al. Dynamic contrast-enhanced magnetic resonance imaging as a surrogate marker of tumor response to anti-angiogenic therapy in a xenograft model of glioblastoma multiforme. J Magn Reson Imaging. 2002;15:233–240. doi: 10.1002/jmri.10072. [DOI] [PubMed] [Google Scholar]
- 16.Simon GH, Fu Y, Berejnoi K, et al. Initial computed tomography imaging experience using a new macromolecular iodinated contrast medium in experimental breast cancer. Invest Radiol. 2005;40:614–620. doi: 10.1097/01.rli.0000174477.11541.ce. [DOI] [PubMed] [Google Scholar]
- 17.Cailleau R, Olive M, Cruciger QV. Long-term human breast carcinoma cell lines of metastatic origin: preliminary characterization. In Vitro. 1978;14:911–915. doi: 10.1007/BF02616120. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Nitecki DE, Maltby D, et al. Dendritic iodinated contrast agents with PEG-cores for CT imaging: synthesis and preliminary characterization. Bioconjug Chem. 2006;17:1043–1056. doi: 10.1021/bc060019c. [DOI] [PubMed] [Google Scholar]
- 19.Shames DM, Kuwatsuru R, Vexler V, Muhler A, Brasch RC. Measurement of capillary permeability to macromolecules by dynamic magnetic resonance imaging: a quantitative noninvasive technique. Magn Reson Med. 1993;29:616–622. doi: 10.1002/mrm.1910290506. [DOI] [PubMed] [Google Scholar]
- 20.van Dijke CF, Brasch RC, Roberts TP, et al. Mammary carcinoma model: correlation of macromolecular contrast-enhanced MR imaging characterizations of tumor microvasculature and histologic capillary density. Radiology. 1996;198:813–818. doi: 10.1148/radiology.198.3.8628876. [DOI] [PubMed] [Google Scholar]
- 21.Fournier LS, Novikov V, Lucidi V, et al. MR monitoring of cyclooxygenase-2 inhibition of angiogenesis in a human breast cancer model in rats. Radiology. 2007;243:105–111. doi: 10.1148/radiol.2431050658. [DOI] [PubMed] [Google Scholar]
- 22.Turetschek K, Preda A, Floyd E, et al. MRI monitoring of tumor response to a novel VEGF tyrosine kinase inhibitor in an experimental breast cancer model. Acad Radiol. 2002;9(Suppl 2):S519–520. doi: 10.1016/s1076-6332(03)80281-7. [DOI] [PubMed] [Google Scholar]
- 23.Wiart M, Fournier LS, Novikov VY, et al. Magnetic resonance imaging detects early changes in microvascular permeability in xenograft tumors after treatment with the matrix metalloprotease inhibitor Prinomastat. Technol Cancer Res Treat. 2004;3:377–382. doi: 10.1177/153303460400300408. [DOI] [PubMed] [Google Scholar]
- 24.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ. Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–499. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 25.Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999;72:701–703. doi: 10.1259/bjr.72.859.10624328. [DOI] [PubMed] [Google Scholar]
- 26.Stacul F, Cova M, Assante M, Hougens Grynne B, Haider T. Comparison between the efficacy of dimeric and monomeric non-ionic contrast media (iodixanol vs iopromide) in urography in patients with mild to moderate renal insufficiency. Br J Radiol. 1998;71:918–922. doi: 10.1259/bjr.71.849.10195004. [DOI] [PubMed] [Google Scholar]
- 27.Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104–1108. doi: 10.1093/ndt/gfk062. [DOI] [PubMed] [Google Scholar]
- 28.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology. 2007;242:647–649. doi: 10.1148/radiol.2423061640. [DOI] [PubMed] [Google Scholar]
- 29.Roberts TP, Helbich TH, Ley S, et al. Utility (or not) of Gd-DTPA-based dynamic MRI for breast cancer diagnosis and grading. Acad Radiol. 2002;9(Suppl 1):S261–265. doi: 10.1016/s1076-6332(03)80452-x. [DOI] [PubMed] [Google Scholar]
- 30.Turetschek K, Preda A, Novikov V, et al. Tumor microvascular changes in antiangiogenic treatment: assessment by magnetic resonance contrast media of different molecular weights. J Magn Reson Imaging. 2004;20:138–144. doi: 10.1002/jmri.20049. [DOI] [PubMed] [Google Scholar]
- 31.Daldrup-Link HE, Kaiser A, Helbich T, et al. Macromolecular contrast medium (feruglose) versus small molecular contrast medium (gadopentetate) enhanced magnetic resonance imaging: differentiation of benign and malignant breast lesions. Acad Radiol. 2003;10:1237–1246. doi: 10.1016/s1076-6332(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 32.Daldrup-Link HE, Shames DM, Wendland M, et al. Comparison of Gadomer-17 and gadopentetate dimeglumine for differentiation of benign from malignant breast tumors with MR imaging. Acad Radiol. 2000;7:934–944. doi: 10.1016/s1076-6332(00)80175-0. [DOI] [PubMed] [Google Scholar]
- 33.Dvorak HF, Brown LF, Detmar M, Dvorak AM. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]