Introduction
Stargardt disease (STGD1) is the most common cause of juvenile-onset macular degeneration and is caused by mutations in the ABCA4 gene.1 It is characterized by retinal pigment epithelial subretinal pisciform flecks and geographic atrophy (GA). Onset of visual symptoms usually occurs by the mid-teenage years.2,3 Reports have suggested that abnormalities in STGD1 may be detectable in the photoreceptors using spectral domain-optical coherence tomography (SD-OCT) prior to the detection of retinal pigment epithelium (RPE) abnormalities.2 We present the case of a 5-year-old girl with normal appearing fundi who carried pathogenic ABCA4 variants on both chromosomes and where thickening of the external limiting membrane (ELM) was the only abnormality detected on SD-OCT.
Materials and methods
This patient was asymptomatic at the time of enrollment, but she was included as both her mother and maternal uncle had been diagnosed with STGD1. Her father was asymptomatic but was also enrolled to complete the pedigree (Fig. 1). All subjects had detailed medical and ophthalmic histories obtained, and were examined by a retinal specialist (ST). Direct and indirect fundoscopy was performed following pupil dilation with Tropicamide 1% eye drops.
FIGURE 1.

The pedigree for this family.
Infrared (IR) and SD-OCT images were acquired using the Spectralis HRA+OCT (Heidelberg Engineering, Dossenheim, Germany). Given the inability of the patient to tolerate fundus autofluorescence (FAF) imaging using the Spectralis, FAF images were acquired using the CX-1 Digital Retinal Camera (Canon, Tokyo). These were then registered to IR images which had been acquired simultaneously with the SD-OCT. Image registration was performed using a previously described method.4
All subjects were recruited at the Edward Harkness Eye Institute, Columbia University. This research was carried out with the approval of the Institutional Review Board of Columbia University, and all patients were enrolled in accordance with the tenets set out in the Declaration of Helsinki. Informed consent was obtained prior to enrollment.
Results
This 5-year-old girl had best-corrected visual acuities of OD: 20/30 and OS: 20/40. Anterior segment examinations and intraocular pressures were normal. No retinal, vasculature, pigmentary or optic nerve head abnormalities were detected on clinical examination.
Horizontal SD-OCT line scans acquired through the fovea revealed intact inner segment ellipsoid band (ISe) of the photoreceptors (formerly known as the inner segment-outer segment junction).5 Furthermore, there was no focal abnormality observed in the outer nuclear layer (ONL) or RPE (Fig. 2C). The ELM in the central macula appeared thickened with indistinct borders, particularly along its inner border (Fig. 2C). The maximum horizontal diameter for the region of thickened ELM was 1113 μm as measured using the Heidelberg Explorer software (double headed white arrow). FAF revealed Bull’s Eye Maculopathy (BEM) (Fig. 2B). The external boundary of the hyperautofluorescent region corresponded with the outer limits of the region with abnormally thickened ELM. There was no FAF evidence of flecks or GA. Furthermore, IR imaging did not reveal any foveal abnormality (Fig. 2A).
FIGURE 2.
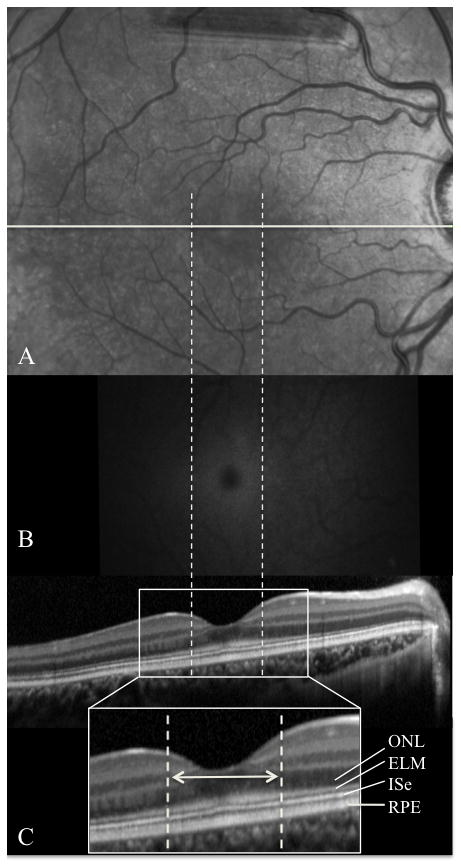
Corresponding infrared (IR) (A), fundus autofluorescence (B, registered to A), and spectral domain-optical coherence tomography (SD-OCT) (C) images of the right eye of the proband are presented. The position of the SD-OCT image on the IR image is indicated by the horizontal white line. The borders of the region with thickening of the external limiting membrane is indicated by the dashed vertical lines.
The patient’s mother was 41 years old at examination and had an age of onset of symptoms at 9 years. Her visual acuities were CF in both eyes. Fundus photographs and FAF images revealed advanced disease (Fig. 3). She was homozygous for a (severe) splice site mutation, IVS 35+2 T>C. The proband was, as expected, heterozygous for this variant and had also inherited the G1961E variant from her father (Fig. 1) as determined by direct sequencing of the entire coding region of the ABCA4 gene.
FIGURE 3.
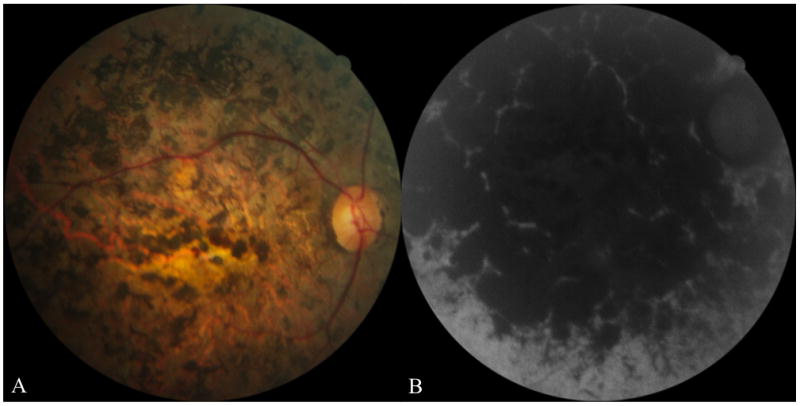
Images from the right eye of the proband’s mother. Fundus photograph (A) and fundus autofluorescence (B) images revealed marked atrophy of the retina, retinal pigment epithelium and choroid. There was also marked intraretinal pigment migration.
Discussion
This case demonstrates that thickening of the ELM may be visible prior to photoreceptor and RPE abnormalities in the early stages of STGD1, as detected by SD-OCT. It is the authors’ experience that the ELM does not appear abnormally thickened in the macula of age-similar unaffected children. The RPE-photoreceptor complex, ISe and ELM have been reported to be “brighter” and “thickened” on SD-OCT, in the fovea compared with the perifoveal region, in a 27-year-old female with BEM secondary to STGD1.6 The SD-OCT findings in our case were also associated with a BEM lesion visible only on FAF. The detection of BEM was not surprising as this patient carried the common G1961E mutation, which is known to yield a BEM phenotype in STGD1.7
The significance of thickening of the ELM remains, as yet, unclear. We hypothesize that the observed change is a response of the Müller cells to structural changes within the photoreceptors secondary to ABCA4 protein dysfunction. Further study of patients at even earlier stages in the disease process is necessary to determine if such changes precede visual impairment or the development of FAF abnormalities in STGD1.
Acknowledgments
The authors wish to acknowledge Dr. Joseph Carroll for providing images of controls, as well as for insightful comments on the case. We also wish to acknowledge Dr. Stanley Chang and Canon (Tokyo, Japan) for the use of the CX-1 Digital Retinal Camera. This research was supported by The Eye Surgery Fund; National Eye Institute Grants EY-018213, EY-015520, EY-021163, EY-019861, EY-13435, and EY-019007 (Core Support for Vision Research), unrestricted funds from Research to Prevent Blindness (New York, NY); the Foundation Fighting Blindness (Owings Mills, Maryland); New York State Grant N09G-302; United States Department of Defense Grant TS080017; the Schneeweiss Stargardt Fund; and The Starr Foundation.
Footnotes
Declaration of Interest: The authors have no conflict of interest to declare.
References
- 1.Allikmets R, Singh N, Sun H, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15:236–246. doi: 10.1038/ng0397-236. [DOI] [PubMed] [Google Scholar]
- 2.Burke TR, Rhee DW, Smith RT, et al. Quantification of peripapillary sparing and macular involvement in Stargardt Disease (STGD1) Invest Ophthalmol Vis Sci. 2011;52:8006–8015. doi: 10.1167/iovs.11-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anastasakis A, Fishman GA, Lindeman M, Genead MA, Zhou W. Infrared Scanning Laser Ophthalmoscope Imaging of the Macula and Its Correlation with Functional Loss and Structural Changes in Patients with Stargardt Disease. Retina. 2011;31:949–958. doi: 10.1097/IAE.0b013e3181f441f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith RT, Gomes NL, Barile G, Busuioc M, Lee N, Laine A. Lipofuscin and autofluorescence metrics in progressive STGD. Invest Ophthalmol Vis Sci. 2009;50:3907–3914. doi: 10.1167/iovs.08-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomograhy: literature review and model. Retina. 2011;31:1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockey RR. Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol. 1999;117:504–510. doi: 10.1001/archopht.117.4.504. [DOI] [PubMed] [Google Scholar]
- 7.Cella W, Greenstein VC, Zernant-Rajang J, et al. G1961E mutant allele in the Stargardt disease gene ABCA4 causes bull’s eye maculopathy. Exp Eye Res. 2009;89:16–24. doi: 10.1016/j.exer.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


