Introduction
Age-related macular degeneration (AMD) is the leading cause of adult blindness in industrialized countries, with a reported 1.47% prevalence and 1.75 million people affected in the United States alone.1, 2 Both “wet” AMD, otherwise known as choroidal neovascularization (CNV), and geographic atrophy (GA), the advanced “dry” form of AMD, are considered advanced AMD. While anti-vascular endothelial growth factor therapy is currently available to treat CNV, there are no proven forms of therapy for GA. Initially, antioxidants and vitamins were suggested as a preventative therapy for those with signs of “dry” AMD; however, later analysis by the Age-Related Eye Disease Study Research Group demonstrated that this therapy has no real effect on GA progression.3 Therefore, it is important to characterize “dry” AMD to elucidate its natural history and facilitate advancements in treatment and outcomes.
Drusen, concentrated deposits of extracellular material found around the macula, are an established marker for AMD. A different lesion type has been characterized and associated with AMD. Mimoun et al. first described “les pseudodrusen bleus” in 1990, referring to indistinct, interlacing yellowish lesions in the outer macula which are enhanced by blue light.4 These were considered a type of drusen and were included in the Wisconsin Age-Related Maculopathy Grading System as a separate entity called “reticular drusen,” based on their characteristic appearance.5 However, initial histopathological analysis suggested that these lesions are associated with a loss of choroidal layers of small vessels and increased spacing between large choroidal veins not typical of AMD-associated drusen.6
Reticular drusen, otherwise referred to as “reticular pseudodrusen,” are difficult to visualize using color fundus photography; therefore, since the development of scanning laser ophthalmoscope (SLO) imaging, including autofluorescence (AF) scans, near-infrared reflectance (NIR) scans, and indocyanine green angiography, these lesions have been more clearly characterized.7, 8 Notable spatial correspondence of individual lesions using different imaging modalities was observed; from this observation, it was concluded that perhaps a single disease entity is responsible.9 The term “reticular macular disease” was defined as reticular pseudodrusen in color or red-free photography and/or a reticular pattern on SLO imaging.9 Spectral domain optical coherence tomography (SD-OCT) scans further extended the phenotypic characterization and, with histology, suggested anatomic correlation with lesions in the subretinal space, known as “subretinal drusenoid deposits.”10-13 The etiology of these lesions and the etiology of reticular pseudodrusen remain unclear. Other histopathologic and blood flow studies of AMD describe degenerative changes in the choriocapillaris and choroidal circulation insufficiency,14-17 and multimodal imaging of patients with reticular lesions has suggested an alteration in choriocapillaris blood flow.9, 18, 19
There may be genetic risk factors for the reticular pseudodrusen subtype of AMD. Two major AMD risk alleles have been identified through genome-wide scanning and the candidate gene approach.20-23 In a retrospective study, one of the two major AMD risk alleles, complement factor H (CFH, rs1061170) 402H, was less associated with reticular pseudodrusen than with the absence of reticular pseudodrusen (39.6% frequency in AMD with reticular pseudodrusen vs. 58.6% in AMD without reticular pseudodrusen, P = 0.003). The other major risk allele, age-related maculopathy susceptibility 2 (ARMS2, rs10490924) 69S, was associated with an enhanced risk of reticular pseudodrusen (44.0% frequency in AMD with reticular pseudodrusen vs. 31.3% in AMD without reticular pseudodrusen, P = 0.045).24 In a larger study, no significant difference was found in the frequency of either risk allele between eyes with AMD and reticular pseudodrusen and eyes with AMD and no reticular pseudodrusen.25
While patients with reticular pseudodrusen make up only about 7-8% of patients with AMD, as many as 32% of patients with CNV and 21% of patients with GA were found to have reticular pseudodrusen on color fundus photography.26-28 In another study, reticular pseudodrusen were found in more than 60% of patients with AMD on AF imaging, implying that the prevalence of reticular pseudodrusen may be higher than previously recognized,29 and more recently, reticular pseudodrusen were found in over 90% of patients with GA.30 Interestingly, reticular pseudodrusen have been associated not only with both forms of late AMD, particularly with conversion to CNV, but also with female gender, systemic disease, and increased mortality.28
The etiology of reticular pseudodrusen is unclear. Recent discoveries suggest that this AMD phenotype is complex and multifactorial, with possible genetic, environmental, and systemic factors. We hypothesize that this is indeed the case, as previous epidemiologic studies have suggested that reticular pseudodrusen are associated with cardiovascular risk factors and the ARMS2 risk allele. This study aimed to investigate genetic, environmental, and systemic risk factors in prospectively identified subjects with AMD and the phenotypes of (a) reticular pseudodrusen without large soft drusen and (b) large soft drusen without reticular pseudodrusen.
Methods
This prospective cross-sectional study was carried out with approval from the Institutional Review Board of New York University School of Medicine and in accordance with the Health Insurance Portability and Accountability Act of 1996. Informed consent, obtained from all study participants, included consent for participation in the study and permission to review relevant medical records. Between July 2012 and February 2013, study participants were recruited from two clinician's practices (KBF and RTS) and identified using clinical examination, SD-OCT imaging, and SLO imaging with characteristics and methods as described previously.9 Briefly, NIR images were obtained using a confocal SLO (Spectralis, Heidelberg Engineering, Inc., Vista, CA). Two independent graders (SB and MM) detected reticular pseudodrusen on IR imaging as groups of hyporeflectant lesions against a mildly hyperreflectant background, in well-defined and regular patterns, and confirmed reticular pseudodrusen by the presence of subretinal drusenoid deposits on SD-OCT imaging obtained with the same instrument (Figure 1). Large (≥125 μm) soft drusen were identified on color photographs by their characteristic yellow, indistinct appearance and confirmed by the presence of mounds of deposits under the retinal pigment epithelium on SD-OCT imaging (Figure 2). Any discrepancies were resolved by a senior grader (KBF or RTS). Based on these findings, patients were classified into two study groups: (a) subjects with an AMD phenotype of reticular pseudodrusen without large soft drusen and (b) subjects with an AMD phenotype of large soft drusen without reticular pseudodrusen.
Figure 1.
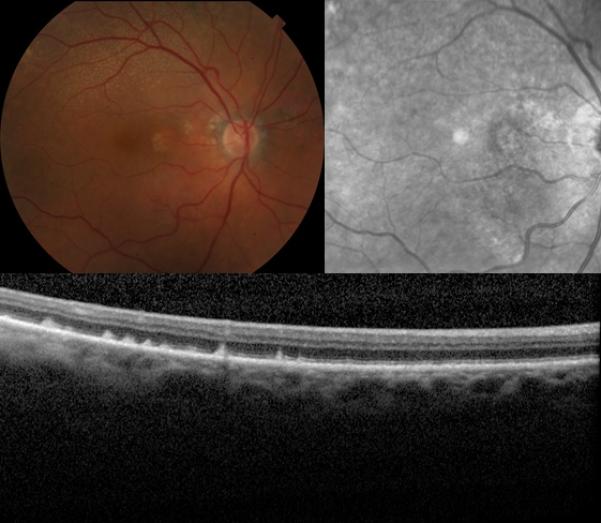
Retinal fundus photographs of the right eye of an 89-year-old female with the reticular pseudodrusen phenotype of age-related macular degeneration. The color photograph (top left) demonstrates the classic presentation of reticular pseudodrusen, identified as yellow or light interlacing networks. Also pictured are the corresponding near-infrared scanning laser ophthalmoscope image of the same eye (top right), showing reticular pseudodrusen as groups of hyporeflectant lesions against a mildly hyperreflectant background in a well-defined pattern, and the optical coherence tomography scan (bottom) of the same eye, showing reticular pseudodrusen as subretinal drusenoid deposits.
Figure 2.
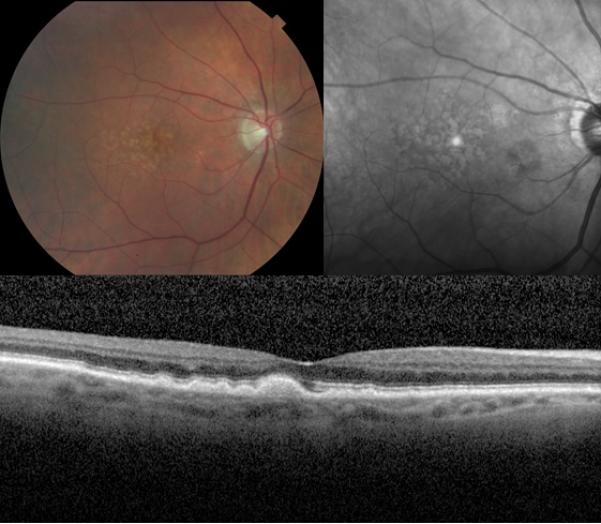
Retinal fundus photographs of the right eye of an 85-year-old male with the large soft drusen phenotype of age-related macular degeneration. The color photograph (top left) demonstrates the classic presentation of large soft drusen characterized by a yellow, indistinct appearance. Also pictured are the corresponding near-infrared scanning laser ophthalmoscope image (top right) of the same eye, showing large soft drusen as hyperreflectant lesions, and the optical coherence tomography scan (bottom) of the same eye, showing large soft drusen as mounds of deposits under the retinal pigment epithelium.
Accordingly, inclusion criteria were AMD and a phenotype of reticular pseudodrusen without large soft drusen or AMD and a phenotype of large soft drusen without reticular pseudodrusen. Exclusion criteria were media opacity that resulted in poor image quality, photographic artifacts, or history of retinal vascular occlusion, retinal detachment, vitreo-retinal or glaucoma surgery, retinal pigment epithelium tear, macular hole, central serous chorioretinopathy, or high myopia (spherical equivalent greater than -6 diopters).
All patients completed an extensive questionnaire that collected demographic information (age, race, and gender), personal and family history of AMD, smoking history, and history of cardiovascular risk factors, including hypertension, diabetes, and hyperlipidemia, as well as the use of medications being used to control these conditions. Elicited personal history of AMD included the age at diagnosis. A patient was defined as having a family history of AMD if he or she had any genetically related family member with AMD. Smoking history included ascertaining if the patient had ever smoked cigarettes for a total duration of 6 months or more. Hypertension was defined as having been previously diagnosed with elevated arterial blood pressure, which is being controlled by at least one hypertensive medication. Hyperlipidemia was defined as having been previously diagnosed with elevated blood lipid levels, which are being controlled by medication. Diabetes mellitus was defined as having been previously diagnosed with elevated blood glucose, which is being controlled by diet or medication.
Definitive CFH and ARMS2 genotyping was performed on all patients as follows: DNA was screened for haplotype-tagging single-nucleotide polymorphisms in CFH (Y402H, rs1061170) and ARMS2 (A69S, rs10490924). Genotyping was performed using polymerase chain reaction-restriction fragment length polymorphism analysis using a master mix (Applied Biosystems, Life Technologies, Norwalk, CT). The thermal cycling conditions consisted of an initial hold at 95° C for 10 minutes, followed by 40 cycles of a 15-second 92° C denaturation step and a 1-minute 60° C annealing and extension step. Results were acquired using Applied Biosystems ViiA 7 RUO Software (Life Technologies, Norwalk, CT).
Allele frequencies were compared between the two study groups. We used the nonparametric Kruskal-Wallis test to evaluate differences of quantitative variables between the two groups. For categorical variables, we report odds ratios (ORs) and 95% confidence intervals (CIs), with P-values calculated from the chi-squared test or Fisher's exact test. Age and age at AMD onset were analyzed both as continuous variables and as categorical variables, derived by dividing the overall distribution into tertiles. For ordinal variables, we used Mantel's test for trend to statistically test for evidence of increasing risk with increasing values.31 We performed multivariable analysis using exact logistic regression as implemented in PROC LOGISTIC (SAS 9.3, 2002-2010, SAS Institute, Inc., Cary, NC).
Results
Thirty patients with a phenotype of reticular pseudodrusen and no evidence of large soft drusen and 43 patients with a phenotype of large soft drusen and no evidence of reticular pseudodrusen were classified, with an inter-observer grading concordance of 90%, and enrolled in the study. The median age of patients included in the analysis was 87 years in the reticular pseudodrusen group and 81 years in the soft drusen group (P = 0.04). Patients with reticular pseudodrusen also had a significantly later age of AMD onset than patients with soft drusen (83 years vs. 70 years, respectively, P = 0.0005). The final analysis set comprised 46/73 (60.9%) women, with a significantly larger proportion of females in the reticular pseudodrusen group than in the soft drusen group (25/30 (83.3%) vs. 21/43 (48.8%), respectively, P = 0.003). All patients with soft drusen (43/43, 100%) and most patients with reticular pseudodrusen (29/30, 96.7%) were of European ancestry; 10/30 patients (33.3%) in the reticular pseudodrusen group and 9/43 patients (20.9%) in the soft drusen group reported an immediate family member with AMD (P = 0.3). The distribution of the ARMS2 risk allele did not significantly differ (P = 0.4) between the reticular pseudodrusen (homozygous = 20.0%; heterozygous = 56.7%) and the large soft drusen (homozygous = 19.0%; heterozygous = 42.9%) phenotypes. Similarly, the distribution of the CHF risk allele did not significantly differ (P = 0.7) between the reticular pseudodrusen (homozygous = 26.7%; heterozygous = 56.7%) and the large soft drusen (homozygous = 21.4%; heterozygous = 66.7%) phenotypes (See Table 1).
Table 1.
Description of subjects with the age-related macular degeneration phenotypes of (a) reticular pseudodrusen without large soft drusen and (b) large soft drusen without reticular pseudodrusen.
| AMDa with RPDb and without large soft drusen, n (%) | AMD with large soft drusen and without RPD, n (%) | P-value | |
|---|---|---|---|
| Gender | 0.003c | ||
| Male | 5 (16.7) | 22 (51.2) | |
| Female | 25 (83.3) | 21 (48.8) | |
| Race | 0.4c | ||
| Non-Hispanic Caucasian | 29 (96.7) | 43 (100.0) | |
| Hispanic | 1 (3.4) | 0 (0.0) | |
| Age (years), median (q1-q3) | 87 (81-89) | 81 (74-88) | 0.04d |
| Categorical age (years) | 0.02e | ||
| ≤ 80 | 6 (20.0) | 19 (44.2) | |
| 81-86 | 7 (23.3) | 13 (30.2) | |
| ≥ 87 | 17 (56.7) | 11 (25.6) | |
| Age at AMD onset (years), median (q1-q3) | 83 (77-86) | 70 (66-78) | 0.0005d |
| Categorical age at AMD onset (years) | 0.0006e | ||
| ≤ 70 | 3 (10.0) | 22 (51.2) | |
| 71-82 | 12 (40.0) | 12 (27.9) | |
| ≥ 83 | 15 (50.0) | 9 (20.9) | |
| Years since AMD diagnosis, median (q1-q3) | 3 (2-6) | 7 (4-11) | 0.002d |
| Family member with AMD | 10 (33.3) | 9 (20.9) | 0.3c |
| Hypertension | 0.08c | ||
| No | 7 (23.3) | 19(44.2) | |
| Yes | 23 (76.7) | 24(55.8) | |
| ARMS2f risk allele (rs10490924) | 0.4c | ||
| −/− | 7 (23.3) | 16 (38.1) | |
| +/− | 17 (56.7) | 18 (42.9) | |
| +/+ | 6 (20.0) | 8 (19.0) | |
| CFHg risk allele (rs1061170) | 0.7c | ||
| −/− | 5 (16.7) | 5 (11.9) | |
| +/− | 17 (56.7) | 28 (66.7) | |
| +/+ | 8 (26.7) | 9 (21.4) |
Age-related macular degeneration.
Reticular pseudodrusen.
P-value from Fisher's exact test.
Kruskal-Wallis P-value.
P-value from Mantel's test for trend.31
Age-related maculopathy susceptibility 2 gene; ARMS2 genotyping for one individual with large soft drusen was indeterminate and is not shown in this data.
Complement factor H gene; CFH genotyping for one individual with large soft drusen was indeterminate and is not shown in this data.
In Table 2, we show univariable analyses of risk factors for reticular pseudodrusen, presenting the total sample of patients with AMD as having different values for (or presence/absence of) each factor. For each risk factor, we show the frequency and percentage of patients with reticular pseudodrusen, as well as ORs and 95% CIs for reticular pseudodrusen among those with a particular value of the risk factor compared to the reference group of those without the risk factor. ORs for reticular pseudodrusen associated with the ARMS2 and CFH risk alleles were not significantly different from the null value of 1.0. ORs for reticular pseudodrusen were significantly increased among older patients and patients with later ages of AMD onset; the test for trend was significant in both cases.
Table 2.
Prevalence of reticular pseudodrusen, odds ratios, 95% confidence intervals, and P-values among patients with age-related macular degeneration presenting with different levels of risk factors at enrollment.
| Risk factor | Number of subjects | RPD,a n (%) | ORb (95% CIc) | P-valued |
|---|---|---|---|---|
| Gender | 0.003 | |||
| Male | 27 | 5 (18.5) | 1 | |
| Female | 46 | 25 (54.4) | 5.2 (1.7-16) | |
| Categorical age (years) | 0.007e | |||
| ≤ 80 | 25 | 6 (24.0) | 1 | |
| 81-86 | 20 | 7 (35.0) | 1.7 (0.46-6.2) | |
| ≥ 87 | 28 | 17 (60.7) | 4.9 (1.5-16) | |
| Categorical age at AMDf onset (years) | 0.0003e | |||
| ≤ 70 | 25 | 3 (12.0) | 1 | |
| 71-82 | 24 | 12 (50.0) | 7.3 (1.7-31.2) | |
| ≥ 83 | 24 | 15 (62.5) | 12 (2.8-53) | |
| Family member with AMDg | 0.3 | |||
| No | 53 | 19 (36.9) | 1 | |
| Yes | 19 | 10 (52.6) | 2.0 (0.69-5.7) | |
| Smoking (> 6 months) | 0.6 | |||
| No | 30 | 11 (36.7) | 1 | |
| Yes | 43 | 19 (44.2) | 1.4 (0.53-3.6) | |
| Hypertension | 0.08 | |||
| No | 26 | 7 (26.9) | 1 | |
| Yes | 47 | 23 (48.9) | 2.6 (0.92-7.3) | |
| Hyperlipidemia | 0.5 | |||
| No | 35 | 16 (45.7) | 1 | |
| Yes | 38 | 14 (36.8) | 0.69 (0.27-1.8) | |
| Diabetes mellitus | 0.2 | |||
| No | 66 | 29 (43.9) | 1 | |
| Yes | 7 | 1 (14.3) | 0.21 (0.02-1.9) | |
| ARMS2h risk allele (rs10490924) | 0.5 | |||
| −/− | 23 | 7 (30.4) | 1 | |
| +/− | 35 | 17 (48.6) | 2.2 (0.71-6.5) | |
| +/+ | 14 | 6 (42.9) | 1.7 (0.43-6.8) | |
| CFHi risk allele (rs1061170) | 0.6 | |||
| −/− | 10 | 5 (50.0) | 1 | |
| +/− | 45 | 17 (37.8) | 0.61 (0.15-2.4) | |
| +/+ | 17 | 8 (47.1) | 0.89 (0.19-4.2) |
Reticular pseudodrusen.
Odds ratio.
Confidence interval.
P-values are from Fisher's exact test unless otherwise noted.
P-value from Mantel's test for trend.
Age-related macular degeneration.
One individual with reticular pseudodrusen could not provide data on family history of AMD.
Age-related maculopathy susceptibility 2 gene; ARMS2 genotyping for one individual with large soft drusen was indeterminate and is not shown in this data.
Complement factor H gene; CFH genotyping for one individual with large soft drusen was indeterminate and is not shown in this data.
There was a suggestion of an increased risk of reticular pseudodrusen with a history of hypertension (OR = 2.6, 95% CI = 0.92 – 7.3, P = 0.08). A lifetime history of smoking for 6 months or longer was associated with a non-significantly elevated OR for reticular pseudodrusen (OR = 1.4, 95% CI = 0.53 – 3.6, P = 0.6). A history of diabetes mellitus was associated with a non-significantly reduced, or protective, OR for reticular pseudodrusen (OR = 0.21, 95% CI = 0.02 – 1.9, P = 0.2).
Exact multiple logistic regression analysis found statistically significant and independent risks for reticular pseudodrusen associated with female gender and age at AMD onset (Table 3). Age, treated either as a three-level categorical variable or as a quantitative variable, did not enter the multivariable model following entry of age at AMD onset and gender.
Table 3.
Exact multiple logistic regression model of risk factors for reticular pseudodrusen among patients with age-related macular degeneration.
| Risk factor | ORa (95% CIb) | P-value |
|---|---|---|
| Age at AMDc onset (years) | 0.001 | |
| ≤ 70 | 1 | |
| 71-82 | 7.7 (2.3-55) | |
| ≥ 83 | 12 (2.3-85) | |
| Gender | 0.009 | |
| Male | 1 | |
| Female | 5.3 (1.4-23) |
Odds ratio.
Confidence interval.
Age-related macular degeneration.
Discussion
A previous retrospective study found an association between reticular pseudodrusen and the ARMS2 risk allele, but not the CFH risk allele, among its two groups of patients: Group 1 comprised patients with AMD and reticular pseudodrusen (n = 67), and Group 2 comprised patients with AMD and no reticular pseudodrusen (n = 64). The frequencies of the ARMS2 69S risk allele were 44.0% in Group 1 and 31.3% in Group 2 (OR = 1.73, P = 0.045). The frequencies of the CFH 402H risk allele were 39.6% in Group 1 and 58.6% in Group 2 (OR = 0.46, P = 0.003).24 A recent larger study found no significant association between reticular pseudodrusen and either risk allele among its three groups of patients: Group 1 comprised patients with AMD and reticular pseudodrusen (n = 105), Group 2 comprised patients with AMD and no reticular pseudodrusen (n = 414), and Group 3 comprised controls with no AMD and no reticular pseudodrusen (n = 430). The ORs for individuals homozygous for the CFH risk allele were 4.0 (P < 0.0004) for Group 1 and 4.3 (P < 0.0004) for Group 2, compared with Group 3. The ORs for individuals homozygous for the ARMS2 risk allele for Groups 1 and 2 compared with Group 3 were 16.3 (P < 0.0004) and 11.9 (P < 0.0004), respectively.25
To our knowledge, the association between reticular pseudodrusen and the ARMS2 and CFH risk alleles has not been looked at prospectively until now. In addition, patients with AMD and reticular pseudodrusen in the previous studies discussed above are not truly representative of the reticular pseudodrusen phenotype as these patients may have had soft drusen in addition to reticular pseudodrusen. In contrast, our study examined the genotypes of carefully selected patients with AMD and reticular pseudodrusen but without any evidence of soft drusen. In our study, there does not appear to be a significant association between either of the two major risk alleles and reticular pseudodrusen.
The mechanism of ARMS2 is still unknown. There is an association of ARMS2 with elevated C-reactive protein,32 which may be relevant in the present context because C-reactive protein is associated with both AMD and coronary artery disease. Prior studies have suggested that reticular pseudodrusen are associated with systemic cardiovascular disease, such as hypertension and angina, with an increased mortality rate in this population separate from comorbidities.9, 24, 28 Our study suggested an elevated risk of reticular pseudodrusen among patients with AMD with a history of hypertension. Hypertension did not, however, enter our multiple logistic regression model after entry of gender and age at AMD onset. If hypertension were a risk factor for reticular pseudodrusen, this would be consistent with a disease process arising from vascular insufficiency.
Disturbances in the ocular circulation in AMD have been reported,33-39 and several histopathological and blood flow studies of AMD have described degenerative changes in the choriocapillaris and choroidal circulation insufficiency,14-17,40-43 supporting the role of hemodynamic abnormalities, such as systemic hypertension, in AMD. In fact, Metelitsina et al. reported that choroidal blood flow is approximately 16.7% lower in patients with AMD with systemic hypertension than in patients with AMD without systemic hypertension.44 Sohrab et al. provided results suggesting that the arrangement and pattern of reticular pseudodrusen are related closely to the choroidal stroma and the choroidal vasculature.45 Arnold et al. related the presence of reticular drusen to abnormalities in the inner choroid.6
In addition to possible vascular substrates for reticular pseudodrusen, the phenotypic association with subretinal drusenoid deposits is also under investigation. An elegant and recent two-lesion two-compartment model suggests that subretinal drusenoid deposits are a sign of retinal pigment epithelium lipid recycling pathways resulting in a lipid and protein spill into the subretinal space, with ensuing photoreceptor damage and ultimately geographic atrophy.46 Querques et al. have suggested a unifying theory in which derangement of the retinal pigment epithelium because of underlying choroidal atrophy and fibrosis may lead to the accumulation of photoreceptor outer segments above the retinal pigment epithelium, creating subretinal deposits.18
Our finding that later age of AMD onset is an independently significant risk factor for the reticular pseudodrusen phenotype of AMD, in the setting of growing data supporting the vascular insufficiency hypothesis of reticular pseudodrusen etiology, raises important questions and warrants further study. Megnien et al., in a study published in Hypertension, found that high blood pressure, and, in particular, the duration of exposure to high blood pressure, promotes the presence and overall extent of coronary calcium, a potential predictor of sudden coronary death.47 As evidenced previously in the ophthalmology literature, reticular pseudodrusen have been linked to systemic diseases, such as hypertension and angina, as well as increased mortality independent of comorbidities.9, 24, 28 It would be of interest to study the role, if any, that the duration of exposure to high blood pressure has in promoting choroidal insufficiency, choroidal atrophy, and choroidal fibrosis and in the eventual manifestation of reticular pseudodrusen,18 a potential predictor of geographic atrophy.48 It would also be of interest to study whether the later age of AMD onset in patients with the reticular pseudodrusen phenotype is related to these same factors.
Another interesting observation is the overwhelming proportion of females presenting with the reticular pseudodrusen phenotype of AMD. We found a strong association of risk for reticular pseudodrusen with female gender that was not affected by adjustment for age at AMD onset or age. This finding has been reported elsewhere, but the reason for this discrepancy between the genders has not been established.28 Given the growing evidence linking cardiovascular disease and reticular pseudodrusen, it is possible that males are underrepresented in this older population because of earlier death from cardiovascular disease, which is in turn due to the lack of the cardio-protective effect of estrogen.49
There has also been recent interest in CFH haplotype analysis. Certain haplotypes may in fact be protective against AMD, and it would be of interest to investigate whether any haplotypes are associated with reticular pseudodrusen.50
Several potential weaknesses of the present study dictate the need for further research on reticular pseudodrusen. The case-case design of the study provided estimates of ORs for reticular pseudodrusen associated with various risk factors using a comparison group of AMD without reticular pseudodrusen. Both the group of interest, i.e., those with reticular pseudodrusen, and the referent group were patients with AMD. This would have increased the frequency of both risk alleles in both groups. Hence, the study was designed to look for a differential expression of the risk alleles in AMD depending on whether the underlying early phenotype was reticular pseudodrusen or soft drusen. ORs for reticular pseudodrusen from our study thus could be quite different from those obtained from a study whose comparison group was healthy individuals without AMD, i.e., a case-control study. Another weakness of the study was the small sample size, resulting in poor statistical power. The small numbers of subjects could explain why some risk factors failed to be statistically significant in multiple logistic regression analyses that included age at AMD onset and gender.
Recruitment of patients from tertiary referral medical practices may have biased the results of our study. If, for example, primary care providers were more inclined to refer difficult-to-treat AMD cases or AMD cases among the elderly to these practices, then our findings may not reflect what would be found in an unbiased, population-based study.
Finally, the cross-sectional design of the study made it susceptible to prevalence-incidence bias.51 Specifically, our study of prevalent cases of reticular pseudodrusen diagnosed in an ophthalmologic practice may have missed information that would have been obtained in an incidence study designed to capture information from patients who died soon after development of reticular pseudodrusen. If, for example, reticular pseudodrusen are associated with late-stage cardiovascular disease, then higher mortality from cardiovascular disease at younger ages among men compared to women might explain the significantly elevated OR for female gender in the present study. In other words, our study may have provided ORs associated with survival to an age advanced enough to develop reticular pseudodrusen in the context of AMD, rather than ORs for reticular pseudodrusen itself. Prospective cohort studies of healthy individuals without AMD who are enrolled and then monitored regularly for incident disease would avoid prevalence-incidence bias and thus would have the best chance of elucidating our novel finding of the increased risk of reticular pseudodrusen with later age of AMD onset.
In summary, reticular pseudodrusen were significantly associated with increased age, later age of AMD onset, and female gender. Our data also suggests an elevated risk of reticular pseudodrusen among patients with hypertension, consistent with a disease process arising from vascular insufficiency. The increased age and high proportion of women among patients with reticular pseudodrusen clearly merits further investigation, perhaps by studying a middle-aged cohort of men with cardiovascular disease for signs of reticular pseudodrusen, as this population may be underrepresented at later ages. Further investigation of all these factors is warranted because better understanding of the mechanism of reticular pseudodrusen can potentially lead to a better understanding of the pathophysiology of AMD and novel therapies for dry AMD.
ACKNOWLEDGMENTS/DISCLOSURE
a. Funding/Support: The research was supported by National Institutes of Health (NIH)/National Eye Institute (NEI) grant R01 EY015520 (RTS), unrestricted funds from Research to Prevent Blindness (RTS), an individual investigator research award from the Foundation Fighting Blindness (RTS), and The Macula Foundation, Inc. (KBF).
Financial Disclosures: K. Bailey Freund: Genentech, Inc. (advisor); Regeneron Pharmaceuticals, Inc. (advisor); Heidelberg Engineering (advisor); Optos plc (advisor). R. Theodore Smith: NIH/NEI (grant support); Research to Prevent Blindness (grant support); Foundation Fighting Blindness (grant support); Boehringer-Ingelheim (consultant). Other authors: none.
Other Acknowledgments: none
Footnotes
Contributions of Authors: Design of the study (RTS, KBF); conduct of the study (RTS, KBF); collection, management, analysis, and interpretation of the data (SB, MDL, MM, M Marmor, KBF, RTS); and preparation, review, or approval of the manuscript (SB, MDL, MM, M Marmor, KBF, RTS).
REFERENCES
- 1.Hyman L. Epidemiology of eye disease in the elderly. Eye (Lond) 1987;1(Pt 2):330–341. doi: 10.1038/eye.1987.53. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Research Group A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119(10):1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mimoun G, Soubrane G, Coscas G. [Macular drusen]. J Fr Ophthalmol. 1990;13(10):511–530. [PubMed] [Google Scholar]
- 5.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 6.Arnold JJ, Sarks SH, Killingsworth MC, Sarks JP. Reticular pseudodrusen: a risk factor in age-related maculopathy. Retina. 1995;15(3):183–191. [PubMed] [Google Scholar]
- 7.Arnold JJ, Quaranta M, Soubrane G, Sarks SH, Coscas G. Indocyanine green angiography of drusen. Am J Ophthalmol. 1997;124(3):344–356. doi: 10.1016/s0002-9394(14)70826-8. [DOI] [PubMed] [Google Scholar]
- 8.Lois N, Owens SL, Coco R, Hopkins J, Fitzke FW, Bird AC. Fundus autofluorescence in patients with age-related macular degeneration and high risk of visual loss. Am J Ophthalmol. 2002;133(3):341–349. doi: 10.1016/s0002-9394(01)01404-0. [DOI] [PubMed] [Google Scholar]
- 9.Smith RT, Sohrab MA, Busuoic M, Barile G. Reticular macular disease. Am J Ophthalmol. 2009;148(5):733–743.e2. doi: 10.1016/j.ajo.2009.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010;117(2):303–312.e1. doi: 10.1016/j.ophtha.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Zweifel SA, Imamura Y, Spaide TC, Fujiwara T, Spaide RF. Prevalence and significance of subretinal drusenoid deposits (reticular pseudodrusen) in age-related macular degeneration. Ophthalmology. 2010;117(9):1775–1781. doi: 10.1016/j.ophtha.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30(9):1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz-Valckenberg S, Steinberg JS, Fleckenstein M, Visvalingam S, Brinkmann CK, Holz FG. Combined confocal scanning laser ophthalmoscopy and spectral-domain optical coherence tomography imaging of reticular drusen associated with age-related macular degeneration. Ophthalmology. 2010;117(6):1169–1176. doi: 10.1016/j.ophtha.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS. Posterior ciliary artery circulation in health and disease: the Weisenfeld lecture. Invest Ophthalmol Vis Sci. 2004;45(3):749–757. 748. doi: 10.1167/iovs.03-0469. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS. Controversies on submacular choroidal circulation. Ophthalmologica. 1981;183(1):11–19. doi: 10.1159/000309127. [DOI] [PubMed] [Google Scholar]
- 16.Hayreh SS. Macular lesions secondary to choroidal vascular disorders. Int Ophthalmol. 1983;6(2):161–170. doi: 10.1007/BF00127645. [DOI] [PubMed] [Google Scholar]
- 17.Foos RY, Trese MT. Chorioretinal juncture. Vascularization of Bruch's membrane in peripheral fundus. Arch Ophthalmol. 1982;100(9):1492–1503. doi: 10.1001/archopht.1982.01030040470020. [DOI] [PubMed] [Google Scholar]
- 18.Querques G, Querques L, Forte R, Massamba N, Coscas F, Souied EH. Choroidal changes associated with reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2012;53(3):1258–1263. doi: 10.1167/iovs.11-8907. [DOI] [PubMed] [Google Scholar]
- 19.Rudolf M, Malek G, Messinger JD, Clark ME, Wang L, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008;87(5):402–408. doi: 10.1016/j.exer.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14(21):3227–3236. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 22.Jakobsdottir J, Conley YP, Weeks DE, Mah TS, Ferrell RE, Gorin MB. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77(3):389–407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308(5720):421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 24.Smith RT, Merriam JE, Sohrab MA, et al. Complement factor H 402H variant and reticular macular disease. Arch Ophthalmol. 2011;129(8):1061–1066. doi: 10.1001/archophthalmol.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puche N, Blanco-Garavito R, Richard F, et al. Genetic and environmental factors associated with reticular pseudodrusen in age-related macular degeneration. Retina. 2013;33(5):998–1004. doi: 10.1097/IAE.0b013e31827b6483. [DOI] [PubMed] [Google Scholar]
- 26.Smith RT, Chan JK, Busuioc M, Sivagnanavel V, Bird AC, Chong NV. Autofluorescence characteristics of early, atrophic, and high-risk fellow eyes in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47(12):5495–5504. doi: 10.1167/iovs.05-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen SY, Dubois L, Tadayoni R, Delahaye-Mazza C, Debibie C, Quentel G. Prevalence of reticular pseudodrusen in age-related macular degeneration with newly diagnosed choroidal neovascularisation. Br J Ophthalmol. 2007;91(3):354–359. doi: 10.1136/bjo.2006.101022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BE. The epidemiology of retinal reticular drusen. Am J Ophthalmol. 2008;145(2):317–326. doi: 10.1016/j.ajo.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2011;52(9):5009–5015. doi: 10.1167/iovs.11-7235. [DOI] [PubMed] [Google Scholar]
- 30.Xu L, Blonska AM, Pumariega NM, et al. Reticular macular disease is associated with multilobular geographic atrophy in age-related macular degeneration. Retina. 2013;33(9):1850–1862. doi: 10.1097/IAE.0b013e31828991b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mantel N. Chi-square tests with one degree of freedom: extensions of the Mantel-Haenszel procedure. J Am Stat Assoc. 1963;58(303):690–700. [Google Scholar]
- 32.Yasuma TR, Nakamura M, Nishiguchi KM, et al. Elevated C-reactive protein levels and ARMS2/HTRA1 gene variants in subjects without age-related macular degeneration. Mol Vis. 2010;16:2923–2930. [PMC free article] [PubMed] [Google Scholar]
- 33.Grunwald JE, Hariprasad SM, DuPont J, et al. Foveolar choroidal blood flow in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1998;39(2):385–390. [PubMed] [Google Scholar]
- 34.Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46(3):1033–1038. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 35.Boker T, Fang T, Steinmetz R. Refractive error and choroidal perfusion characteristics in patients with choroidal neovascularization and age-related macular degeneration. Ger J Ophthalmol. 1993;2(1):10–13. [PubMed] [Google Scholar]
- 36.Chen JC, Fitzke FW, Pauleikhoff D, Bird AC. Functional loss in age-related Bruch's membrane change with choroidal perfusion defect. Invest Ophthalmol Vis Sci. 1992;33(2):334–340. [PubMed] [Google Scholar]
- 37.Pauleikhoff D, Spital G, Radermacher M, Brumm GA, Lommatzsch A, Bird AC. A fluorescein and indocyanine green angiographic study of choriocapillaris in age-related macular disease. Arch Ophthalmol. 1999;117(10):1353–1358. doi: 10.1001/archopht.117.10.1353. [DOI] [PubMed] [Google Scholar]
- 38.Ciulla TA, Harris A, Chung HS, et al. Color Doppler imaging discloses reduced ocular blood flow velocities in nonexudative age-related macular degeneration. Am J Ophthalmol. 1999;128(1):75–80. doi: 10.1016/s0002-9394(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 39.Ciulla TA, Harris A, Kagemann L, et al. Choroidal perfusion perturbations in nonneovascular age related macular degeneration. Br J Ophthalmol. 2002;86(2):209–213. doi: 10.1136/bjo.86.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarks SH. Changes in the region of the choriocapillaris in aging and degeneration. In: Shimizu K, Oosterhuis JA, editors. XXIII Concilium Ophthalmologicum Kyoto 1978. Excerpta Medica; Amsterdam: 1978. pp. 228–238. [Google Scholar]
- 41.Sarks JP, Sarks SH, Killingsworth MC. Evolution of geographic atrophy of the retinal pigment epithelium. Eye (Lond) 1988;2(Pt 5):552–577. doi: 10.1038/eye.1988.106. [DOI] [PubMed] [Google Scholar]
- 42.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch's membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35(6):2857–2864. [PubMed] [Google Scholar]
- 43.Kornzweig AL. Changes in the choriocapillaris associated with senile macular degeneration. Ann Ophthalmol. 1977;9(6):753–762. [PubMed] [Google Scholar]
- 44.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS. Effect of systemic hypertension on foveolar choroidal blood flow in age related macular degeneration. Br J Ophthalmol. 2006;90(3):342–346. doi: 10.1136/bjo.2005.082974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sohrab MA, Smith RT, Salehi-Had H, Sadda SR, Fawzi AA. Image registration and multimodal imaging of reticular pseudodrusen. Invest Ophthalmol Vis Sci. 2011;52(8):5743–5748. doi: 10.1167/iovs.10-6942. [DOI] [PubMed] [Google Scholar]
- 46.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):265–276. doi: 10.1097/IAE.0b013e31827e25e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Megnien JL, Simon A, Lemariey M, Plainfossé MC, Levenson J. Hypertension promotes coronary calcium deposit in asymptomatic men. Hypertension. 1996;27(4):949–954. doi: 10.1161/01.hyp.27.4.949. [DOI] [PubMed] [Google Scholar]
- 48.Marsiglia M, Boddu S, Bearelly S, Xu L, Breaux BE, Jr, Freund KB, Yannuzzi LA, Smith RT. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Investigative Ophthalmol Vis Sci. 2013;54(12):7362–7369. doi: 10.1167/iovs.12-11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrett-Connor E, Bush TL. Estrogen and coronary heart disease in women. JAMA. 1991;265(14):1861–1867. [PubMed] [Google Scholar]
- 50.Sivakumaran TA, Igo RP, Jr, Kidd JM, et al. A 32 kb critical region excluding Y402H in CFH mediates risk for age-related macular degeneration. PLoS One. 2011;6(10):e25598. doi: 10.1371/journal.pone.0025598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32(1-2):51–63. doi: 10.1016/0021-9681(79)90012-2. [DOI] [PubMed] [Google Scholar]


