Abstract
Objective:
The intervertebral disc (IVD) undergoes biochemical and morphologic degenerative changes during the process of aging. Aquaporins (AQPs) are a family of water channel proteins that facilitate water and small solute movement in tissues and may have a potential role in the aging degeneration of IVDs. One of the important problems in understanding disc degeneration is to find cellular molecules which contribute to the pathogenesis of IVDs. XThe aim of this study was to demonstrate the expression of aquaporin 1 and 3 in nucleus pulposus (NP), annulus fibrosus (AF) cells of rat lumbar intervertebral discs from both young and aged animals using immunohistochemistry.
Material and Methods:
Twenty Wistar-albino rats were included in the study. The rats were separated into two groups: 2-month-old rats (n=10) as the young group, 18-month-old rats (n=10) as the old group. The intervertebral disc tissues obtained from the lumbar spine (L1–L4, 4 discs) were used for immunohistochemical staining of AQP-1 and 3.
Results:
This study demonstrated that AQP-1 and AQP-3 immunoreactivity significantly decreased in NP and AF of aged rats compared to the young rats.
Conclusion:
We suggest that AQP-1 and 3 may contribute to the age related degeneration of the intervertebral disc.
Keywords: Intervertebral disc, aquaporin 1 and aquaporin 3, immunohistochemistry, aging, rat
Introduction
The intervertebral disc (IVD) has morphologically three distinct regions: The central nucleus pulposus (NP), the outer annulus fibrosus (AF) and the inferior cartilage endplates (1). The NP is a highly hydrated gel-like matrix composed of negatively charged aggregating proteoglycans, randomly organized collagen fibers and radially oriented elastin fibers. The surrounding AF consists of a series of concentric lamellae of predominantly type-I collagen fibers (2). Proteoglycans (PG) in the NP have a high osmotic potential because of large negative charge, which attracts mainly cations such as Na+, K+ and Ca2+ and consequently large amount of water in to extracellular matrix (ECM) of the NP (3).
Intervertebral disc undergoes biochemical and morphologic degenerative changes during the process of aging. The incidence of degeneration, increases severely with age and is regarded as a major cause of disc originated low back pain (4). One of the important problems in understanding disc degeneration and regeneration is to determine the cellular signaling molecules contributing the IVD structures. Despite the multitude of disc disease, disc degeneration is still a puzzle that is fully disclosed (5).
Degeneration involves all parts of the disc. Although degeneration of the AF, NP and the cartilaginous endplates have been shown that alterations of signaling molecules, subsequent changes with aging are not clarified. There is reason to believe that loss of aquaporins (AQPs) may be the cause of degeneration. AQPs bi-directional water-permeable trans-membrane channels and play a vital role for a rapid water movement (4, 6). The expression patterns of aquaporins in different tissues such as kidney, eye, brain, the digestive, respiratory, genito-urinary tracts, articular cartilage and human IVD proves their differing properties and their specific cell membrane transport functions (7–9).
The expression of AQP-1 and 3 were also demonstrated in different tissues including articular cartilage and human IVD (2, 10). In a recent study, AQP-1 has been shown to have important roles in degeneration of IVDs with aging (6). Li et al. (11) demonstrated that AQP-3 decreased with increasing age in both skin and NHEK samples. Despite above findings based on AQP expressions in different systems, little is known of the expression pattern of AQP-1 and AQP-3 in older IVDs of rats. Therefore, the aim of this study was to demonstrate the expression of AQP-1 and 3 in IVDs in young and aged animals using immunohistochemistry.
Material and Methods
Animals and surgical procedures
Twenty Wistar albino rats were obtained from Gaziosmanpasa University Experimental Animal Research Laboratory. Rats were handled in the laboratory according to institutional guidelines, as well as the Guide for Care and Use of Laboratory Animals of the National Research Council. All experimental protocols were approved by the by the ethics committee (HADYEK-013). All rats were observed for several days to ascertain their health prior to sample collection. Rats were reared with their dams and they were kept in a temperature-controlled room (20–23°C) on a 12-hour light/dark cycle, with food (commercial rat chow) and fresh water available adlibitum. The rats were separated into two equal groups: 2-month-old rats (n=10) as the young group, 18-month-old rats (n=10) mature as the old group. These rats were gently killed in our laboratory with an overdose of phenobarbital, and the lumbar spine was removed aseptically within 0.5 h. In each group, the lumbar spine (L1–L4, 4 discs) of rats was used for immunohistochemistry to confirm AQP-1 and 3 expressions in the NP and AF.
Briefly, the lumbar discs removed from the adjacent vertebral body bone and fixed in 10% neutral formalin solution for 2 days, decalcified in 20% EDTA acid for 2 weeks, and embedded in paraffin for immunohistochemistry and histochemistry. The rat kidney tissue was prepared as positive control.
Immunohistochemistry
Immunohistochemistry was performed according to the procedure previously described with minor modification (12). Briefly, 5 μm-thick serial sections were collected on poly-L-lysine-coated slides (Sigma-Aldrich, St. Louis, MO, USA) and incubated overnight at 56°C. Tissue sections were deparaffinised in xylene and rehydrated in a graded series of ethanol. Sections were then treated in a microwave oven in 10 mM citrate buffer, pH 6.0 for 2 minutes twice and left to cool for 15 min. After three washes in phosphate buffered saline (PBS), endogenous peroxidase activity was quenched by 3% hydrogen peroxide in PBS for 20 min, and the sections were washed again three times in PBS. The sections were then incubated in a blocking serum (Ultra V Block, ScyTek Laboratories, Utah, USA) for 10 min. to block non-specific binding. Subsequently, sections were incubated overnight at 4°C with rabbit polyclonal AQP-1 (cat no: sc-32738, 1: 50, SantaCruz, USA) and AQP-3 (cat no: ab85903, 1: 200, Abcam, UK). The sections were then washed three times in PBS and incubated with biotinylated anti-rabbit (BA-1000; 1:400 Dilution; Vector Laboratories) secondary antibodies for 45 min. at room temperature. After three washes with PBS, the antigen–antibody complexes were detected using a streptavidin–peroxidase complex (TP-060-HL; LabVision, Fremont, CA, USA) for 15 min, followed by three rinses with PBS. Bound peroxidase was developed with 3-amino-9-ethylcarbazol (AEC) (ScyTek Laboratories, USA) chromogen, and sections were counterstained with Mayer’s hematoxylin (ScyTek Laboratories, Utah, USA) and mounted with Permount (ScyTek Laboratories, Utah, USA) on glass slides. For controls, sections were treated with the appropriate isotype of rabbit and mouse IgGs. Photomicrographs were collected with a Leica microscope (Leica DM2500, Nussloch, Germany).
Evaluation of immunohistochemistry
Evaluation of the immunohistochemical labeling was performed using H-SCORE analyses as previously described (13). The intensity of AQP-1 and AQP-3 immunoreactivity was semi-quantitatively evaluated using the following intensity categories: 0 (no staining), 1+ (weak but detectable staining), 2+ (moderate or distinct staining), and 3+ (intense staining). For each tissue, an H-SCORE value was derived by, first, calculating the sum of the percentages of cells that stained at each intensity category, and then, multiplying that value by the weighted intensity of the staining using the formula H-SCORE=∑Pi(i+ l), where ‘i’ represents the intensity scores and ‘Pi’ is the corresponding percentage of the cells. On each slide, five randomly selected areas were evaluated under a light microscope (40x objective). The percentage of the cells at each intensity within these areas was determined by two investigators, at different times, who were not informed about the type and source of the tissues. The average score of both observers was used.
Statistical analysis
The multiple comparisons were analyzed with non-parametric ANOVA followed by post-hoc tests. Statistical calculations were performed using SigmaStat for Windows, version 2.0 (Jandel Scientific Corp., San Rafael, CA). Statistical significance was defined as p<0.05.
Results
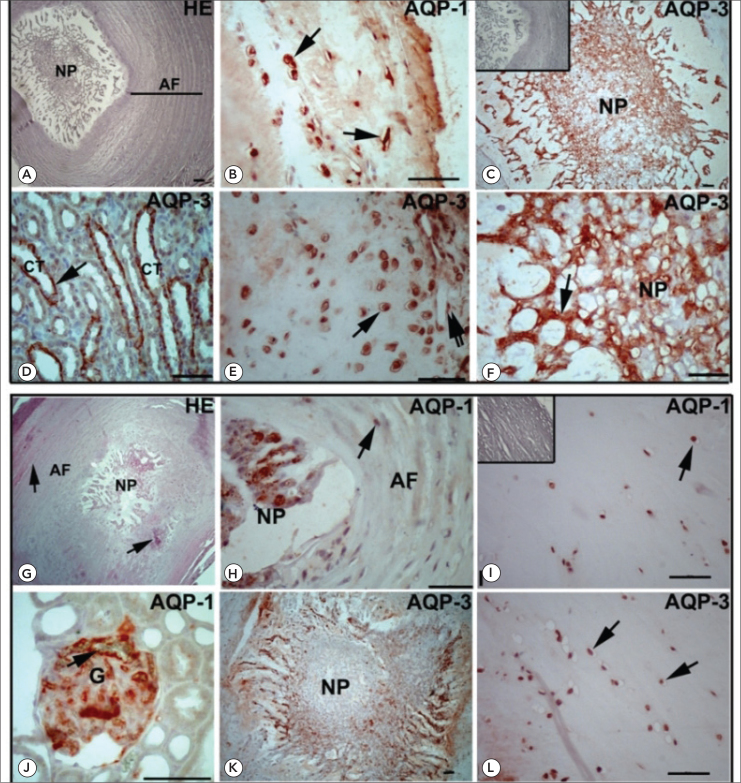
The intervertebral disks from 2-month-old and 18-month-old rats were stained with hematoxylin and eosin (HE) and examined by light microscopy (Figure 1A and G). The intervertebral disk structure is complete and composed with the laminated annulus fibrosus (AF) and the central nucleus pulposus (NP) in 2-month-old rats (Figure 1A). However, the laminated structure of AF was deteriorated and the demarcation between NP and the AF was disappeared in some regions of IVDs of 18-month-old rats. Additionally, NP structure was constant and no degeneration was observed in NP (Figure 1G). The definition of the degree of degenerative changes based on histological appearances in older IVDs of rat was determined as previously published (1).
Figure 1.
Hematoxylin and eosin (HE) staining of intervertebral discs of 2-month-old (A) and 18-month-old rats (G). A: Note the clear demarcation between annulus fibrosus (AF) and nucleus pulposus (NP). AF shows the laminated structure. G: The presence of slits and chondrocyte clusters (arrow) is appeared in 18-month-old rats. The distinction between the AF and NP is started to lost in some region of IVD. Immunohistochemical staining shows the expression of AQP-1 and AQP-3 in the IVD of 2-month-old (B–C and E–F) and 18-month-old (H–I and K–L) rats. No significant staining was observed in the negative controls (C, I, inserts). B: Positive immunostaining of AQP-1 is demonstrated in chondrocytes (arrows) of AF in 2-month-old rats. C: NP shows strong AQP-3 immunoreactivity in 2-month-old rats. D: Collecting tubules (CT) of rat kidney are immunopositive for AQP-3. E: Chondrocytes (arrow) and endothelium (double arrow) of blood vessels are strongly immunpositive for AQP-3. F: AQP-3 is highly expressed in NP of IVD in 2-month-old rats. H: AQP-1 is weakly expressed in AF (arrow) and moderately expressed in NP of 18-month-old rats. I: Chondrocytes (arrow) exhibit weak to moderate immunoreactivity in AF. J: AQP-1 expression is seen in the basal membranes of kidney glomerule as positive control. K and L: AQP-3 is moderately expressed in NP and weakly expressed in AF (arrow) of 18-month-old rats. Scale bars: 50 μm
Immunohistochemistry was used to show the localization of AQP-1 and AQP-3 in 2-month-old and 18-month-old rats as depicted in Figure 1B–F and H–L. In the 2-month-old rats, AQP-1 and AQP-3 were mainly localized in the NP and the inner segment of the AF (Figure 1 B, C, E, F). The NP of IVD and the inner segment of AF showed stronger immunoreactivity however, the outer part of the AF showed weak immunostaining for AQ1 and AQ3 (Figure 1B–F). The intensity of AQP-1 and AQP-3 was found to be the similar in different region of IVD in the 2-month-old rats. As a negative control for specificity of detection, omission of primary anti-AQP-1 and AQP-3 antibodies and their substitution by rabbit IgG (Figure 1C and I, insets) resulted in negative staining. As a positive control, rat kidney tissues were used (Figure 1D and J). AQP-3 was detected in the basolateral membrane of the kidney collecting ducts (Figure 1D) and AQP-1 was found to localize in the glomerular membranes (Figure 1J).
H-SCORE analysis revealed that the staining intensity and the number of cells positively stained for AQP-1 and AQP-3 in IVD significantly decreased in 18-month-old rats (Figure 2). In older animals, weak to moderate immunostaining was observed in AF and NP (Figure 1H–I and K–L).
Figure 2.
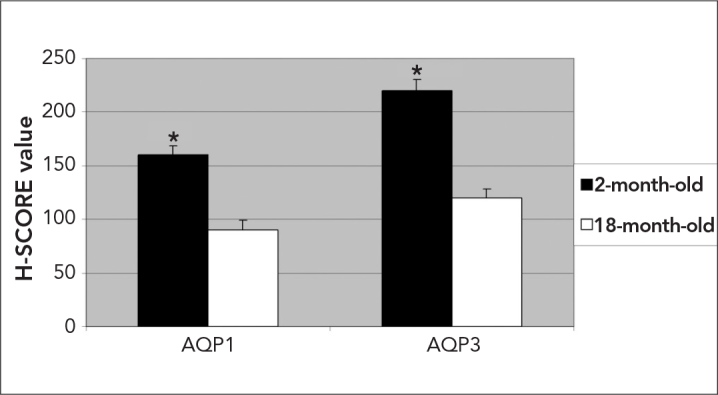
H-SCORE of the AQP-1 and AQP-3 immunostaining intensities in 2-month-old and 18-month-old rats. The data are represented as the means±SEM. Star: p<0.05, 2-month-old vs. 18-month-old day for AQP-1 and AQP-3 expression
Discussion
In this study, the expression of AQP-1 and AQP-3 significantly decreased from 2-month-old to 18-month-old rats in the IVD. Our data provided evidence of early and late expression of AQP-1 and AQP-3 in the IVD of rat. Although many studies showed the expression pattern of AQP-1 and AQP-3 in human IVD, according to our knowledge, this study is the first to compare the distribution of AQP-3 in the young and old IVD of rats using immunohistochemistry.
Intervertebral disc is a highly specialized and organized tissue that is normally well integrated with its adjacent tissues but it is continually changing because of development, aging, and some disorder (14). Intervertebral discs go through age-linked degenerative changes that contain decreasing nutrition of the central disc that further compromises cell function and may cause cell death (15). Progressive changes in intervertebral disc histology with aging include an increased number and extent of fissures and tears, the presence of granular material, and neovascularization from the outer aspect of the annulus inwards. Loss of demarcation between the annulus and the nucleus also increasingly occurs with aging (14). In accordance with above-mentioned studies, we have also noticed some of those changes in the IVD of 18-month old rats. Especially, the demarcation between AF and NP was partly disappeared and the fissures in the AF were detected in older IVD of rats.
It has been reported that with aging, the proteoglycans significantly decreases in the nucleus pulposus, which is believed to be a critical factor in intervertebral disc degeneration (4, 16). Proteoglycans attract fluid, which works to reduce mechanical stresses in the solid matrix of the nucleus and provide a hydrostatic pressure to the annulus fibrosus, whose fibrous nature accommodates this stress (16, 17). Subsequent decrease in the load bearing capability of the disc as the proteoglycan content decreases, the osmotic pressure of the disc falls and the disc is less able to maintain hydration under load. All of these changes, which were detected with aging, could also affect the protein distribution of AQPs in the IVD. Therefore, we were interested in whether the expression of AQPs changed with old IVDs of rats.
AQP-1 is permeable to water and O2, which inhibits quick volume deformation under osmotic stress and accelerates O2 diffusion across the plasma membrane (6). AQP-1 may be important for cell volume regulation, the flow of matrix and metabolic water across the membrane in chondrocytes. Immunohistochemical staining demonstrated AQP-1 localization in articular chondrocytes, synoviocytes, and synovial capillary vascular endothelial cells. The presence of AQP-1 in chondrocytes supports a role for AQP-1-mediated water transport across the synovial micro vessels and the plasma membrane of chondrocytes in load-bearing joints (18). AQP-3 is also highly expressed in many of the human tissues such as kidney, tracheal and bronchial and epithelium, choroid plexus, articular chondrocytes, subchondral osteoblasts and synovium. AQP-3 has been proposed to transport water and small solutes such as glycerol and urea (9). Expression of AQP-1 and 3 demonstrate in equine articular chondrocytes using by immunohistochemistry, western blotting and quantitative flow cytometry (10). As it is obvious from above studies, AQP-1 and 3 were extensively studied in many systems. However, according to our knowledge this study is the first demonstrating the decreased production of AQP-3 in older IVDs of rats. This could be an important observation because it indicates a potential therapeutic target in the management of degenerative disease of the intervertebral disc.
A lots of study were demonstrated the number and function of AQPs in different tissues with aging (11, 19, 20). The gene expression and protein level changing of AQ4 in the cochlea and inferior colliculus (21), AQP2-3 in kidney (19, 20), and AQP5 in parotid glands (22) were demonstrated in previous studies. In addition to these studies, Wang and Zhu also demonstrated changes of AQP-1 expression with aging in IVDs of rabbits (6). Although all of these studies show the role of AQPs with aging, AQP-1 expression together with AQP-3 in older IVDs of rat gives additional information about the possible roles of AQP-3 in IVDs of rats.
The localization of AQP-1 and AQP-3 was demonstrated in the NP and inner AF of IVD using by immunohistochemistry (2). However, the authors found lacked expression of AQP-1 and very low AQP-3 expression in the outer AF. Our results showed similar results; interestingly we also detected the expression of AQP-1 and AQP-3 in the outer layer AF. This can be explained with antibody specificity, different protocols or tissues since our experiments were performed with different antibodies.
Conclusion
In the present study, we examined the expression of AQP-1 and AQP-3 in 2-month-old and 18-month-old IVDs of rats. AQP-1 and AQP-3 might have significant roles for the development of the age-related IVD degeneration. Further functional studies need to clarify the main role of AQP-1 and AQP-3 in IVD pathogenesis. Ongoing experiments within our research group are focused on examining the other AQPs and their roles within the intervertebral discs using cell culture and Western blotting.
Footnotes
This study presented in the 4th International Symposium of Clinical and Applied Anatomy in Ankara between 28 June–1 July 2012.
Conflict of Interest
No conflict of interest was declared by the authors.
References
- 1.Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–7. doi: 10.1136/mp.55.2.91. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson SM, Knowles R, Marples D, Hoyland JA, Mobasheri A. Aquaporin expression in the human intervertebral disc. J Mol Histol. 2008;39:303–9. doi: 10.1007/s10735-008-9166-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 3.Iatridis JC, MacLean JJ, O’Brien M, Stokes IA. Measurements of proteoglycan and water content distribution in human lumbar intervertebral discs. Spine (Phila Pa 1976) 2007;32:1493–7. doi: 10.1097/BRS.0b013e318067dd3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Podichetty VK. The aging spine: the role of inflammatory mediators in intervertebral disc degeneration. Cell Mol Biol. 2007;53:4–18. [PubMed] [Google Scholar]
- 5.Lee CR, Sakai D, Nakai T, Toyama K, Mochida J, Alini M, et al. A phenotypic comparison of intervertebral disc and articular cartilage cells in the rat. Eur Spine J. 2007;16:2174–85. doi: 10.1007/s00586-007-0475-y. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Zhu Y. Aquaporin-1: a potential membrane channel for facilitating the adaptability of rabbit nucleus pulposus cells to an extracellular matrix environment. J Orthop Sci. 2011;16:304–12. doi: 10.1007/s00776-011-0055-1. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 7.King LS, Yasui M, Agre P. Aquaporins in health and disease. Mol Med Today. 2000;6:60–5. doi: 10.1016/s1357-4310(99)01636-6. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 8.Agre P, Kozono D. Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett. 2003;555:72–8. doi: 10.1016/s0014-5793(03)01083-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 9.Mobasheri A, Wray S, Marples D. Distribution of AQP-2 and AQP-3 water channels in human tissue microarrays. J Mol Histol. 2005;36:1–14. doi: 10.1007/s10735-004-2633-4. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 10.Mobasheri A, Trujillo E, Bell S, Carter SD, Clegg PD, Martín-Vasallo P, et al. Aquaporin water channels AQP-1 and AQP-3, are expressed in equine articular chondrocytes. Vet J. 2004;168:143–50. doi: 10.1016/j.tvjl.2003.08.001. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 11.Li J, Tang H, Hu X, Chen M, Xie H. Aquaporin-3 gene and protein expression in sun-protected human skin decreases with skin ageing. Australas J Dermatol. 2010;51:106–12. doi: 10.1111/j.1440-0960.2010.00629.x. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 12.Sati L, Seval-Celik Y, Demir R. Lung surfactant proteins in the early human placenta. Histochem Cell Biol. 2010;133:85–93. doi: 10.1007/s00418-009-0642-9. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 13.Cayli S, Eyibilen A, Gurbuzler L, et al. Jab1 expression is associated with TGF-beta1 signaling in chronic rhinosinusitis and nasal polyposis. Acta Histochem. 2011;114:12–7. doi: 10.1016/j.acthis.2011.01.005. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 14.Roberts S, Evans H, Trivedi J, Menage J. Histology and pathology of the human intervertebral disc. J Bone Joint Surg Am. 2006;88:10–4. doi: 10.2106/JBJS.F.00019. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 15.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976) 1995;20:1307–14. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Massey CJ, van Donkelaar CC, Vresilovic E, Zavaliangos A, Marcolongo M. Effects of aging and degeneration on the human intervertebral disc during the diurnal cycle: A finite element study. J Orthop Res. 2012;30:122–8. doi: 10.1002/jor.21475. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 17.Massey CJ, van Donkelaar CC, Vresilovic E, Zavaliangos A, Marcolongo M. Effects of aging and degeneration on the human intervertebral disc during the diurnal cycle: A finite element study. J Orthop Res. 2010;30:122–8. doi: 10.1002/jor.21475. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 18.Mobasheri A, Marples D. Expression of the AQP-1 water channel in normal human tissues: a semiquantitative study using tissue microarray technology. Am J Physiol Cell Physiol. 2004;286:C529–37. doi: 10.1152/ajpcell.00408.2003. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 19.Combet S, Gouraud S, Gobin R, Berthonaud V, Geelen G, Corman B, et al. Aquaporin-2 downregulation in kidney medulla of aging rats is posttranscriptional and is abolished by water deprivation. Am J Physiol Renal Physiol. 2008;294:F1408–14. doi: 10.1152/ajprenal.00437.2007. [CrossRef] [DOI] [PubMed] [Google Scholar]
- 20.Preisser L, Teillet L, Aliotti S, Gobin R, Berthonaud V, Chevalier J, et al. Downregulation of aquaporin-2 and -3 in aging kidney is independent of V(2) vasopressin receptor. Am J Physiol Renal Physiol. 2000;279:F144–52. doi: 10.1152/ajprenal.2000.279.1.F144. [DOI] [PubMed] [Google Scholar]
- 21.Christensen N, D’Souza M, Zhu X, Frisina RD. Age-related hearing loss: aquaporin 4 gene expression changes in the mouse cochlea and auditory midbrain. Brain Res. 2009;1253:27–34. doi: 10.1016/j.brainres.2008.11.070. [CrossRef] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue N, Iida H, Yuan Z, Ishikawa Y, Ishida H. Age-related decreases in the response of aquaporin-5 to acetylcholine in rat parotid glands. J Dent Res. 2003;82:476–80. doi: 10.1177/154405910308200614. [CrossRef] [DOI] [PubMed] [Google Scholar]