Abstract
Background:
Bacterial Translocation is believed to be an important factor on mortality and morbidity in Obstructive Jaundiced.
Aims:
We investigated the probable or estimated positive effects of tauroursodeoxycholic acid, which has antibacterial and regulatory effects on intestinal flora, together with glutamine on BT in an experimental obstructive jaundiced rat model.
Study Design:
Animal experimentation.
Methods:
Forty adult, male, Sprague Dawley rats were used in this study. Animals were randomised and divided into five groups of eight each: sham (Sh); control (common bile duct ligation, CBDL); and supplementation groups administered tauroursodeoxycholic acid (CBDL+T), glutamine (CBDL+G), or tauroursodeoxycholic acid plus glutamine (CBDL+TG). Blood and liver, spleen, MLN, and ileal samples were taken via laparotomy under sterile conditions for investigation of bacterial translocation and intestinal mucosal integrity and hepatic function tests on the tenth postoperative day.
Results:
There were statistically significant differences in BT rates in all samples except the spleen of the CBDL+TG group compared with the CBDL group (p=0.041, p=0.026, and p=0.041, respectively).
Conclusion:
It is essential to protect hepatic functions besides maintaining intestinal mucosal integrity in the active struggle against BT occurring in obstructive jaundice. The positive effect on intestinal mucosal integrity can be increased if glutamine is used with tauroursodeoxycholic acid, which also has hepatoprotective and immunomodulatory features.
Keywords: Bacterial translocation, glutamine, tauroursodeoxycholic acid
Introduction
Despite current modern diagnostic and therapeutic approaches, obstructive jaundice is still a clinical entity with up to 56% morbidity and 10–25% mortality rates due to septic complications. Two points play a key role in the pathophysiology of septic complications. One is physical disruption of the intestinal barrier function and the other is an impaired immune system, especially the reticuloendothelial system (RES) and metabolic functions of the liver because of cholestasis (1–9).
Cholestasis emerging in biliary obstruction is characterised by the accumulation of toxic substances, especially hydrophobic bile acids in hepatocytes and bile trunks, owing to disability in the secretion of bile into the intestine. The products eliminated via bile such as bilirubin increase in the systemic circulation and tissues (10, 11).
A healthy gut barrier prevents the spread of intraluminal bacteria and endotoxins to the extra-intestinal tissues, and portal and systemic circulation (1, 9). However, significant structural and functional changes in the gut such as increased intestinal permeability appear in biliary obstruction. Eventually, gut barrier function failure and disappearance of antibacterial and detergent functions of bile acids due to failure of their enterohepatic circulation cause alterations that lead to bacterial translocation (BT) (6, 11–14). BT is described as the passage of viable intestinal bacteria and their products from the gut barrier to normally sterile extra-intestinal tissues such as the mesenteric lymph nodes (MLNs), liver, spleen, and bloodstream (6, 7, 15–17). In biliary obstruction, the infection starts as a slight cholangitis and can turn into sepsis rapidly because BT is usually caused by Gram-negative bacteria. At the end of this process, systemic inflammatory response syndrome, multiple organ failure, and even death may result (11). Therefore, from the moment the biliary obstruction diagnosis is made, it is important to begin supportive treatment as soon as possible to prevent BT in patients with obstructive jaundice.
Bile acids are known to inhibit the proliferation of intestinal bacteria and may conduce to the regulation of intestinal flora (18). Tauroursodeoxycholic acid (TUDCA), which is the taurine conjugate of ursodeoxycholic acid (UDCA), is a cytoprotective bile acid frequently used to remedy cholestatic disorders to protect hepatocytes from apoptosis induced by a variety of agents, such as hydrophobic bile acids. The cytotoxicity of a bile acid decreases with an increase of its hydrophilicity. Therefore, TUDCA, which is considerably more hydrophilic than UDCA, displaces and dilutes endogenous hydrophobic bile acids (19–22).
Over the past 25 years, a great number of products that improve gut barrier function to avoid BT have been investigated. The most investigated product is glutamine, which is the main energetic substrate for rapidly proliferating cells such as enterocytes and lymphocytes. Its plasma concentration reduces during catabolic conditions such as biliary sepsis. Sepsis damages intestinal glutamine metabolism and debilitates the gut mucosal barrier. The gut barrier is a complex of mechanisms such as peristalsis, mucus, microflora, bile acids, and gut-associated lymphoid tissue (GALT). Glutamine is a potent immune stimulator that preserves gut mucosal integrity and supports the host immune system (14, 18, 23–28). Glutamine also plays a major role in enterocyte oxidative metabolism by counteracting glutathione depletion, as it supports intestinal glutathione biosynthesis. Glutamine deficiency may contribute to glutathione depletion. At the same time, glutathione has been demonstrated to influence the protection of intestinal mucosal integrity (26, 29).
Eventually, supporting the gut lumen with nutrients, which strengthens intestinal barrier function, is very important to obtaining normal intestinal mucosal structure and function during catabolic situations. There is no research in the literature demonstrating a relationship between TUDCA and BT from extra-hepatic biliary obstruction. The aim of this study was to investigate the probable or estimated beneficial effects of TUDCA and glutamine together on BT and intestinal mucosal villus integrity in an experimental model of obstructive jaundice.
Material and Methods
This experimental study was approved by the Trakya University Animal Ethics Committee.
Experimental animals and groups
Forty healthy male Sprague Dawley rats, weighing 200 to 250 g and having the same biological and physiological properties, were provided by the Experimental Research Center of the Medical Faculty of Trakya University. All rats were housed in stainless steel cages and under standardised laboratory conditions of temperature 21±2°C, relative humidity 50–60%, and 12h dark/light cycles. They were fed with standard rat chow and fresh tap water ad libitum. All animals were exposed to human care appropriate to the criteria outlined in the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. A total of 40 male Sprague Dawley rats were divided into five groups containing eight rats each: sham (Sh); control (common bile duct ligation, CBDL); and CBDL plus TUDCA supplementation (CBDL+T), CBDL plus glutamine supplementation (CBDL+G), and CBDL plus supplementation with both TUDCA and glutamine (CBDL+TG).
Experimental procedures
All procedures were performed under a heat lamp and the rats’ body temperatures were kept at 35–36°C constantly. The same surgeon conducted all operations with the same technique. Foods were removed 12 hours before anaesthesia, and animals were released only for water 2 hours before anaesthesia. The rats were anaesthetised with 90 mg/ kg body weight ketamine hydrochloride (Ketalar® flk; Pfizer, İstanbul, Turkey) plus 10 mg/kg body weight xylazine (Rompun®; Bayer, İstanbul, Turkey) intramuscularly. The abdomen was shaved and disinfected with 10% povidone iodine solution before the operation. The common bile duct (CBD) was detected through a 3-cm midline abdominal incision in rats under sterile conditions. In the Sh group, rats underwent incision of the abdominal cavity and only dissection of the CBD without ligation. In the other groups, obstructive jaundice was achieved by a double ligation with 5/0 silk and transection of the CBD in the supraduodenal region between the lower most tributary of the bile duct and the upper most tributary of the pancreatic duct. The operation was finished by closing the midline incision, fascia, and skin separately by continuously absorbable 4/0 polyglactin sutures (Vicryl®; Ethicon, Johnson & Johnson Company, USA) or by using 3/0 atraumatic silk sutures (Sterisilk®; SSM, İstanbul, Turkey). After surgical intervention, all the rats were separated into groups and placed in special cages under controlled conditions.
TUDCA and glutamine were administered once a day to the supported groups. TUDCA (Taurolite®; Biogen, Ankara, Turkey) was administered to the CBDL+T and CBDL+TG groups for 10 days, starting 12 hours following CBD ligation at a daily dose of 10 mg/kg via the orogastric route (o.g.) with a 7 gauge feeding tube. Glutamine (Resource® Glutamin; Nestle Nutrition, İstanbul, Turkey) was administered to the CBDL+G and CBDL+TG groups for 10 days at a daily dose of 1/g/kg via o.g. under the same conditions. Standard rat chow was also given to these three groups as in the Sh and CBDL groups. The animals were sacrificed on the tenth postoperative day. After opening the abdominal cavity, samples from the liver, MLNs, spleen, and tissue of the terminal ileum and blood samples were collected from all rats with sterile instruments under aseptic conditions for microbiological, biochemical, and histopathological investigation.
Microbiological and biochemical analyses
Direct serum bilirubin (DB), alanine transaminase (ALT), and gamma-glutamyl transferase (GGT) values were measured as parameters indicative of hepatic function using standard biochemical techniques on the tenth postoperative day. ALT and GGT results are expressed as IU/L. DB levels are expressed as mg/dL.
Priority was given to blood culture in order to prevent the contamination of 5–7 mL blood samples taken by cardiac puncture. The blood samples taken were put into aerobic and anaerobic culture flasks and incubated for a maximum of 7 days in the microbiological culture analyzer (Bact/ Alert®;Biomerieux, Marcy l’Etoile, France) blood culture device. When a reproduction signal was received, they were transferred to their appropriate media and incubated at 37°C in aerobic and anaerobic environments. Bacteria reproducing in the media were named with conventional methods and automatized identification system (VITEK 2®; Biomerieux, Marcy l’Etoile, France).
Two millilitre samples of blood, which were residual from the first blood collection on the tenth postoperative day, were used to measure the levels of serum DB, ALT, and GGT. After the tissue samples (MLNs, liver, and spleen) were removed in sterile conditions, approximately 1 g tissue was pulverised in a sterile mortar with addition of 1 mL thioglycollate, and homogenised. Subsequently, 0.01 mL was taken from this homogenate and planted in suitable media. After transfer to media, incubation was continued in aerobic and anaerobic environment at 37°C for 72 hours. Reproducing colonies were counted and the density of bacteria in the tissue was calculated as CFU/gram. Bacteria reproducing in the media were named with conventional methods and the VITEK 2 (Biomerieux, France) automatised identification system. Microbiological data were evaluated by a microbiologist who was blinded to the study design.
Histopathological evaluation
All of the terminal ileum samples obtained after sacrificing of the animals were fixed in 10% neutral buffered formalin solution for histological evaluation. Later, they were embedded in paraffin, and sections of 5µm thickness were cut and stained with haematoxylin-eosin (HE). Then, the specimens were examined under a light microscope. Histopathological examinations were performed by a pathologist who was blinded to the study design and photographs were taken with Zeiss Axioplan 2 imaging and Nikon E600. In all groups, the number of villi per centimetre (V/cm) and the total mucosal thickness (in µm) were assessed to examine histological structural changes in the terminal ileum. Mucosal thickness was measured in a minimum of 20 well-preserved villi in each randomly selected sample from each tissue block.
Statistical analysis
Numerical values are expressed as mean±standard deviation (SD). The differences among groups were evaluated by Kruskal Wallis variance analysis. If the p-value was statistically significant after Bonferroni correction, pairwise comparisons were performed using Mann Whitney U test. Statistical significance was set at p<0.005. Univariate statistical analyses were performed using Fisher’s exact test (c2) for categorical variables. Statistical significance was set at p<0.05. All analyses were performed with the Statistical Package for Social Sciences ver. 15.0 (SPSS; IBM, Chicago, USA).
Results
Two rats from the CBDL+G group and one rat from the CBDL+T group died during the experiment. No new rat was added to replace the dead rats. Clinically jaundice consisted in all CBDL groups on the third postoperative day. Dilated CBDs were observed in all rats on the day of operation at the end of experiment.
Biochemical findings
DB, ALT, and GGT levels were evaluated on the tenth postoperative day. As expected, the results were normal in the Sh group. The distribution of variance in liver function tests for all the other groups is shown in Table 1. There was no significant difference between the CBDL and the study groups in terms of hepatic function tests, in spite of the values being lower in the CBDL+T and CBDL+TG groups. DB, ALT, and GGT levels were significantly elevated in the study and control groups compared with the sham group (p<0.01).
Table 1.
The biochemical parameters for each group (mean±standard deviation)
| Variants | Sh (n=8) | CBDL (n=8) | CBDL+T (n=7) | CBDL+G (n=6) | CBDL+TG (n=8) | pa |
|---|---|---|---|---|---|---|
| DB | 0.03±0.02 | 6.48±1.24† | 6.14±1.64† | 6.58±1.71† | 6.18±2.18† | <0.001 |
| ALT | 59.50±5.42 | 145.13±23.53† | 132.28±34.07† | 154.16±37.76† | 132.13±23.50† | <0.001 |
| GGT | 3.63±0.74 | 15.25±2.82† | 13.29±2.81† | 17.17±6.49† | 14.88±2.95† | <0.001 |
DB: direct bilirubin (mg/dL); ALT: alanine transaminase (IU/L); GGT: gamma-glutamyl transferase (IU/L); Sh: sham group; CBDL: common bile duct ligation control group; CBDL+T: tauroursodeoxycholic acid -treated group;
CBDL+G: glutamine-treated group; CBDL+TG: tauroursodeoxycholic acid plus glutamine-treated group
Kruskal Wallis test
Mann Whitney U test. The difference vs Sh is statistically significant (p<0.005)
Microbiological findings
The most commonly isolated bacterium among all positive cultures was Escherichia coli (58.53%). The other identified microorganisms were Enterococcus faecalis (41.46%). Thirty-eight growths of bacteria in quantitative culture occurred in 37 rats. Proliferation of both E. coli and E. faecalis was found in four cultures. Anaerobic reproduction was not detected. The BT rates of the groups and statistically values are shown in Table 2. There was no BT in any of the specimens in the Sh group. The rate of BT in the CBDL group was higher than in the other groups. There were statistically significant differences in BT rates in the liver, MLNs, and blood samples, except in the spleen of group CBDL+TG compared with group CBDL (p=0.041, p=0.026, and p=0.041, respectively). In the CBDL+G group, only the BT rate in MLNs was decreased significantly compared with the CBDL group (p=0.031), in spite of the BT rate being lower in the other samples. There was no statistically significant difference between the CBDL+T and CBDL groups in spite of the BT rate being lower in group CBDL+T (p>0.05).
Table 2.
Bacterial translocation rates of the groups [mean (percentage)]
| Variants | Sh (n=8) | CBDL (n=8) | CBDL+T (n=7) | CBDL+G (n=6) | CBDL+TG (n=8) |
|---|---|---|---|---|---|
| Blood | 0 (0%) | 6 (75.0%)† | 2 (28.6%) | 2 (33.3%) | 1 (12.5%)‡ |
| Liver | 0 (0%) | 6 (75.0%)† | 2 (28.6%) | 2 (33.3%) | 1 (12.5%)‡ |
| Spleen | 0 (0%) | 5 (62.5%)† | 2 (28.6%) | 1 (16.7%) | 1 (12.5%) |
| MLNs | 0 (0%) | 5 (62.5%)† | 2 (28.6%) | 0 (0%)‡ | 0 (0%)‡ |
MLNs: mesenteric lymph nodes; Sh: sham group; CBDL: common bile duct ligation control group; CBDL+T: tauroursodeoxycholic acid -treated group; CBDL+G: glutamine-treated group; CBDL+TG: tauroursodeoxycholic acid plus glutamine-treated group
p values(χ2)
The difference vs Sh is statistically significant (p<0.05)
The difference vs CBDL is statistically significant (p<0.05)
Histopathological findings
Histopathological examination (Figure 1) showed that the main structure of the mucosa in the terminal ileum specimens was normal in all the Sh group rats. The mean number of villi per centimetre and mucosal thickness of the groups are presented at length in Table 3. The mean number of villi per centimetre (Figure 2) and mucosal thickness (Figure 3) were decreased the most in the CBDL group. The mean number of villi per centimetre in ileal specimens were increased significantly only in the CBDL+TG group compared with the sham group (p=0.008). In contrast, there were significant increments in all the study groups compared with group CBDL (p=0.047, p=0.033, and p=0.002, respectively). When we evaluated the mean mucosal thickness, the increment was not significant in the CBDL+G group compared with the CBDL group (p=0.061), but the mean mucosal thickness in ileal specimens were increased significantly in the CBDL+TG group compared with the CBDL group (p=0.015).
Figure 1.
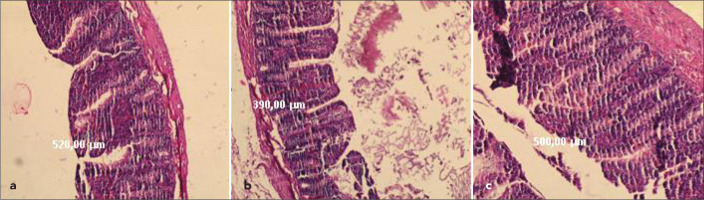
a–c. Hematoxylin-eosin stained sections (HE×50) of tissue samples from terminal ileum of the animals showing that the total mucosal thicknes and the number of the villi per cm. (a) CBDL+TG rats administered Tauroursodeoxycholic acid plus Glutamin. (b) CBDL (Control) rats showing damaged mucosal construction and (c) sham-operated rats illustrating a normal mucosal construction
Table 3.
Mean number of villi per cm and mean height of mucosa in μm (mean±standard deviation)
| Variants | Sh (n=8) | CBDL (n=8) | CBDL+T (n=7) | CBDL+G (n=6) | CBDL+TG (n=8) | pa |
|---|---|---|---|---|---|---|
| Mean number of villi per cm | 65.00±7.56 | 57.50±10.35 | 70.00±10.00‡ | 73.33±12.11‡ | 77.50±7.07†‡ | < 0.007 |
| Mean mucosal thickness | 482.50±72.26 | 381.25±93.87 | 401.43±19.52 | 471.66±30.60 | 497.50±57.50‡ | < 0.003 |
Sh: sham group; CBDL: common bile duct ligation control group; CBDL+T: tauroursodeoxycholic acid- treated group; CBDL+G: glutamine-treated group; CBDL+TG: tauroursodeoxycholic acid plus glutamine-treated group
Kruskal Wallis test
Mann Whitney U test. The difference vs Sh is statistically significant (p<0.005)
Mann Whitney U test. The difference vs CBDL is statistically significant (p<0.005)
Figure 2.
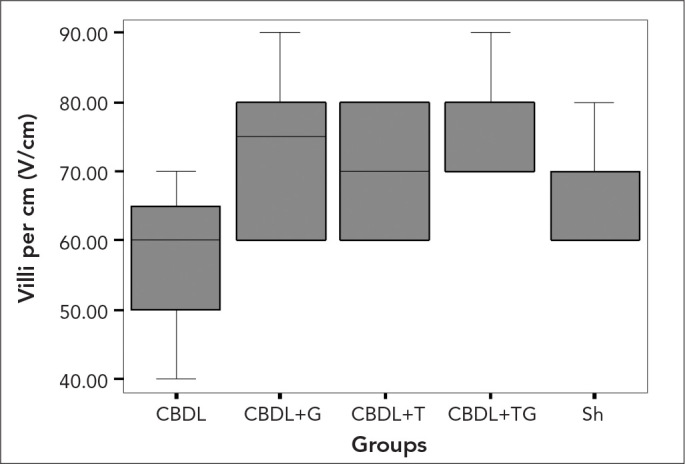
The values of the mean number of villi per centimeter in the experimental groups. Kruskal Wallis test, p=0.007
Figure 3.
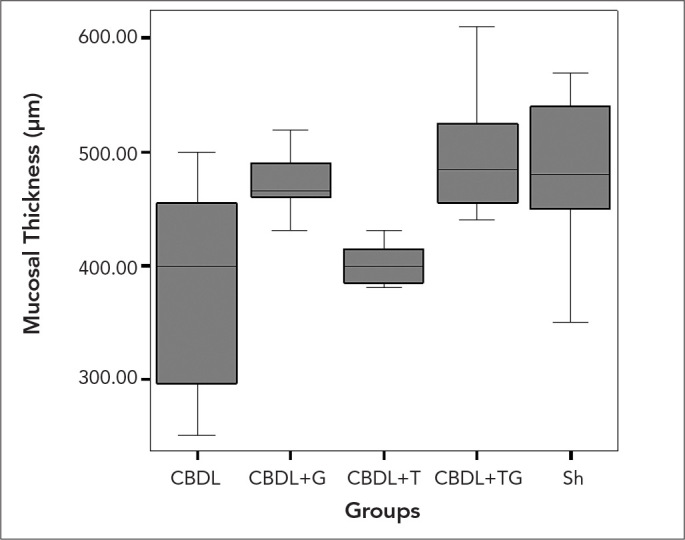
The values of the mean mucosal thickness in the experimental groups. Kruskal Wallis test, p=0.003
Discussion
In biliary tract obstruction, hepatocyte damage occurs as a result of the toxic effect of bile acids in the liver due to cholestasis. Clinically, it leads to an increase in liver function tests (11, 17). UDCA and TUDCA lead to an improvement in liver function tests. However, regression in bilirubin and GGT values occur at a later period than the regression in ALT levels (30). ALT levels were measured as a marker of hepatocellular damage, and GGT and DB levels were measured as markers of cholestasis.
Ishizaki et al. (21) speculated that UDCA and its taurine conjugate might have a marked effect on the liver damage observed in obstructive jaundiced rats. In contrast, Zimmermann and Reichen (31) reported that bile acids such as UDCA had no marked effect on liver damage and dysfunction in experimental bile duct ligation in rats. Therefore, there are contradictory studies in the literature about the protective effect of bile acids on the liver dysfunction seen in obstructive jaundiced rats. Aldemir et al. (18) reported that there were no significant changes in bilirubin, ALT, and GGT levels in groups to which they applied glutamine and UDCA separately, whereas in a similar study, Bildik et al. (11) reported that a significant reduction occurred in these parameters. Karatepe et al. (10) detected a significant reduction in ALT and GGT levels apart from bilirubin in the group to which they applied glutamine support. In our study, in the groups where TUDCA was applied individually and TUDCA support was provided with glutamine, the reduction in hepatic function tests relative to the control group was not as statistically significant as the results of the study of Aldemir et al. (18).
The physical barrier function of the intestinal mucosa is one of the major factors in preventing and inspecting BT (8). The gut barrier is a compound that consists of ingredients such as intestinal flora, bile acids, and GALT (32). In obstructive jaundice, gut barrier failure stems from increased intestinal permeability and translocation due to impaired immune cell function within the intestinal wall and GALT (33). The other important factor is a repressed host immune system. In cholestasis emerging after biliary obstruction, the RES seems to be ruined and phagocytic function is depressed. Kupffer cell activation plays a basic role at the beginning of an efficacious immune response (5, 8, 11, 17).
UDCA is found at a lower rate than 3% in the human bile acid reserve and is formed from chenodesoxycholic acid, which is a primary bile acid with an effect on colon bacteria. Transferring the hydroxyl group from the α position to the β position leads to the transformation of a hydrophobic bile acid to a hydrophilic condition and it becoming less toxic. TUDCA formed with the taurine conjugation of UDCA has a more hydrophilic structure. Accumulation of non-toxic hydrophilic bile acids in hepatocytes causes a damage-reducing effect. UDCA and TUDCA used as a choleretic agent try to limit the toxic effects of hydrophobic bile acids, enabling the stabilisation of hepatocyte membrane, with a hepatoprotective effect. Healthy hepatocytes do not express HLA class 1 or 2 antigens. However, in the presence of cholestasis, HLA class 1 antigen expression and subsequently cytotoxic T cell-dependent hepatocyte destruction occur. In vitro studies suggest that hydrophilic bile acids inhibit HLA class 1 antigen expression and reduce the damage to minimum. Furthermore, they obstruct the absorption of hydrophobic endogenous bile acids by the terminal ileum and also have immunomodulatory effects (30). Non-toxic bile acids in the intestinal lumen have antibacterial, anti-adherence, and detergent effects. It has been asserted that increased absorption of bacterial endotoxins is related to the absence of the luminal flow of bile acids, which have lytic effects on intraluminal bacteria. The enterohepatic circulation of bile acids has also been considerable in the improvement of phagocytic function. In addition, bile debilitates the adherence of bacteria to enterocytes and catalyses the lysis of endotoxins (18).
Glutamine is primarily formed and stored in skeletal muscle and is the main metabolic energy source for rapidly proliferating cells such as enterocytes and immune cells. This amino acid is also necessary for a normal GALT function. It becomes an essential amino acid under stress and sepsis. Supplementary glutamine has been demonstrated to be a considerable dietary constituent for maintaining intestinal mucosal integrity. It increases intestinal villus height and stimulates gut mucosal cellular proliferation (7, 17, 34–36).
Glutamine prevents increased intestinal permeability and BT-related biliary sepsis in biliary obstruction (36). At the same time, bile acids such as UDCA and its taurine conjugate have efficacious effects on bile duct-ligated animals (21). Although there are no studies in the literature investigating the effect of TUDCA on BT, there are a few studies on UDCA. Different results were reported in studies investigating the positive effects of both glutamine and UDCA on BT. Aldemir et al. (18) detected a significant reduction of BT rates in the blood, with MLNs and spleen tissue samples in the groups separately supported with glutamine and UDCA. White et al. (26) detected a significant reduction only in liver tissue samples, whereas Karatepe et al. (10) detected a significant reduction only in the blood. In some studies, a significant reduction in BT rates was observed in the MLNs and liver tissue samples in the groups supported with glutamine (7, 9, 18). On the other hand, in the study of Bildik et al. (11) it was reported that there was a significant reduction in BT rates in the blood and MLN samples in the group supported with glutamine. Similar to the study of Bildik et al. (11), in our study there was a significant reduction only in MLN samples in the group supported with glutamine, but no significant difference was detected, although there was a reduction of BT rates in the blood and other tissue samples. On the other hand, in the group supported with TUDCA, no significant difference was detected, although there was a reduction in BT rates in the blood and all tissue samples. In contrast, in the group that was given TUDCA and glutamine together, BT rates were reduced by a significant level in the blood and other tissue samples, except for the spleen, although there was a reduction in BT rates in spleen samples. While glutamine by itself only caused a significant regression in the translocation occurring in the MLNs, when it was used with TUDCA, it caused a significant regression in the liver tissue samples as well as MLN samples. We think that this stems from the positive additive effects of both substrates and the hepatoprotective and immunomodulatory features of TUDCA.
Considering blood and all tissue samples, similar to other studies (10, 18) in the literature, the most frequently reproducing bacterium was E. coli in our study. Morphometric confirmation of ileal mucosal injury has been observed with a reduction in villus height and total mucosal thickness in obstructive jaundiced rats (37). The number of villi per centimetre and mucosal thickness measurements were evaluated in terminal ileum sections. In the literature, different results on mucosal integrity were reported in the studies on glutamine support. While in the study of White et al. (26) the increase in villus height was not significant, a significant increase was found in other studies (10, 18). In their study, Aldemir et al. (18) reported a significant increase in villus height in the group provided with UDCA support. In our study, in the groups that received supportive treatment, an increase was detected relative to the control group both in mucosal integrity and the number of villi per centimetre. However, while there was a significant increase only in the number of villi per centimetre in the groups separately supported with glutamine and TUDCA, a significant increase in both mucosal integrity and the number of villi was detected in the group provided with glutamine and TUDCA together. Thus, there was an additive effect on mucosal thickness when TUDCA and glutamine were applied together compared with the positive effect when they were applied individually.
In conclusion, it is essential to protect hepatic functions besides maintaining intestinal mucosal integrity in the active struggle against BT occurring in obstructive jaundice. The positive effect of glutamine on intestinal mucosal integrity can be increased if it is used with TUDCA, which also has hepatoprotective and immunomodulatory features.
Acknowledgments
The authors would like to thank Dr. Esin Seçkin Sayhan, for the help she has provided during the statistical analysis process.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author contributions: Concept – A.R.H., S.O.; Design – A.R.H., S.O.; Supervision – A.R.H., S.O., C.E.; Resource – A.R.H., S.O.; Materials – A.R.H., D.A., T.S.; Data Collection&/or Processing – A.R.H., S.O., D.A.; Analysis&/or Interpretation – Ş.G., T.Y.; Literature Search – S.O., D.A., Y.A.S.; Writing – S.O., A.R.H., D.A.; Critical Reviews – A.R.H., S.O., D.A.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study received no financial support.
References
- 1.Assimakopoulos SF, Vagi anos CE, Charonis A, Nikolopoulou VN, Scopa CD. Intestinal failure in obstructive jaundice. World J Gastroenterol. 2005;11:3806–7. doi: 10.3748/wjg.v11.i24.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assimakopoulos SF, Scopa CD, Vagianos CE. Pathophysiology of increased intestinal permeability in obstructive jaundice. World J Gastroenterol. 2007;13:6458–64. doi: 10.3748/wjg.v13.i48.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assimakopoulos SF, Thomopoulos KC, Patsoukis N, Georgiou CD, Scopa CD, Nikolopoulou VN, et al. Evidence for intestinal oxidative stress in patients with obstructive jaundice. Eur J Clin Invest. 2006;36:181–7. doi: 10.1111/j.1365-2362.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 4.Seki S, Habu Y, Kawamura T, Takeda K, Dobashi H, Ohkawa T, et al. The liver as a crucial organ in the first line of host defense:the roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol Rev. 2000;174:35–46. doi: 10.1034/j.1600-0528.2002.017404.x. [DOI] [PubMed] [Google Scholar]
- 5.Andersson R, Andersson B, Andersson E, Eckerwall G, Norden M, Tingstedt B. Immunomodulation in surgical practice. HPB. 2006;8:116–23. doi: 10.1080/13651820410016660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sileri P, Morini S, Sica GS, Schena S, Rastellini C, Gaspari AL, et al. Bacterial translocation and intestinal morphological findings in jaundiced rats. Dig Dis Sci. 2002;47:929–34. doi: 10.1023/a:1014733226337. [DOI] [PubMed] [Google Scholar]
- 7.Zulfikaroglu B, Zulfikaroglu E, Ozmen MM, Ozalp N, Berkem R, Erdogan S, et al. The effect of immunonutrition on bacterial translocation, and intestinal villus atrophy in experimental obstructive jaundice. Clinical Nutrition. 2003;22:277–81. doi: 10.1016/s0261-5614(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 8.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–91. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 9.Margaritis VG, Filos KS, Michalaki MA, Scopa CD, Spiliopoulou I, Nikolopoulou VN, et al. Effect of oral glutamine administration on bacterial tanslocation, endotoxemia, liver and ileal morphology, and apoptosis in rats with obstructive jaundice. World J Surg. 2005;29:1329–34. doi: 10.1007/s00268-005-7721-4. [DOI] [PubMed] [Google Scholar]
- 10.Karatepe O, Acet E, Battal M, Adas G, Kemik A, Altiok M, et al. Effects of glutamine and curcumin on bacterial translocation in jaundiced rats. World J Gastroenterol. 2010;16:4313–20. doi: 10.3748/wjg.v16.i34.4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bildik N, Cevik A, Kadioglu B, Ekinci H, Altıntas M, Dalkilic G, et al. The effects of ursodeoxycholic acid and glutamine on bacterial translocation, liver functions and hepatic histopathology in rats with obstructive jaundice. Kartal Eğitim ve Araştırma Hastanesi Tıp Dergisi. 2009;20:13–21. [Google Scholar]
- 12.Assimakopoulos SF, Vagianos CE, Patsoukis N, Georgiou C, Nikolopoulou V, Scopa CD. Evidence for intestinal oxidative stress in obstructive jaundice-induced gut barrier dysfunction in rats. Acta Physiol Scand. 2004;180:177–85. doi: 10.1046/j.0001-6772.2003.01229.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaya O, Ozdemir F, Atlı M, Aslan V, Cagatay M, Anlar M, et al. The effects of ciprofloxacin and ursodeoxycholic acid on bacterial translocation in obstructive jaundice. Turk J Gastroenterol. 2009;20:186–91. doi: 10.4318/tjg.2009.0005. [DOI] [PubMed] [Google Scholar]
- 14.Sheen-Chen SM, Hung KS, Ho HT, Chen WJ, Eng HL. Effect of glutamine and bile acid on hepatocyte apoptosis after bile duct ligation in the rat. World J Surg. 2004;28:457–60. doi: 10.1007/s00268-004-7189-7. [DOI] [PubMed] [Google Scholar]
- 15.Steinberg SM. Bacterial translocation: what it is and what it is not. Am J Surg. 2003;186:301–5. doi: 10.1016/s0002-9610(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 16.Abdeldayem H, Ghoneim E, Ahmad-El Refaei A, Abou-Gabal A. Obstructive jaundice promotes intestinal-barrier dysfunction and bacterial translocation: experimental study. Hepatol Int. 2007;1:444–8. doi: 10.1007/s12072-007-9018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuru B, Dinc S, Altinok G, Aksoz T, Camlibel M, Gulcelik MA, et al. Effect of Different Enteral Nutrients on Bacterial Translocation in Experimental Obstructive Jaundice. Eur Surg Res. 2004;36:45–52. doi: 10.1159/000075074. [DOI] [PubMed] [Google Scholar]
- 18.Aldemir M, Geyik MF, Kökoğlu OF, Büyükbayram H, Hoşoğlu S, Yağmur Y. Effects of ursodeoxycholic acid, glutamine and polyclonal immunoglobulins on bacterial translocation in common bile duct ligated rats. ANZ J Surg. 2003;73:722–6. doi: 10.1046/j.1445-2197.2003.02749.x. [DOI] [PubMed] [Google Scholar]
- 19.Setchell KDR, Rodrigues CMP, Podda M, Crosignani A. Metabolism of orally administered tauroursodeoxycholic acid in patients with primary biliary cirrhosis. Gut. 1996;38:439–46. doi: 10.1136/gut.38.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benz C, Angermuller S, Otto G, Sauer P, Stremmel W, Stiehl A. Effect of tauroursodeoxycholic acid on bile acid-induced apoptosis in primary human hepatocytes. Eur J Clin Invest. 2000;30:203–9. doi: 10.1046/j.1365-2362.2000.00615.x. [DOI] [PubMed] [Google Scholar]
- 21.Ishizaki K, Kinbara S, Miyazawa N, Takeuchi Y, Hirabayashi N, Kasai H, et al. Effect of sodium tauroursodeoxycholate UR-906 on liver dysfunction in bile duct-ligated rats. Eur J Pharmacol. 1997;333:207–13. doi: 10.1016/s0014-2999(97)01143-6. [DOI] [PubMed] [Google Scholar]
- 22.Larghi A, Crosignani A, Battezzati PM, De Valle G, Allocca M, Invernizzi P, et al. Ursodeoxycholic and tauroursodeoxycholic acids for the treatment of primary biliary cirrhosis: a pilot crossover study. Aliment Pharmacol Ther. 1997;11:409–14. doi: 10.1046/j.1365-2036.1997.124295000.x. [DOI] [PubMed] [Google Scholar]
- 23.Salvalaggio PRO, Campos ACL. Bacterial Translocation and Glutamine. Nutrition. 2002;18:435–7. doi: 10.1016/s0899-9007(02)00770-0. [DOI] [PubMed] [Google Scholar]
- 24.Wiest R, Rath HC. Gastrointestinal disorders of the critically ill. Bacterial translocation in the gut. Best Pract Res Clin Gastroenterol. 2003;17:397–425. doi: 10.1016/s1521-6918(03)00024-6. [DOI] [PubMed] [Google Scholar]
- 25.White JS, Hoper M, Parks RW, Clements WD, Diamond T. Glutamine improves intestinal barrier function in experimental biliary obstruction. Eur Surg Res. 2005;37:342–7. doi: 10.1159/000090334. [DOI] [PubMed] [Google Scholar]
- 26.Belmonte L, Coëffier M, Le Pessot F, Miralles-Barrachina O, Hiron M, Leplingard A, et al. Effects of glutamine supplementation on gut barrier, glutathione content and acute phase response in malnourished rats during inflammatory shock. World J Gastroenterol. 2007;13:2833–40. doi: 10.3748/wjg.v13.i20.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro SR, Junior PEP, Miranda AC, Bromberg SH, Lopasso FP, Irya K. Weight loss and morphometric study of intestinal mucosa in rats after massive intestinal resection. Influence of a glutamine-enriched diet. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:349–56. doi: 10.1590/s0041-87812004000600007. [DOI] [PubMed] [Google Scholar]
- 28.Roth E. Nonnutritive Effects of Glutamine. J Nutr. 2008;138:2025–31. doi: 10.1093/jn/138.10.2025S. [DOI] [PubMed] [Google Scholar]
- 29.Santos RGC, Viana ML, Generoso SV, Arantes RE, Correia MITD, Cardoso VN. Glutamine supplementation decreases intestinal permeability and preserves gut mucosa integrity in an experimental mouse model. JPEN J Parenter Enteral Nutr. 2010;34:408–13. doi: 10.1177/0148607110362530. [DOI] [PubMed] [Google Scholar]
- 30.Aydogdu S. Kolestazda Medikal Tedavi. The Journal of Current Pediatrics. 2006;4:125–32. [Google Scholar]
- 31.Zimmermann H, Reichen J. Ursodeoxycholate has no beneficial effect on liver function or histology in biliary cirrhosis in the rat. J Hepatol. 1992;16:355–9. doi: 10.1016/s0168-8278(05)80669-5. [DOI] [PubMed] [Google Scholar]
- 32.MacFie J. Enteral versus parenteral nutrition:the significance of bacterial translocation and gut barrier function. Nutrition. 2000;16:606–11. doi: 10.1016/s0899-9007(00)00249-5. [DOI] [PubMed] [Google Scholar]
- 33.Welsh FK, Ramsden CW, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, et al. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg. 1998;227:205–12. doi: 10.1097/00000658-199802000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudsk KA. Immunonutrition in surgery and critical care. Annu Rev Nutr. 2006;26:463–79. doi: 10.1146/annurev.nutr.26.061505.111230. [DOI] [PubMed] [Google Scholar]
- 35.Alexander JW. Nutritional pharmacology in surgical patients. Am J Surg. 2002;183:349–52. doi: 10.1016/s0002-9610(02)00807-3. [DOI] [PubMed] [Google Scholar]
- 36.Miller AL. Therapeutic considerations of L-glutamine: a review of the literature. Altern Med Rev. 1999;4:239–48. [PubMed] [Google Scholar]
- 37.Parks RW, Stuart Cameron CH, Gannon CD, Pope C, Diamond T, Rowlands BJ. Changes in gastrointestinal morphology associated with obstructive jaundice. J Pathol. 2000;192:526–32. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH787>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]


