Abstract
Background: Type 1 diabetes management has evolved from meal plans towards flexible eating with carbohydrate counting. With this shift, youth with type 1 diabetes may consume excess fat and insufficient fiber, which may impact glycemic control. Few studies consider whether insulin regimen influences associations between dietary intake and hemoglobin A1c.
Patients and Methods: In this cross-sectional study, 252 youth (52% male; age, 13.2±2.8 years; body mass index z-score [z-BMI], 0.7±0.8) with type 1 diabetes completed 3-day food records. Dietary intake was compared with published guidelines. Logistic regression predicted the odds of suboptimal glycemic control (an A1c level of ≥8.5%) related to fat and protein intake or fiber intake according to insulin regimen (pump vs. injection) adjusting for age, sex, diabetes duration, z-BMI, insulin dose, glucose monitoring frequency, and total energy intake (TEI).
Results: Youth had a mean TEI of 40.9±15.4 kcal/kg/day and excess fat and insufficient fiber intake compared against published guidelines. Pump-treated youth consuming the highest quartile of fat intake (as percentage TEI) had 3.6 (95% confidence interval, 1.3–9.7) times the odds of a suboptimal A1c than those in the lowest quartile. No such association was found in injection-treated youth. In the total sample, youth with the lowest quartile of fiber intake had 3.6 (95% confidence interval, 1.4–9.0) times the odds of a suboptimal A1c, but this association did not differ by insulin regimen. There was no association between protein intake and A1c.
Conclusions: Higher fat intake in pump-treated youth and lower fiber intake in all youth were associated with an A1c level of ≥8.5%. Improving dietary quality may help improve A1c.
Introduction
While youth with type 1 diabetes were once placed on strict meal plans, nutritional management of type 1 diabetes in the current era has become more liberalized with a focus on carbohydrate estimation and general healthy eating. Although diets with sufficient fiber and limited fat are associated with less cardiovascular disease (CVD) risk in the general population,1 the focus on carbohydrate intake for individuals with type 1 diabetes may detract attention from healthful eating.2 Thus, youth with type 1 diabetes may not be eating diets that conform to nutritional guidelines.
Youth with type 1 diabetes already have an increased risk of future CVD. Furthermore, excess fat3 and insufficient fiber intake4 have been associated with suboptimal glycemic control in type 1 diabetes. Although carbohydrates provide the major contribution to postprandial glycemic excursions, dietary fats also impact glycemic excursions by producing prolonged hyperglycemic effects.5 Thus, youth with type 1 diabetes may benefit on multiple levels from optimizing diet quality by increasing fiber intake and limiting dietary fat intake.
The aim of this study was to evaluate macronutrient intake in a large sample of youth with type 1 diabetes in comparison with national and international guidelines. We studied whether the relative intake of different macronutrients was associated with suboptimal glycemic control. Youth treated with insulin pumps may bolus at different intervals relative to their food intake, for instance, by taking additional boluses for extra carbohydrate during meals or by using extended bolus features that distribute the insulin bolus over a greater time interval. These may affect postprandial glycemic excursions and hemoglobin A1c. As such, we considered the impact of macronutrients on glycemic control according to insulin regimen (pump versus injection-based treatment).
Patients and Methods
This was a cross-sectional study of 252 youth with type 1 diabetes recruited from a pediatric diabetes center. Youth, ≥8 to 18 years of age, were recruited at the time of a routine diabetes visit. Inclusion criteria were type 1 diabetes confirmed by a pediatric endocrinologist, diabetes duration of ≥1 year, and English speaking. Youth who were on medications such as daily systemic steroids or who had chronic illness or major gastrointestinal disease (e.g., celiac disease, ulcerative colitis, Crohn's disease) that would substantially interfere with nutritional management or glucose metabolism were excluded. Of 455 youth who were approached to participate in the study, 153 declined participation. Of the 302 individuals enrolled, 11 were siblings of participants and excluded from analysis; data from the sibling with the longer diabetes duration were retained. Among the 291 youth remaining, 39 participants did not complete food records, leaving 252 youth included in the analysis. Informed consent and assent were obtained before any study procedures were initiated.
Youth height and weight were measured using calibrated stadiometer and scale, and body mass index z-score (z-BMI) was calculated according to age and sex using Centers for Disease Control and Prevention normative values.6 The electronic medical record provided diabetes history and treatment details. Blood glucose monitoring frequency was based on provider report in the medical record. We assessed glycemic control by A1c (Tosoh, San Francisco, CA) (reference range, 4–6%). An A1c level of ≥8.5% was termed suboptimal as 8.5% is often reported as the mean A1c of pediatric study samples.7,8 A1c levels were most often measured in the week before the food records were obtained (median time, 4 days).
Youth dietary intake was assessed by 3-day diet records. During the review of study procedures, youth and families received explanations as to how to complete the diet records and that the study was aimed at improving the understanding of diet and diabetes management; there was no specific mention that fat and fiber intake would be specifically analyzed. A research assistant trained youths and families to record detailed dietary information for 2 weekdays and 1 weekend day (two youths completed only 2 days of food records). Two research assistants reviewed the data before the data were entered into the nutritional software. The Nutrition Data System for Research software program (University of Minnesota, Minneapolis, MN) was used to analyze food record data and obtain nutrient information. Macronutrient intake was expressed as a percentage of total energy intake (% TEI).
Macronutrient intake was compared against three national and international guidelines: International Society for Pediatric and Adolescent Diabetes (ISPAD) guidelines,9 American Diabetes Association (ADA) guidelines,10 and dietary reference intakes (DRIs) established by the U.S. Department of Agriculture (USDA).11 ISPAD goals for fat intake are 30–35% TEI with saturated fat and trans fat together as <10% TEI. The guidelines describe two possible goals for fiber intake. We chose the fiber intake goal (in g) of greater than or equal to the child's age in years +5. Youth intake was also compared against the USDA DRIs, which describe the acceptable macronutrient distribution range for dietary fat intake of 25–35% TEI. These guidelines indicate that saturated and trans fats should be minimized but do not give acceptable levels of intake. They state that adequate intake for fiber is 14 g/1,000 kcal/day. We also compared participants' reported intake versus the ADA recommendation for <7% of TEI from saturated fat.10
Statistical methods
Descriptive analyses are presented as mean±SD values or percentages as appropriate. In comparing differences between pump- and injection-treated youth and differences between youth with A1c <8.5% versus youth with A1c ≥8.5%, Fisher's exact test was used for proportions, and unpaired t tests were used for continuous comparisons. Because so few youth met goals for fiber intake, we compare actual fiber intake and not the proportion meeting goal intake for fiber. To evaluate the association between macronutrient intakes and A1c after adjusting for potential confounding variables, we conducted multivariate logistic regression analyses with suboptimal glycemic control (A1c ≥8.5%) as the dependent variable. All multivariate analyses adjusted for age, sex, diabetes duration, daily insulin dose (units/kg/day), blood glucose monitoring frequency (times per day), and z-BMI. We divided weight-adjusted caloric intake (kcal/kg), fat intake (% TEI), protein intake (% TEI), and fiber intake (g) into quartiles in multivariate analyses in order to facilitate the calculation of odds ratios and allow a better understanding of the clinical implications of our findings. We used the multivariate nutrient density model12 to account for varied TEI because of variation in body size across children. Both protein and fat were included in the macronutrient model simultaneously, and thus these analyses can be interpreted as the odds of a suboptimal A1c associated with the substitution of either fat or protein for carbohydrate. In separate models, tests for interaction were performed between insulin regimen and nutrient intake. For these tests of interaction, nutrient intakes were treated as continuous and not divided into quartiles. Additional analyses were stratified by treatment regimen (pump vs. injection-based) to evaluate the impact of regimen on the association between dietary intake and glycemic control. We performed a Wald χ2 test of trend on our logistic regression models to determine if there was a stepwise association with different quartiles of intake. A value of P<0.05 was considered significant. Statistical analyses were done in SAS version 9.2 software (SAS Institute, Inc., Cary, NC).
Results
Study population
The sample consisted of 252 youth (52% male) with a mean A1c of 8.5±1.3% and type 1 diabetes duration of 6.3±3.4 years (Table 1). Sixty-nine percent of youth used insulin pump therapy. Of those on injection therapy, 91% used three or more injections daily. Youth had a mean TEI of 40.3±15.4 kcal/kg/day. Youth on injection-based therapy had a higher daily insulin dose, performed less frequent blood glucose monitoring, and had a higher A1c than youth on pump-based therapy (Table 1).
Table 1.
Baseline Characteristics in the Total Sample and by Insulin Regimen
| Total sample (n=252) | Injections (n=79) | Pump (n=173) | P value (injections vs. pump) | |
|---|---|---|---|---|
| Clinical variable | ||||
| Age (years) | 13.2±2.8 | 13.4±3.0 | 13.2±2.8 | 0.6 |
| Sex (% male) | 51.6 | 48.1 | 53.2 | 0.5 |
| Ethnicity (% non-white) | 8.4 | 17.9 | 4.0 | <0.0001 |
| z-BMI (SDS) | 0.7±0.8 | 0.7±0.8 | 0.7±0.8 | 0.8 |
| Diabetes duration (years) | 6.3±3.4 | 5.9±3.6 | 6.5±3.3 | 0.1 |
| Insulin dose (units/kg/day) | 0.9±0.3 | 1.0±0.3 | 0.8±0.2 | 0.0005 |
| BG monitoring (times/day) | 5.5±2.2 | 4.6±2.2 | 5.9±2.1 | <0.0001 |
| A1c (%) | 8.5±1.3 | 9.1±1.7 | 8.2±1.0 | <0.0001 |
| Dietary variable | ||||
| TEI (kcal/kg/day) | 40.3±15.4 | 40.5±16.4 | 40.2±15.0 | 0.9 |
| Carbohydrate intake (% TEI) | 49.0±6.3 | 48.4±6.3 | 49.2±6.3 | 0.4 |
| Protein intake (% TEI) | 16.1±3.0 | 16.2±3.3 | 16.0±2.9 | 0.6 |
| Fat intake (% TEI) | 35.0±5.5 | 35.3±5.9 | 34.8±5.3 | 0.5 |
| Saturated fat (% TEI) | 12.4±2.6 | 12.4±2.6 | 12.4±2.6 | 0.9 |
| Trans fat (% TEI) | 2.2±0.9 | 2.1±1.0 | 2.3±0.9 | 0.2 |
| Fiber intake (g/day) | 16.2±5.9 | 16.2±5.7 | 16.2±6.0 | 1.0 |
A1c, hemoglobin A1c; BG, blood glucose; SDS, SD score; TEI, total energy intake; z-BMI, body mass index z-score.
Dietary intake in comparison with nutritional guidelines
Few youth met guidelines for macronutrient intake (Table 2). Only 11.5% of youth simultaneously met the goals for protein, carbohydrate, and fat intake presented in the ISPAD consensus guidelines for nutritional management.9 Two-thirds of youth had insufficient fiber intake according to the ISPAD guidelines. Only 4.4% of youth met the ISPAD recommendation to limit saturated and trans fat to <10% of TEI. Less than half of youth (46.8%) met the goal intake for dietary fat when compared against USDA DRIs.11 Only 2.8% had sufficient fiber intake for age and sex when compared against the USDA DRIs. Only 1.6% of youth met the ADA recommendation to limit saturated fat to <7% of TEI.10
Table 2.
Nutritional Goals and Percentage of Participants Meeting Goals
| ISPAD | USDA DRIs | ADA | ||||
|---|---|---|---|---|---|---|
| Goals | % meeting goals | Goals | % meeting goals | Goals | % meeting goals | |
| All macronutrient goalsa | 11.5 | 44.8 | ||||
| Carbohydrate | 50–55% of TEI | 32.1 | 45–65% of TEI | 72.2 | ||
| Fat | 30–35% TEI | 32.9 | 25–35% TEI | 46.8 | ||
| Saturated fat and trans fat | <10% of TEI | 4.4 | As low as possible | NA | <7% of TEIb | 1.6 |
| Protein | 10–15% of TEI | 39.7 | 10–30% of TEI | 98.8 | ||
| Fiber | ≥age (years)+5 | 33.7 | 14 g/1,000 kcal | 2.8 | ||
All macronutrient goals refer to meeting goals for carbohydrate, fat, and protein simultaneously.
American Diabetes Association (ADA) goal is for saturated fat only.
ISPAD, International Society for Pediatric and Adolescent Diabetes; NA, not applicable; TEI, total energy intake; USDA DRIs, U.S. Department of Agriculture dietary reference intakes.
Associations between nutritional intake and A1c
Youth with an A1c <8.5% reported higher caloric intake, lower fat intake, and higher fiber intake versus those with a suboptimal A1c (Table 3). There were no associations between A1c and protein intake in bivariate analyses.
Table 3.
Clinical and Dietary Variables According to Glycemic Control
| A1c <8.5% (n=143) | A1c ≥8.5% (n=109) | P value | |
|---|---|---|---|
| Clinical variable | |||
| Age (years) | 12.8±2.8 | 13.8±2.8 | 0.004 |
| Sex (% male) | 55.9 | 45.9 | 0.1 |
| Race/ethnicity (% non-white) | 6.3 | 11.1 | 0.2 |
| z-BMI (SDS) | 0.6±0.8 | 0.8±0.8 | 0.06 |
| Diabetes duration (years) | 6.1±3.2 | 6.6±3.6 | 0.3 |
| Insulin dose (units/kg/day) | 0.8±0.2 | 0.9±0.3 | 0.02 |
| BG monitoring (times/day) | 6.2±2.1 | 4.5±1.9 | <0.0001 |
| Pump-treated | 78.3% | 56.0% | <0.0001 |
| A1c (%) | 7.6±0.6 | 9.6±1.2 | <0.0001 |
| Dietary variable | |||
| TEI (kcal/kg/day) | 43.2±15.6 | 36.5±14.4 | 0.0006 |
| Carbohydrate intake (% TEI) | 49.7±6.1 | 48.0±6.4 | 0.04 |
| Protein intake (% TEI) | 16.1±2.8 | 16.1±3.2 | 1.0 |
| Fat intake (% TEI) | 34.3±5.3 | 35.9±5.7 | 0.02 |
| Saturated fat intake (% TEI) | 12.3±2.7 | 12.5±2.5 | 0.6 |
| Trans fat intake (% TEI) | 2.2±0.8 | 2.2±1.1 | 1.0 |
| Fiber intake (g/day) | 17.0±5.6 | 15.0±6.1 | 0.007 |
A1c, hemoglobin A1c; BG, blood glucose; SDS, SD score; TEI, total energy intake; z-BMI, body mass index z-score.
In analyses by regimen subgroup, the association of greater TEI with lower A1c was seen among both youth treated with insulin pumps and those treated with injections (42.6±15.1 vs. 35.8±13.9 kcal/kg/day [P=0.004] and 45.2±17.6 vs. 37.5±15.1 kcal/kg/day [P=0.04], respectively). Among insulin pump users, an A1c level of<8.5% was associated with less fat intake (33.9±5.0% vs. 36.6±5.6% TEI [P=0.001]); this association was not present in injection-treated youth (35.7±6.2% vs. 35.1±5.8% TEI [P=0.7]). In youth treated by pump therapy, there was an association between an A1c level of <8.5% and more daily fiber (16.9±5.4 vs. 14.7±6.6 g/day [P=0.02]) that was not present in youth receiving injections (17.3±6.2 vs. 15.4±5.4 g/day [P=0.1]).
In multivariate analyses adjusting for age, sex, diabetes duration, daily insulin dose, blood glucose monitoring frequency, and z-BMI, we evaluated for interaction by insulin regimen. There was no significant interaction between insulin regimen and total energy intake (P=0.7), insulin regimen and protein intake (P=0.2), or insulin regimen and fiber intake (P=0.8). Interaction was demonstrated between insulin regimen and fat intake (P=0.0498), suggesting that pump use modifies the relationship between fat intake and suboptimal glycemic control.
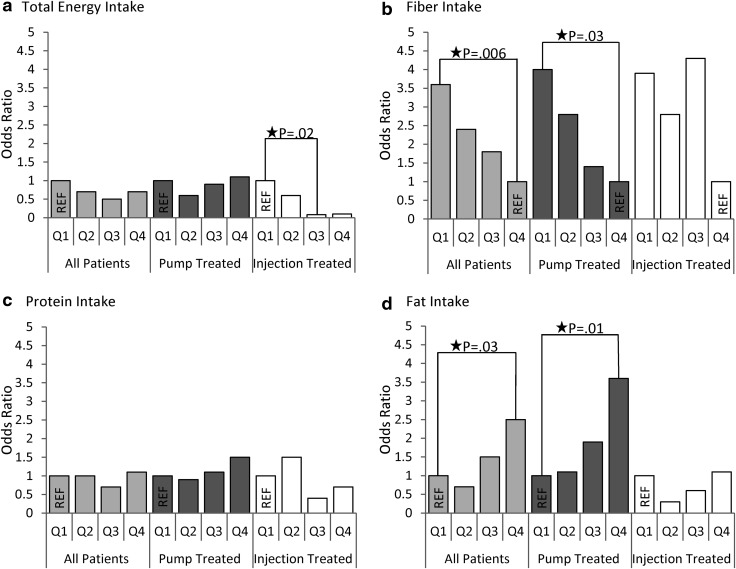
There was no association between increasing quartiles of TEI and the likelihood of a suboptimal A1c in the entire sample (Fig. 1 and Table 4). However, injection-treated youth who reported higher TEI had lower odds of a suboptimal A1c (P for test of trend=0.03). Pump-treated youth did not demonstrate this association. Across all youth, those in the highest quartile of fat intake had 2.5 (95% confidence interval, 1.1–5.5) times the odds of a suboptimal A1c than youth in the lowest quartile in multivariate analyses (P for test of trend=0.009). In pump-treated youth, those in the highest quartile of fat intake had 3.6 (95% confidence interval, 1.3–9.7) times the odds of a suboptimal A1c than those in the lowest quartile (P for test of trend=0.007). No association between fat intake and suboptimal A1c was found for injection-treated youth (P for test of trend=0.7). There were no associations between protein intake and suboptimal A1c in multivariate analyses of the entire sample with or without stratification by insulin regimen. Youth who reported the lowest quartile of fiber intake had 3.6 (95% confidence interval, 1.4–9.0) times the odds of a suboptimal A1c (P for test of trend=0.006). However, stratified analyses by insulin regimen only demonstrated an association between lower fiber intake and increased odds of a suboptimal A1c by test of trend in pump-treated youth (P for test of trend=0.01).
FIG. 1.
Odds of suboptimal glycemic control (hemoglobin A1c ≥8.5%) by quartiles (Q) of nutrient intake for the entire sample and stratified by youth treated by pump (n=173) or injections (n=79): (a) total energy intake, (b) fiber intake, (c) protein intake, and (d) fat intake. All analyses adjusted for age, sex, diabetes duration, daily insulin dose (units/kg/day), blood glucose monitoring frequency (times/day), and body mass index z-score. Both protein and fat were included in the macronutrient model simultaneously. REF, reference.
Table 4.
Odds of Hemoglobin A1c >8.5% by Quartile
| All patients (n=252) | Pump-treated (n=173) | Injection-treated (n=79) | |||||
|---|---|---|---|---|---|---|---|
| Dietary variable, quartile | Value | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| TEI (kcal/kg/day) | |||||||
| 1st | 0–27.8 | 1 | 1 | 1 | |||
| 2nd | >27.8–38.4 | 0.7 | 0.3–1.6 | 0.6 | 0.2–1.8 | 0.6 | 0.1–3.1 |
| 3rd | >38.4–49.4 | 0.5 | 0.2–1.2 | 0.9 | 0.3–2.6 | 0.08 | 0.01–0.6 |
| 4th | >49.4 | 0.7 | 0.2–2.0 | 1.1 | 0.3–4.5 | 0.1 | 0.01–1.2 |
| P by test of trend | 0.4 | 0.8 | 0.03 | ||||
| Fiber (g/day) | |||||||
| 1st | 0–11.6 | 3.6 | 1.4–9.0 | 4.0 | 1.3–12.4 | 3.9 | 0.7–21.1 |
| 2nd | >11.6–15.5 | 2.4 | 1.0–5.8 | 2.8 | 0.9–8.9 | 2.8 | 0.6–13.5 |
| 3rd | >15.5–19.7 | 1.8 | 0.8–4.2 | 1.4 | 0.5–4.1 | 4.3 | 0.7–25.0 |
| 4th | >19.7 | 1 | 1 | 1 | |||
| P by test of trend | 0.006 | 0.01 | 0.2 | ||||
| Protein (% TEI) | |||||||
| 1st | 0–14.1 | 1 | 1 | 1 | |||
| 2nd | >14.1–15.9 | 1.0 | 0.4–2.2 | 0.9 | 0.3–2.6 | 1.5 | 0.3–7.7 |
| 3rd | >15.9–17.9 | 0.7 | 0.3–1.5 | 1.1 | 0.4–3.0 | 0.4 | 0.1–2.1 |
| 4th | >17.9 | 1.1 | 0.5–2.5 | 1.5 | 0.5–4.4 | 0.7 | 0.1–3.5 |
| P by test of trend | 0.9 | 0.4 | 0.3 | ||||
| Fat (% TEI) | |||||||
| 1st | 0–31.5 | 1 | 1 | 1 | |||
| 2nd | >31.5–35.0 | 0.7 | 0.3–1.7 | 1.1 | 0.4–3.0 | 0.3 | 0.05–1.6 |
| 3rd | >35.0–38.3 | 1.5 | 0.7–3.4 | 1.9 | 0.7–5.4 | 0.6 | 0.1–3.0 |
| 4th | >38.3 | 2.5 | 1.1–5.5 | 3.6 | 1.3–9.7 | 1.1 | 0.2–6.1 |
| P test of trend | 0.009 | 0.007 | 0.7 | ||||
All analyses were adjusted for age, sex, diabetes duration, daily insulin dose (units/kg/day), blood glucose monitoring frequency (times/day), and body mass index z-score. Both protein and fat were included in the macronutrient model simultaneously.
CI, confidence interval; OR, odds ratio; TEI, total energy intake.
Discussion
Few youth in a large sample of contemporary youth with type 1 diabetes met national and international nutritional guidelines. Most youth consumed excess dietary fat, especially saturated and trans fat. Youth with the lowest quartile of fat intake had better glycemic control than youth with the highest quartile of fat intake. The association between fat intake and glycemic control only existed in youth treated with an insulin pump, even in this sample where injection-treated youth were overwhelmingly on intensive insulin therapy. Most youth reported insufficient fiber intake. Youth who reported the highest quartile of fiber intake had better glycemic control than youth with the lowest quartile of fiber intake.
Findings comparing dietary intake in youth with type 1 diabetes with that in youth without diabetes are mixed. Some studies suggest better dietary quality among youth with type 1 diabetes,13–15 whereas others find the opposite. A study of 132 U.S. adolescents with type 1 diabetes described higher fat and protein intake in these youth when compared with 131 healthy youth of similar sex, race, ethnicity, and age based on 3 days of dietary recall.16 Additionally, a study of 177 Norwegian youth with type 1 diabetes showed greater fat and saturated fat intake in these youth when compared with 1,809 healthy same-age control subjects based on 4-day dietary records.17 Many studies spanning the United States and Europe describe excess fat intake14,17–19 and insufficient fiber intake14,16–18 relative to national guidelines.
The associations of fat and fiber intake with glycemic control are consistent with previous research. Associations between increased dietary fat and higher A1c in type 1 diabetes have been previously demonstrated.14 An examination of 532 individuals in the intensively treated arm of the Diabetes Control and Complications Trial showed an association between higher dietary fat and poorer glycemic control.3 In 114 youth with type 1 diabetes, the odds of having an A1c level of ≥7.5% increased with greater saturated fat intake.15 Higher fiber intake has also been associated with better glycemic control. The EURODIAB study demonstrated a significant association between greater fiber intake and lower A1c level in 2,065 adults with type 1 diabetes.4 Furthermore, in a randomized trial, 29 adults with type 1 diabetes randomized to a high fiber diet (50 g/day) had a lower A1c level after 24 weeks than the 25 adults with type 1 diabetes following a low fiber diet (15 g/day).20
In this study, the association between higher fat intake and higher A1c level was present only in the subset of youth treated with an insulin pump. Few studies evaluate whether an association between dietary fat and glycemic control varies by insulin regimen. In this sample, youth on an insulin pump may be more adherent to their diabetes management as demonstrated by their greater blood glucose monitoring frequency and lower A1c level. Youth who transition to an insulin pump receive additional education, including a review of carbohydrate counting, which may improve adherence in these youth. It is also possible that youth on an injection-based basal-bolus regimen used higher basal insulin doses to cover some of the extended postprandial hyperglycemia that occurs with higher fat intake.
Excess dietary fat and insufficient fiber intake may affect CVD risk in individuals with type 1 diabetes. In the cross-sectional CACTI (Coronary Artery Calcification in Type 1 Diabetes) study, higher saturated fat intake was associated with greater coronary artery calcification, although this association was no longer evident once models controlled for serum lipid levels.21 In the EURODIAB Prospective Complications Study, there was no association between saturated fat intake and CVD or all-cause mortality over a mean of 7.3 years; however, for every 5 g/day of total dietary fiber, CVD risk decreased 16% (95% confidence interval of hazard ratio, 0.72–0.98), and all-cause mortality decreased 28% (95% confidence interval, 0.55–0.95).22
Our study has several limitations. Almost all participants were on intensive insulin therapy, and thus findings may not be generalizable to youth with type 1 diabetes on other types of regimens. Poor adherence with recording dietary intake may be associated with poor adherence with diabetes management and higher A1c, leading to bias in the associations between TEI and A1c. This may explain the unexpected associations between higher TEI and an A1c level of <8.5%. Although TEI may have been under-reported, we analyzed macronutrient intake as a percentage of TEI, and this measure is less likely to be biased.12 Only the association of fat intake with suboptimal glycemic control demonstrated significant interaction by insulin regimen, and thus the other subgroup analyses by insulin regimen should be interpreted with caution. The study design does not allow us to determine if differences between pump- and injection-treated youth are secondary to differences between the youth in the two groups or secondary to the insulin regimen itself. It is possible that higher fat intake may co-vary with other factors, such as lower socioeconomic status, and it is these other factors that are associated with a higher A1c level. We do not have data on whether participants used advanced bolus features or split insulin doses to manage the extended hyperglycemia of high-fat meals. Only a limited portion of the sample (8.4%) belonged to an ethnic or racial minority, and thus these results may not be generalizable to these groups. The fewer number of injection-treated participants may have limited our ability to detect differences in that group. Finally, the study was cross-sectional, and causation cannot be determined.
These findings have potential implications for clinical practice. As a substitution for high-fat foods, youth with diabetes should consider eating high-fiber foods such as fruits, vegetables, and complex carbohydrates, which may improve glycemic control and reduce CVD risk. Use of extended bolus features, with and without additional insulin, may help prevent postprandial hyperglycemia after high-fat meals.23,24 There may be potential to improve glycemic control in pump-treated youth by extending the timing of the meal bolus for high-fat meals or by encouraging patients to decrease their fat intake to be in compliance with national standards.
In a large sample of U.S. youth with type 1 diabetes, youth reported excess dietary fat and insufficient fiber intake. High fat and low fiber intakes were each associated with suboptimal glycemic control (A1c ≥8.5%). These dietary characteristics may influence CVD risk both inherently and through their influence on glycemic control. For pump-treated youth, limiting dietary fat may improve glycemic control. Further nutritional education and emphasis on limiting fat and increasing fiber may improve glycemic control and decrease future CVD risk.
Acknowledgments
We thank all the families for their participation in this investigation. We also thank the research staff for their efforts. This research was supported by the intramural research program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract number HHSN267200703434C) and by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health grants T32 #5T32DK007260-35, 1K12DK094721-01, and grant P30DK036836 to the Joslin Diabetes and Endocrinology Research Center. Additional support came from the Katherine Adler Astrove Youth Education Fund, the Maria Griffin Drury Fund, and the Eleanor Chesterman Beatson Care Ambassador Fund.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Gidding SS, Dennison BA, Birch LL, Daniels SR, Gillman MW, Lichtenstein AH, Rattay KT, Steinberger J, Stettler N, Van Horn L: Dietary recommendations for children and adolescents: a guide for practitioners: consensus statement from the American Heart Association. Circulation 2005;112:2061–2075 [DOI] [PubMed] [Google Scholar]
- 2.Mehta SN, Haynie DL, Higgins LA, Bucey NN, Rovner AJ, Volkening LK, Nansel TR, Laffel LM: Emphasis on carbohydrates may negatively influence dietary patterns in youth with type 1 diabetes. Diabetes Care 2009;32:2174–2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delahanty LM, Nathan DM, Lachin JM, Hu FB, Cleary PA, Ziegler GK, Wylie-Rosett J, Wexler DJ: Association of diet with glycated hemoglobin during intensive treatment of type 1 diabetes in the Diabetes Control and Complications Trial. Am J Clin Nutr 2009;89:518–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buyken AE, Toeller M, Heitkamp G, Vitelli F, Stehle P, Scherbaum WA, Fuller JH: Relation of fibre intake to HbA1c and the prevalence of severe ketoacidosis and severe hypoglycaemia. EURODIAB IDDM Complications Study Group. Diabetologia 1998;41:882–890 [DOI] [PubMed] [Google Scholar]
- 5.Wolpert HA, Atakov-Castillo A, Smith SA, Steil GM: Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate-based bolus dose calculation and intensive diabetes management. Diabetes Care 2013;36:810–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institutes of Health: Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 1998;6(Suppl 2):51S–209S [PubMed] [Google Scholar]
- 7.Paris CA, Imperatore G, Klingensmith G, Petitti D, Rodriguez B, Anderson AM, Schwartz ID, Standiford DA, Pihoker C: Predictors of insulin regimens and impact on outcomes in youth with type 1 diabetes: the SEARCH for Diabetes in Youth study. J Pediatr 2009;155:183–189 [DOI] [PubMed] [Google Scholar]
- 8.Wood JR, Miller KM, Maahs DM, Beck RW, DiMeglio LA, Libman IM, Quinn M, Tamborlane WV, Woerner SE: Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care 2013;36:2035–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smart C, Aslander-van Vliet E, Waldron S: Nutritional management in children and adolescents with diabetes. Pediatr Diabetes 2009;10(Suppl 12):100–117 [DOI] [PubMed] [Google Scholar]
- 10.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, Hoogwerf BJ, Lichtenstein AH, Mayer-Davis E, Mooradian AD, Wheeler ML: Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care 2008;31(Suppl 1):S61–S78 [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine: Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press, 2006 [Google Scholar]
- 12.Willett W: Nutritional Epidemiology, 2nd ed. New York: Oxford University Press, 1998 [Google Scholar]
- 13.Wiltshire EJ, Hirte C, Couper JJ: Dietary fats do not contribute to hyperlipidemia in children and adolescents with type 1 diabetes. Diabetes Care 2003;26:1356–1361 [DOI] [PubMed] [Google Scholar]
- 14.Lodefalk M, Aman J: Food habits, energy and nutrient intake in adolescents with type 1 diabetes mellitus. Diabet Med 2006;23:1225–1232 [DOI] [PubMed] [Google Scholar]
- 15.Maffeis C, Morandi A, Ventura E, Sabbion A, Contreas G, Tomasselli F, Tommasi M, Fasan I, Costantini S, Pinelli L: Diet, physical, and biochemical characteristics of children and adolescents with type 1 diabetes: relationship between dietary fat and glucose control. Pediatr Diabetes 2012;13:137–146 [DOI] [PubMed] [Google Scholar]
- 16.Helgeson VS, Viccaro L, Becker D, Escobar O, Siminerio L: Diet of adolescents with and without diabetes: trading candy for potato chips? Diabetes Care 2006;29:982–987 [DOI] [PubMed] [Google Scholar]
- 17.Overby NC, Flaaten V, Veierod MB, Bergstad I, Margeirsdottir HD, Dahl-Jorgensen K, Andersen LF: Children and adolescents with type 1 diabetes eat a more atherosclerosis-prone diet than healthy control subjects. Diabetologia 2007;50:307–316 [DOI] [PubMed] [Google Scholar]
- 18.Mayer-Davis EJ, Nichols M, Liese AD, Bell RA, Dabelea DM, Johansen JM, Pihoker C, Rodriguez BL, Thomas J, Williams D: Dietary intake among youth with diabetes: the SEARCH for Diabetes in Youth Study. J Am Diet Assoc 2006;106:689–697 [DOI] [PubMed] [Google Scholar]
- 19.Schober E, Langergraber B, Rupprecht G, Rami B: Dietary intake of Austrian diabetic children 10 to 14 years of age. J Pediatr Gastroenterol Nutr 1999;29:144–147 [DOI] [PubMed] [Google Scholar]
- 20.Giacco R, Parillo M, Rivellese AA, Lasorella G, Giacco A, D'Episcopo L, Riccardi G: Long-term dietary treatment with increased amounts of fiber-rich low-glycemic index natural foods improves blood glucose control and reduces the number of hypoglycemic events in type 1 diabetic patients. Diabetes Care 2000;23:1461–1466 [DOI] [PubMed] [Google Scholar]
- 21.Snell-Bergeon JK, Chartier-Logan C, Maahs DM, Ogden LG, Hokanson JE, Kinney GL, Eckel RH, Ehrlich J, Rewers M: Adults with type 1 diabetes eat a high-fat atherogenic diet that is associated with coronary artery calcium. Diabetologia 2009;52:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenaker DA, Toeller M, Chaturvedi N, Fuller JH, Soedamah-Muthu SS: Dietary saturated fat and fibre and risk of cardiovascular disease and all-cause mortality among type 1 diabetic patients: the EURODIAB Prospective Complications Study. Diabetologia 2012;55:2132–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankowska E, Blazik M, Groele L: Does the fat-protein meal increase postprandial glucose level in type 1 diabetes patients on insulin pump: the conclusion of a randomized study. Diabetes Technol Ther 2012;14:16–22 [DOI] [PubMed] [Google Scholar]
- 24.Chase HP, Saib SZ, Mackenzie T, Hansen MM, Garg SK: Post-prandial glucose excursions following four methods of bolus insulin administration in subjects with type 1 diabetes. Diabet Med 2002;19:317–321 [DOI] [PubMed] [Google Scholar]