Abstract
Objective
To determine the effectiveness of Virtual Reality Exposure (VRE) augmented with D-cycloserine (50mg) or alprazolam (0.25mg), compared to placebo, in reducing PTSD due to military trauma in Iraq and Afghanistan.
Method
A double-blind, placebo-controlled randomized clinical trial comparing augmentation methods for VRE for subjects (n= 156) with PTSD was conducted.
Results
PTSD symptoms significantly improved from pre- to post-treatment over the 6-session VRE treatment (p<.001) across all conditions and were maintained at 3, 6, and 12 months follow-up. There were no overall differences between the D-cycloserine group on symptoms at any time-point. The alprazolam and placebo conditions significantly differed on the post-treatment Clinician Administered PTSD scale (p = .006) and the 3-month post-treatment PTSD diagnosis, such that the alprazolam group showed greater rates of PTSD (79.2% alprazolam vs. 47.8% placebo). Between-session extinction learning was a treatment-specific enhancer of outcome for the D-cycloserine group only (p<.005). At post-treatment, the D-cycloserine group was the lowest on cortisol reactivity (p<.05) and startle response during VR scenes (p<.05).
Conclusions
A small number of VRE sessions were associated with reduced PTSD diagnosis and symptoms in Iraq/Afghanistan veterans, although there was no control condition for the VRE. Overall, there was no advantage of D-cycloserine on PTSD symptoms in primary analyses. In secondary analyses, benzodiazepine use during treatment may impair recovery, and D-cycloserine may enhance VRE in patients who demonstrate within-session learning. D-cycloserine augmentation treatment in PTSD patients may reduce cortisol and startle reactivity compared to the alprazolam and placebo treatment, consistent with the animal literature.
Keywords: behavior therapy, biological markers, drug psychotherapy combination, military psychiatry, posttraumatic stress disorder, startle
INTRODUCTION
Operations Iraqi Freedom and Enduring Freedom deployed ~2.5 million troops to Iraq and Afghanistan, approximately 13–20% of whom will develop PTSD (1,2). The PTSD treatment with the most empirical support is prolonged imaginal exposure (PE) (3). PE involves 8–12 individual therapy sessions involving repeated recounting of the traumatic memories, including emotional processing of the content and in vivo exposure (4). Virtual reality exposure therapy (VRE), using head-mounted computer simulations of sights, sounds, vibrations and smells tailored to the patient’s individual trauma, has successfully augmented imaginal exposure (5,6).
Repeated therapeutic activation of the trauma memory allows new information to be encoded, thus reducing the memory-associated fear and anxiety (7). This learning is an active process involving synaptic modification in the amygdala that may be pharmacologically augmented to improve the extinction learning underlying therapy (8). D-cycloserine is an N-methyl-D-aspartate glutamate receptor partial agonist (8) shown to improve the efficacy and durability of exposure therapy when dosed acutely prior to treatment for several anxiety disorders (9,10,11,12,13), although D-cycloserine did not augment a full course of CBT in a large trial with social phobia (14). In contrast, enhancing gamma-aminobutyric acid (GABA) activity with benzodiazepines may interfere with fear extinction (15). However, it is unknown whether benzodiazepines diminish the efficacy of exposure therapy in PTSD patients. This question is important clinically because benzodiazepines remain widely used in PTSD (16), contrary to VA/DOD treatment guidelines (17).
In a civilian PTSD sample, overall PE treatment outcomes were not significantly improved with added D-cycloserine compared to placebo, although there was greater symptom reduction among patients who required more sessions (18). In a recent study of OIF/OEF veterans, D-cycloserine addition to PE produced less improvement compared to placebo (19). Most recently, the combination of VRE with D-cycloserine was superior to placebo in the treatment of civilians with PTSD (20).
An important potential biomarker of treatment outcome is hypothalamic-pituitary axis reactivity, specifically, increased cortisol reactivity to a psychosocial stressor. In women with PTSD, open label serotonin-selective reuptake inhibitors reduced cortisol reactivity to a cognitive challenge (22), but no PTSD study that we are aware of has conducted a randomized control trial with cortisol reactivity as an outcome measure. Another physiological marker is exaggerated startle, a cardinal symptom of PTSD (23), although it has not been examined as a treatment outcome measure. Because startle response can serve as a measure of reactivity in both humans (24) and animals (8), it may function as an indicator of mechanism in exposure therapy.
Veterans with combat-related PTSD were randomized to receive either D-cycloserine, alprazolam, or placebo 30 minutes prior to each of five virtual reality exposure sessions. We hypothesized that treatment outcomes in the patients receiving D-cycloserine would be superior to those receiving placebo, whereas patients randomized to alprazolam would have an inferior outcome relative to placebo. To examine biomarkers of treatment effect, cortisol and startle response were collected. Prior work has demonstrated that the beneficial effects of DCS occur when sufficient extinction learning has occurred (10,25,26). Extinction learning was explored as a mediator of treatment response as a result. Extinction learning was defined in the present study as the average decrease in peak subjective discomfort ratings across exposure sessions.
METHOD
This double-blind, placebo-controlled study consisted of a baseline screening assessment, six treatment visits, and follow-up assessments at 3, 6, and 12 months post-treatment conducted by blind independent evaluators. Subjects were randomized 1:1:1 to one of three treatment conditions: virtual reality exposure (VRE) + D-cycloserine 50 mg, VRE + 0.25 mg alprazolam, or VRE + pill placebo. The compounding pharmacy randomized patients to medications in blocks of 30. Study staff were blind to medication condition. The Emory and Atlanta Veterans Affairs Hospital Institutional Review Boards approved this study.
Participants
Participants were156 medically stable Iraq/Afghanistan veterans between 22 and 55 years who met DSM-IV criteria for PTSD due to military trauma verified via the participant’s discharge papers. Exclusion criteria included a lifetime history of psychosis; bipolar disorder; current suicidal risk; current alcohol or drug dependence; pregnancy; and current use of medications that could confound data (glucocorticoids, benzodiazepines, chronic opioid use). Mild traumatic brain injury was permitted. Patients were required to be off long-acting benzodiazepines for 1 month and short-acting benzodiazepines for 2 weeks before screening. Participants on other psychotropic medication(s) must have been on a stable dose for at least 2 weeks prior to beginning the study and maintain a stable dose throughout the study. As needed pain medication was prohibited on study treatment days. Participants received $50.00 following each assessment visit and $15.00 travel reimbursement per treatment session. After complete description of the study to the subjects, written informed consent was obtained.
Assessments
At the baseline screening visit, a master’s level clinician administered the Clinician Administered PTSD Scale (CAPS) (27) to assess PTSD diagnosis status, using the most traumatic incident identified by the patient. The Mini International Neuropsychiatric Interview (MINI) (28) was administered to evaluate other Axis I disorders. Participants completed several self-report measures, including the PTSD Symptom Scale (PSS, 29). All assessment interviews were videotaped to assess inter-rater reliability. Ten percent of the MINI and CAPS interviews were randomly selected to monitor the reliability of the interview process. All assessments were performed when the participants were study medication-free.
Cortisol and Startle assessments
At each assessment, participants were exposed to standardized 2-minute virtual reality scenes Startle responses were elicited by 40ms, 106dB white noise bursts during each scene, as well as in the presence of blank blue squares at a variable 15–45 sec interstimulus interval. They were measured using electromyography (EMG) of the orbicularis oculi muscle contraction using the EMG module of the Biopac MP150 system. Immediately before, immediately after, and 15 minutes after scene presentation, salivary cortisol samples were collected via Salivette (Sarsedt Inc, Newton, NC). Samples were immediately frozen and batch-processed using a chemiluminescent immunoassay on a Beckman Access analyzer. All samples were run in duplicate and 3 levels of quality control were processed in every assay. The detection limit for the salivary assay was 0.1 nmol/L. The inter-assay and the intra-assay coefficients of variations were under 10%.
Treatment
All participants were seen individually for one 90-minute introductory session (information gathering, treatment planning, explaining treatment rationale) followed by 5 weekly 90-minute Virtual Reality Exposure (VRE) sessions delivered by Ph.D. level clinicians. Participants arrived 30 minutes prior to the VRE session, were given questionnaires to complete and took a single pill supervised by study personnel. During VRE sessions, participants were encouraged to expose themselves to their most traumatic memories, following guidelines for standard exposure therapy (4). The therapist viewed the virtual environments on a video monitor and attempted to match stimuli that the patient described. The therapist encouraged continued exposure (30 to 45 minutes) until anxiety decreased. The participant’s anxiety level was assessed every 5 minutes through the use of Subjective Units of Discomfort (0 = no anxiety, 100 = maximum anxiety). Exposure was followed by 15–20 minutes of processing of the experience, integrating material from the exposure, and making new associations explicit. Other than the VRE, the only differences from standard exposure therapy were: no homework was assigned as the design dictated exposure only on study medication, participant’s eyes were open to view the VRE, and only 6 sessions were administered.
Apparatus
During VRE sessions, the participant wore an eMagin Z800 head-mounted display (eMagin Corp., Bellevue, WA) that included separate screens for each eye, integrated headtracking, and stereo earphones. The participant was presented with a computer-generated view of a Virtual Iraq or Afghanistan environment that changed in a natural way with head/body motion. A handheld controller allowed the participant to navigate within the environment at his own pace to drive a Humvee down a desert highway alone or in a convoy, or to navigate on foot through Iraq- like city scenes. Trigger stimulus options included: Sounds, e.g. weapons fire, explosions, incoming mortars, helicopter flyovers, prayer calls, and radio; Visual stimuli, e.g., night vision, smoke, explosions, civilians, and burned vehicles; olfactory stimuli, e.g., burning rubber, diesel fuel, weapons fire, and Middle Eastern spices. Tactile stimuli (e.g., vibrations) were delivered through a raised platform with a subwoofer driven by an audio amplifier. The therapist delivered trigger stimuli via a network-linked to the VR PC.
Statistical Analyses
A piecewise mixed-effect model was used to test the hypotheses that (1) D-cycloserine would have superior outcomes to placebo and (2) alprazolam would not have superior outcome to placebo. Piecewise models estimate separate slopes for distinct time periods such as active treatment and follow-up. The model included an intercept representing the post-treatment assessment, a slope representing the magnitude of change from baseline to post-treatment, a slope representing the magnitude of change from post-treatment to 12-month follow-up, and dummy-coded variables for D-cycloserine and alprazolam that assessed specified hypotheses using an α=.025. Interaction terms between the dummy-coded variables and the slopes assessed differences in change during treatment and follow-up. PTSD diagnostic rates according to the Clinician Administered PTSD Scale were compared at post-treatment, 3-, 6-, and 12-months using chi-square tests. Outcomes were analyzed using the intent-to-treat sample of all randomized participants.
An exploratory analysis was conducted examining extinction learning, calculated as the difference between peak Subjective Units of Distress in successive sessions, The mean of these differences was used as an index of average extinction learning across treatment. This mean was included as a fixed effect predicting post-treatment scores, as an interaction with the dummy-coded contrasts to assess the mediating effect of extinction learning on outcomes across the conditions. When significant, 95% Confidence bands were constructed to identify the level of extinction learning in which a significant difference between the conditions was detected at α=.05 (30).
Repeated-measures analysis of variance was used to analyze the effects of the VR scenes on cortisol levels across time. Within-subjects variables included virtual reality (VR) sample (pre-VR, post-VR, and 15 min Post-VR) and time (baseline, post-treatment, and 6 month follow-up). Effects of treatment condition (D-cycloserine, alprazolam, and placebo) were examined as a between-groups variable. The same analysis was repeated using startle response as the dependent variable. The time and group condition variables were the same as above, but the VR included 2 levels (VR scene, blue square), and a variable for block (2 blocks were presented).
RESULTS
There were 156 participants randomized to the three conditions (D-cycloserine = 53, Alprazolam = 50, Placebo = 53). Supplemental Figure 1 depicts the flow of participants through the study. There were no significant differences in drop-out rate across conditions at post-treatment (Χ2 (2) = 3.36, p = .19), or 3-month (Χ2 (2) = 3.63, p = .16), 6-month (Χ2 (2) = 2.75, p = .25), and 12-month (Χ2 (2) = 1.11, p = .57) follow-up. Drop-outs did not significantly differ from completers on baseline demographic characteristics and symptom variables. Little’s test suggested that missing cases met the assumption for missing completely at random, Χ2 (127) = 124.89, p = .54. There were no meaningful between group differences at baseline (Table 1). A randomly selected subset (N=47) of diagnostic interviews were reviewed by a master’s level clinician to calculate the inter-rater reliability and agreement for the primary diagnosis across assessments was 100%.
Table 1.
Baseline characteristics of the sample
| D–cycloserine | Alprazolam | Placebo | ||
|---|---|---|---|---|
| N = 53 | N = 50 | N = 53 | ||
| Age, mean (95% CI), years | 34.9 (32.5–37.4) | 36.2 (33.8–38.6) | 34.3 (32.0–36.5) | |
| Sex | ||||
| Male | 49 (92.5) | 49 (98.0) | 50 (94.3) | |
| Female | 4 (7.5) | 1 (2.0) | 3 (5.7) | |
| Race/ethnicity | ||||
| Black | 27 (50.9) | 24 (48) | 28 (52.8) | |
| White | 22 (41.5) | 23 (46) | 20 (37.7) | |
| Hispanic | 3 (5.7) | 3 (6.0) | 2 (3.8) | |
| Asian | 0 (0) | 0 (0) | 1 (1.9) | |
| Other | 1 (1.9) | 0 (0) | 2 (3.8) | |
| Partnered | 22 (41.5) | 24 (48.0) | 20 (37.7) | |
| Comorbid Mood Disorder | 12 (22.6) | 12 (24.0) | 19 (35.8) | |
Efficacy Comparisons
There was a significant effect over the course of the trial for the Clinician Administered PTSD Scale (CAPS) (b = −12.19, CI: −16.04 to −8.33, p < .001, d = 1.56) and the PTSD Symptom Scale (PSS) (b = −4.68, CI: −6.56 to −2.80, p < .001, d = 1.16) across all conditions (Table 2). The effect for the CAPS was maintained over 12-months (b = −1.19, CI: −1.86 to −0.53, p < .001), but not for the PSS (b = −0.22, CI: −0.54 to 0.11, p = .191).
Table 2.
Summary of the piecewise model predicting PTSD symptoms
| D-cycloserine | Alprazolam | Placebo | ||||
|---|---|---|---|---|---|---|
| CAPS | PSS | CAPS | PSS | CAPS | PSS | |
| Baseline | 85.3 (79.3–91.9) | 32.9 (29.7–36.2) | 88.0 (82.3–93.6) | 32.4 (26.0–38.9) | 82.6 (75.8–89.4) | 32.4 (29.1–35.7) |
| Post-tx | 65.9 (60.2–71.6) | 27.1 (24.3–29.9) | 69.6 (63.8–75.4) | 25.6 (22.5–28.7) | 63.8 (56.7–70.9) | 24.2 (20.2–28.1) |
| 3-month | 60.3 (54.4–66.2) | 25.2 (22.0–28.5) | 66.8 (59.6–74.0) | 26.1 (22.6–29.4) | 51.5 (43.6–59.5) | 21.4 (17.1–25.6) |
| 6-month | 56.00 (50.1–62.00) | 24.1 (20.5–27.6) | 63.4 (55.4–71.4) | 26.3 (22.5–30.1) | 46.9 (38.7–55.1) | 20.0 (15.9–24.0) |
| 12-month | 48.0 (41.0–55.0) | 22.6 (19.1–26.1) | 57.2 (50.6–63.8) | 24.2 (20.8–27.6) | 48.4 (41.0–55.8) | 21.7 (17.9–25.4) |
Note: DCS = D-cycloserine; CAPS = Clinician Administered PTSD Scale; PSS = PTSD Symptom Scale; post-tx = post-treatment assessment; All of these value are point estimates with a corresponding confidence interval (CI). The CI is needed to provide a range for the population parameter estimate. The CI around the baseline measure indicates that all of the groups started at the same point.
Comparisons in post-treatment differences were made according to a priori planned contrasts (Figure 1A). There was not a significant difference between D-cycloserine and placebo at post-treatment on the Clinician Administered PTSD Scale (CAPS) (b = 4.46, CI: −1.40 to 10.33, p = .135) or the PTSD Symptom Scale (PSS) (b = 4.76, CI: −0.39 to 9.92, p = .070). The treatment × condition interaction was not significant for the D-cycloserine and placebo conditions for the CAPS (b = 4.46, CI: −4.68 to 14.39, p = .318) or the PSS (b = 3.00, CI: −1.66 to 7.66, p = .207), which suggests that the change from baseline to post-treatment did not differ across D-cycloserine and placebo. The 12-month × condition interaction was also not significant for the D-cycloserine and placebo conditions for the CAPS (b = −0.56, CI: −2.24 to 1.12, p = .514), the PSS (b = −0.23, CI: −1.06 to 0.59, p = .581).
Figure 1. Effect of Treatment on CAPS at each Assessment and Extinction Learning across Treatment.
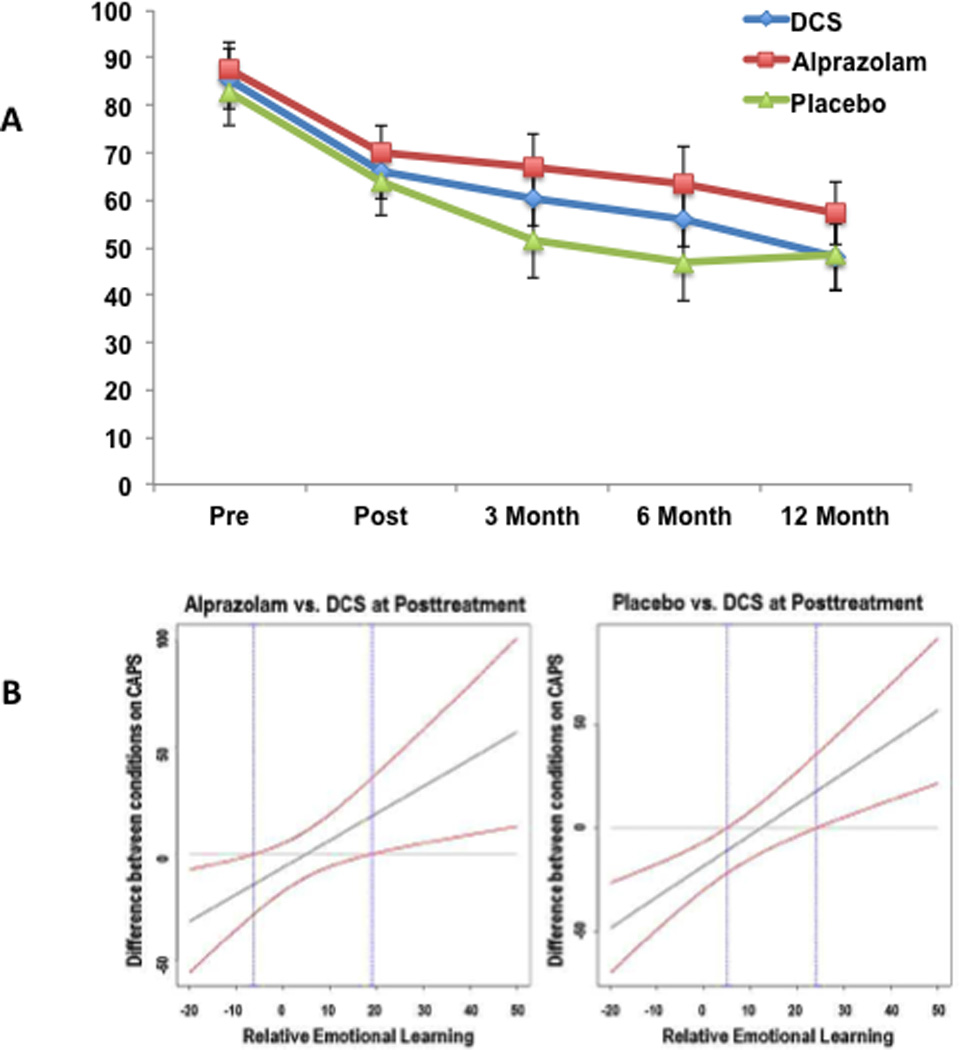
A) CAPS scores at each assessment point across the three conditions. Means are model implied from a mixed effect model that used all participants (n = 156) in the sample. Error bars correspond to 95% confidence intervals for the estimate. B) 95% Confidence bands estimating differences between D-cycloserine (DCS) vs Alprazolam and D-cycloserine vs Placebo at post-treatment. Differences are significant outside of the area defined by the blue lines. The confidence bands indicate that there is a significant difference between the D-cycloserine and Alprazolam conditions when learning was less than −6.29 and greater than 18.99. There is a significant difference between the D-cycloserine and Placebo conditions when learning was less than 4.78 and greater than 23.94.
There was a significant difference between alprazolam and placebo conditions at post-treatment on the CAPS (b = 7.88, CI: 2.28 to 13.48, p = .006), but not on the PTSD Symptom Scale (PSS) (b = 2.61, CI: −2.23 to 7.45, p = .290). The treatment × condition interaction was not significant for the alprazolam and placebo conditions for the Clinician Administered PTSD Scale (CAPS) (b = 3.57, CI: −5.70 to 12.84, p = .449) or the PSS (b = 1.93, CI: −2.61 to 6.48, p = .403). The 12-month×condition interaction was not significant for the alprazolam and placebo conditions for the CAPS (b = 0.07, CI: −.1.51 to 1.65, p = .931) or the PSS (b = 0.04, CI: −0.74 to 0.82, p = .918). Finally, at 3-month follow-up, significantly more alprazolam participants met full PTSD criteria than the D-cycloserine or placebo (Table 3), a difference not observed at the other time points.
Table 3.
Proportion of participants who met criteria for PTSD.
| D-cycloserine (N = 53) |
Alprazolam (N = 50) |
Placebo (N = 53) |
||||
|---|---|---|---|---|---|---|
| Did not meet Criteria |
Met Criteria |
Did not meet Criteria |
Met Criteria |
Did not meet Criteria |
Met Criteria |
|
| post-tx | 6 (21.4)a | 22 (78.6) | 9 (25.7)a | 26 (74.3) | 9 (26.5)a | 24 (73.5) |
| 3-month | 7 (35.0)a | 13 (65.0) | 5 (20.8)b | 19 (79.2) | 12 (52.2)a | 11 (47.8) |
| 6-month | 7 (41.2)a | 10 (58.8) | 6 (24.0)a | 13 (68.4) | 13 (56.5)a | 10 (43.5) |
| 12-month | 9 (52.9)a | 8 (47.1)a | 8 (36.4)a | 11 (57.8) | 9 (45.0)a | 11 (55.0) |
Note: post-tx = post-treatment assessment; Values are raw numbers and scores in parentheses are percentages. Superscripts denote differences across groups within each time points at α of .05
Mediators
Extinction Learning
There was a significant learning (35, 36) x D-cycloserine interaction for the Clinician Administered PTSD Scale (CAPS) (b = −2.19, CI: −3.44 to −0.94, p = .001) and PTSD Symptom Scale (PSS) (b = −0.82, CI: −1.19 to −0.45, p = .001). However, there were not significant interactions for learning×placebo (CAPS: b = 0.59, CI: −0.04 to 1.22, p = .068; PSS: b = 0.11, CI: −0.08 to 0.32, p = .258) or learning×alprazolam (CAPS: b = −0.17, CI: −0.97 to 0.62, p = .665; PSS: b = −0.04, CI: −0.42 to 0.35, p = .849). These findings suggest that extinction learning was associated with post-treatment scores for the D-cycloserine condition, but not for the placebo and alprazolam scores. To further clarify this interaction, 95% confidence bands were calculated for the difference between the D-cycloserine condition and the other conditions (Figure 1B). For the CAPS, those in the D-cycloserine condition reported better post-treatment outcomes than alprazolam and placebo conditions with greater learning (> 18.99 and >23.94 respectively) and worse outcomes with decreased learning (<−6.28 and < 4.78, respectively).
Biomarkers
Cortisol data were available for 104 individuals at baseline, and 39 individuals at all 3 time points (baseline, post-treatment, and 6-month follow-up). Pre-Virtual Reality (VR) cortisol levels did not differ between conditions (p=0.59). A Repeated Measures Analysis of Variance (RM-ANOVA) of cortisol levels revealed a significant interaction of time and VR, F(4,152)=2.43, p=.05. A RM-ANOVA of cortisol change scores (from pre to 15 min post-VR) revealed a significant time × condition interaction, F(4,72)=2.58, p<.05, with the change score decreasing more in the D-cycloserine group post-treatment compared to the alprazolam and placebo groups (both p’s<.05) (Figure 2A). Startle data were available for 117 patients at baseline and 32 patients at all 3 time points. A RM-ANOVA indicated a significant 4-way interaction of time, VR, block and condition, F(4,58)=2.74, p<.05. Follow-up analyses within each treatment condition found that treatment had a significant effect on the startle response during the VR scenes only in the D-cycloserine group, F(2,12)=6.24, p=.01. After correcting for group differences in startle response at baseline, the D-cycloserine group again showed a significant percent change from baseline with time, F(2,12)=51.65, p<.001, while the other groups did not (Figure 2B).
Figure 2. Effect of treatment on Cortisol and Startle Responses.
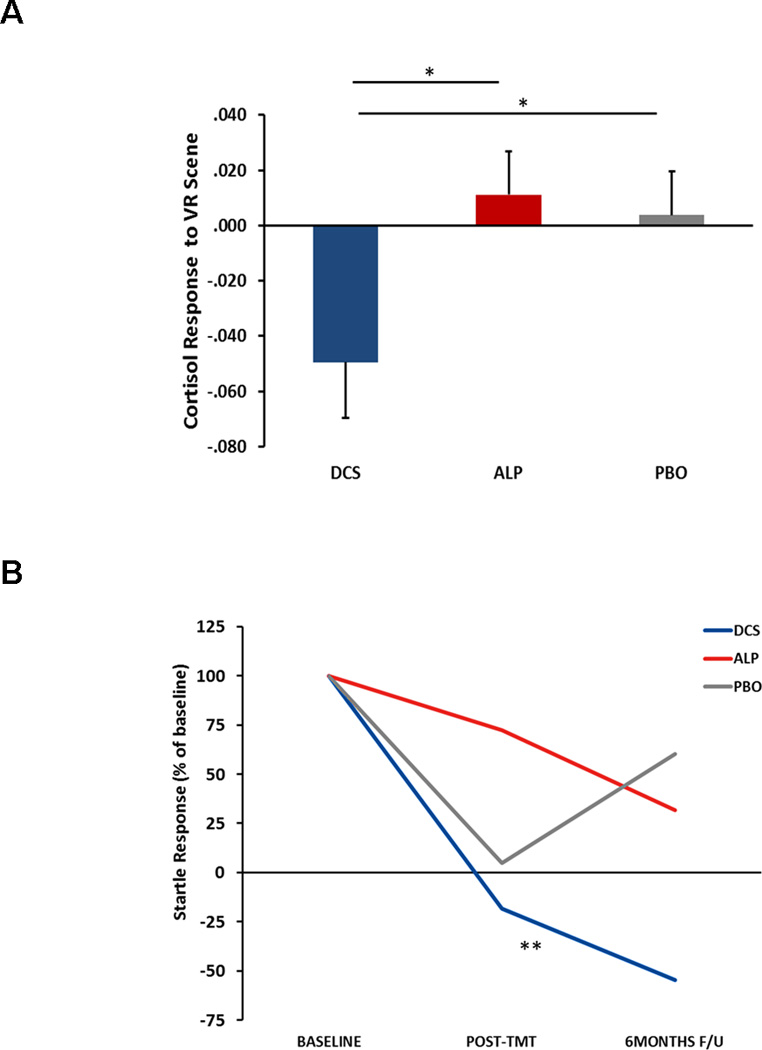
Cortisol response is shown at post-treatment as a function of treatment across D-cycloserine (DCS), alprazolam (ALP), and placebo (PBO) groups. Bars represent mean+SE. *p<.05 between groups. B) Startle response change across time in D-cycloserine (DCS), alprazolam (ALP), and placebo (PBO) groups. Startle is shown as % of baseline startle to correct for group differences pre-treatment. **p<.01 across time.
DISCUSSION
We report the first randomized controlled clinical trial with this cohort of veterans utilizing Virtual Reality Exposure (VRE) combined with D-cycloserine, alprazolam, or placebo. The most significant findings with potential clinical implications include: 1) 6 sessions (five of the VRE) were associated with significant improvement in PTSD symptoms at post-treatment that were maintained at follow-up, although there was no control for the VRE; 2) the primary hypothesis of D-cycloserine enhancement was not supported overall; but D-cycloserine may enhance outcomes of PTSD symptoms in those with more between-session learning; 3) alprazolam use during treatment may diminish the efficacy of exposure therapy, with more severe post-treatment symptoms and higher rates of PTSD diagnosis at 3-month follow-up; and 4) two neurobiological biomarkers, salivary cortisol and startle response, were sensitive to treatment gains and indicated differential treatment response favoring D-cycloserine. The effects of D-cycloserine enhancement of VRE in this veteran PTSD population are inconclusive. The primary hypothesis of overall differences in the Clinician Administered PTSD Scale was not supported, but D-cycloserine demonstrated benefits on secondary measures (cortisol and startle) in exploratory analyses.
These results suggest that VR exposure therapy attenuated cortisol and startle responses to a trauma-relevant scene. Reduced cortisol reactivity after 12 months of paroxetine treatment of PTSD treatment has been observed previously (22); the current study expanded this finding to a placebo-controlled RCT and demonstrated the effect after 6 weeks of treatment. Patients who received D-cycloserine had greater reductions in cortisol and startle responses post-treatment compared to either alprazolam or placebo. Although D-cycloserine has shown enhanced extinction of fear-potentiated startle in animal studies (8), this is the first study we are aware of showing treatment effects of D-cycloserine on fear-potentiated startle in PTSD patients.
As a potential targeted pharmacological agent for the augmentation of extinction learning, which is believed to underlie exposure based psychotherapy, D-cycloserine was initially shown to enhance extinction of conditioned fear in rodent models (8). This finding was rapidly replicated in a number of additional animal studies, which found that D-cycloserine enhances the consolidation phase of fear extinction, the rate of between-session extinction learning, and reduces fear reinstatement (31). These promising preclinical studies were translated across a number of exposure therapy paradigms in humans with clinical anxiety disorders (9,10,11,12,13). Despite these promising findings, studies examining fear extinction in healthy humans using skin conductance response found no D-cycloserine enhancement of extinction training or recall (32,33). However, similar to the rodent studies, D-cycloserine was found to significantly reduce reinstatement of fear after extinction (33) demonstrating potential long-term benefits of extinction learning. Given the role of D-cycloserine in learning, it may have contributed to more long-lasting extinction to the VR scenes (as seen in Figure 2B).
Therefore, we believe reduced reactivity to trauma-related cues following treatment was likely due to treatment-related learning effects (i. e. extinction) and that D-cycloserine enhanced these learning effects. Additionally, our data showing that extinction learning was associated with post-treatment scores for the D-cycloserine condition, but not for the other conditions, is consistent with the increasing literature that the D-cycloserine clinical effect may be best observed through enhancing consolidation of extinction gains made between-session (10,14, 25,26,34) and preventing return of symptoms post-treatment. Together these effects may represent the N-methyl-D-aspartate-dependent augmentation of relatively weak components of extinction learning, which may be difficult to identify in the patients’ clinical ratings in the context of the robust effects of exposure therapy.
Strengths of the current study include the novel treatments offered, the combination of treatments, and the use of biomarkers. Use of Virtual Reality Exposure (VRE) has been advocated for this cohort of Veterans as they are a video-game-playing generation and find VRE more acceptable than talk therapy, and VR is very evocative and may assist emotional engagement in exposure therapy (35). Improvements in only 6 sessions in this study were comparable to other studies in Veterans administering more sessions of prolonged exposure (36). The comparison of D-cycloserine and alprazolam to placebo is novel. The study was well controlled, with objective measures at every assessment point, including long-term assessment by blinded independent assessors.
Weaknesses of the current study include high dropout rate. It should be noted that 31 participants dropped out prior to the first treatment session. Many of these participants came to be assessed after completing the PTSD 101 course at the local Veterans Administration Hospital. Seeking treatment is encouraged after the course. Many of these individuals may have attended the baseline assessment to be compliant and for monetary reimbursement. Although disheartening, the dropout rate is similar to other studies with Veterans and active duty personnel (36). There were also differences in how the data were analyzed between the clinical measures and the biological measures (cortisol, startle) that were necessitated by differences in data type.
In conclusion, it appears we observed an apparent response to a brief course of Virtual Reality Exposure (VRE) in this cohort of Veterans, although there was no VRE-treatment control. As previously thought, we should be cautious in the use of benzodiazepines in patients with PTSD as they seemed to have attenuated overall response in the long term. The D-cycloserine results are less conclusive: there was a clear drug effect favoring D-cycloserine on salivary cortisol and startle biomarkers and a more complicated advantage on clinical symptoms for those who experienced good extinction learning between sessions. There may be a subtype of participants for whom D-cycloserine will enhance learning during exposure therapy.
Supplementary Material
The status of complete enrollment, exclusion, allocation, treatment, and followup subjects are shown.
Acknowledgments
This study was supported by National Institute of Mental Health, Grant No. 1R01MH70880-01-A2, “A Cognitive Enhancer may Facilitate Behavioral Exposure Therapy” awarded to Dr. Rothbaum.
Dr. Rothbaum is a consultant to and owns equity in Virtually Better, Inc. that creates virtual environments; however, Virtually Better did not create the Virtual Iraq environment tested in this study. Drs. Ressler and Davis are founding members of Extinction Pharmaceuticals / Therapade Technologies, which seek to develop D-cycloserine and other compounds for use to augment the effectiveness of psychotherapy. They have received no equity or income from this relationship within the last 3 years. Dr. Dunlop has received grant support from BMS, Forest, GSK, Novartis, and Pfizer in the past 3 years, and has served as a consultant to Roche and BMS.
Dr. Rothbaum also has funding from Department of Defense Clinical Trial Grant No.W81XWH-10-1-1045, “Enhancing Exposure Therapy for PTSD: Virtual Reality and Imaginal Exposure with a Cognitive Enhancer”, National Institute of Mental Health, Grant No. 5 U19 MH069056-03, “The Emory-MSSM-GSK-NIMH Collaborative Mood and Anxiety Disorders Initiative,” National Institute of Mental Health, Grant No. 1R01MH70880-01-A2, “A Cognitive Enhancer may Facilitate Behavioral Exposure Therapy,” NIH Grant No. 1R01MH094757-01, “Prospective Determination of Psychobiological Risk Factors for Posttraumatic Stress,” Brain and Behavior Research Foundation (NARSAD) Distinguished Investigator Grant, “Optimal Dose of early intervention to prevent PTSD”, and McCormick Foundation “Brave Heart: MLB’s Welcome Back Veterans SouthEast Initiative,” and recent previous support from Transcept Pharmaceuticals “A Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study To Evaluate The Efficacy And Safety Of Low-Dose Ondansetron For Adjunctive Therapy In Adult Patients With Obsessive-Compulsive Disorder Who Have Not Adequately Responded To Treatment With A Serotonin Reuptake Inhibitor.” Dr. Rothbaum receives royalties from Oxford University Press, Guilford, APPI, and Emory University and received one advisory board payment from Genentech.
Footnotes
Previous presentation: These data were presented at the 29th Annual Meeting of the International Society for Traumatic Stress Studies (ISTSS), Nov. 7–9, 2013, Philadelphia, PA.
Disclosure Statement
Drs. Jovanovic, Norrholm, Bradley, and Price report no competing interests.
The terms of these arrangements have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
REFERENCES
- 1.Institute of Medicine (IOM) Treatment for posttraumatic stress disorder in military and veteran populations: initial assessment. Washington, DC: The National Academies Press; 2012. Preface. [PubMed] [Google Scholar]
- 2.Hoge CW. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine (IOM) Treatment of posttraumatic stress disorder: an assessment of the evidence. Washington, DC: The National Academies Press; 2008. [Google Scholar]
- 4.Foa EB, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences, Therapist Guide. New York: Oxford University Press; 2007. [Google Scholar]
- 5.Gerardi M, Cukor J, Difede J, Rizzo A, Rothbaum BO. Virtual reality exposure therapy for post-traumatic stress disorder and other anxiety disorders. Curr Psychiatry Rep. 2010;12:298–305. doi: 10.1007/s11920-010-0128-4. [DOI] [PubMed] [Google Scholar]
- 6.Gerardi M, Rothbaum BO, Ressler K, Heekin M, Rizzo A. Virtual reality exposure therapy using a virtual Iraq: case report. J Trauma Stress. 2008;21:209–213. doi: 10.1002/jts.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foa EB, Rothbaum BO. Treating the Trauma of Rape: Cognitive-Behavior Therapy for PTSD. New York: Guilford Press; 1998. [Google Scholar]
- 8.Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration of intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 10.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, Dadds M R. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, Hofmann SG, Eisenmenger K, Krystal JH, Pollack MH. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry. 2010;67:365–370. doi: 10.1016/j.biopsych.2009.07.036. [DOI] [PubMed] [Google Scholar]
- 12.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, McCabe J, Peterson J, Foa EB. D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, Cannistraro P, Jenike MA, Rauch SL. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann S, Smits J, Rosenfield D, Simon N, Otto M, Meuret A, Marques L, Fang A, Tart C, Polacck M. D-cycloserine as an augmentation strategy with cognitive-behavioral therapy for social anxiety disorder. Am J Psychiatry. 2013;170:751–758. doi: 10.1176/appi.ajp.2013.12070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouton ME, Kenney FA, & Rosengard C. State-dependent fear extinction with two benzodiazepine tranquilizers. Behavioral Neuroscience. 1990;104:44–55. doi: 10.1037//0735-7044.104.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Lund BC, Bernardy NC, Vaughan-Sarrazin M, Alexander B, Friedman MJ. Patient and facility characteristics associated with benzodiazepine prescribing for Veterans with PTSD. Psych Serv. doi: 10.1176/appi.ps.201200267. In press. [DOI] [PubMed] [Google Scholar]
- 17.The Management of Post-Traumatic Stress Working Group. VA/DoD Clinical Practice Guideline for Management of Post-Traumatic Stress. Washington, DC: Department of Veterans Affairs, Department of Defense; 2010. [Google Scholar]
- 18.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 19.Litz BT, Salters-Pedneault K, Steenkamp MM, Hermos JA, Bryant RA, Otto MW, Hofmann SG. A randomized placebo-controlled trial of D-cycloserine and exposure therapy for posttraumatic stress disorder. J Psychiatr Res. 2012;46:1184–1190. doi: 10.1016/j.jpsychires.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Difede J, Cukor J, Wyka K, Olden M, Hunter H, Lee FS, Altemus M. D-cycloserine augmentation of exposure therapy for posttraumatic stress disorder: a pilot randomized clinical trial. Neuropsychopharmacol. 2013 doi: 10.1038/npp.2013.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holland JM, Schatzberg AF, O'Hara R, Marquett RM, Gallagher-Thompson D. Pretreatment cortisol levels predict posttreatment outcomes among older adults with depression in cognitive behavioral therapy. Psychiatry Res. 2013;210:444–450. doi: 10.1016/j.psychres.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vermetten E, Vythilingam M, Schmahl C, DE Kloet C, Southwick SM, Charney DS, Bremner JD. Alterations in stress reactivity after long-term treatment with paroxetine in women with posttraumatic stress disorder. Ann N Y Acad Sci. 2006;1071:184–202. doi: 10.1196/annals.1364.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orr S, Roth W. Psychophysiological assessment: clinical applications for PTSD. J Affect Disord. 2000;61:225–240. doi: 10.1016/s0165-0327(00)00340-2. [DOI] [PubMed] [Google Scholar]
- 24.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanau I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biol Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smits JA, Rosenfield D, Otto MW, Powers MB, Hofmann SG, Telch MJ, Pollack MH, Tart CD. D-cycloserine enhancement of fear extinction is specific to successful exposure sessions: evidence from the treatment of height phobia. Biol Psychiatry. 2013;73:1054–1058. doi: 10.1016/j.biopsych.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits JA, Rosenfield D, Otto MW, Marques L, Davis ML, Meuret AE, Simon NM, Pollack MH, Hofmann SG. D-cycloserine enhancement of exposure therapy for social anxiety disorder depends on the success of exposure sessions. J Psychiatr Res. 2013;47:1455–1461. doi: 10.1016/j.jpsychires.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8:75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DV, Lecrubier Y, Sheehan KH. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;1959:22–33. [PubMed] [Google Scholar]
- 29.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J Trauma Stress. 1993;6:459–473. [Google Scholar]
- 30.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression. Multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 31.Ledgerwood L, Richardson R, Cranney H. D-cycloserine and the facilitation of extinction of conditioned fear: consequences for reinstatement. Behav Neurosci. 2004;118:505–513. doi: 10.1037/0735-7044.118.3.505. [DOI] [PubMed] [Google Scholar]
- 32.Guastella AJ, Lovibond PF, Dadds MR, Mitchell P, Richardson R. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Kuriyama K, Honma M, Yoshiike T, Kim Y. Valproic acid but not D-cycloserine facilitates sleep-dependent offline learning of extinction and habituation of conditioned fear in humans. Neuropharmacology. 2013;64:424–431. doi: 10.1016/j.neuropharm.2012.07.045. [DOI] [PubMed] [Google Scholar]
- 34.Chasson GS, Buhlmann U, Toln DF, Rao SR, Reese HE, Rowley T, Welsh KS, Wilhelm S. Need for speed: evaluating slopes of OCD recovery in behavior therapy enhanced with d-cycloserine. Behav Res Ther. 2010;48:675–679. doi: 10.1016/j.brat.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Reger G, Gahm G. Virtual reality exposure therapy for active duty soldiers. J Clin Psychol. 2008;64:940–946. doi: 10.1002/jclp.20512. [DOI] [PubMed] [Google Scholar]
- 36.Eftekhari A, Ruzek J, Crowley J, Rosen C, Greenbaum M, Karlin B. Effectiveness of national implementation of prolonged exposure therapy in veterans affairs care. JAMA Psychiatry. 2013;36 doi: 10.1001/jamapsychiatry.2013.36. Available from: http://archpsyc.jamanetwork.com/article.aspx?articleid=1714401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The status of complete enrollment, exclusion, allocation, treatment, and followup subjects are shown.


