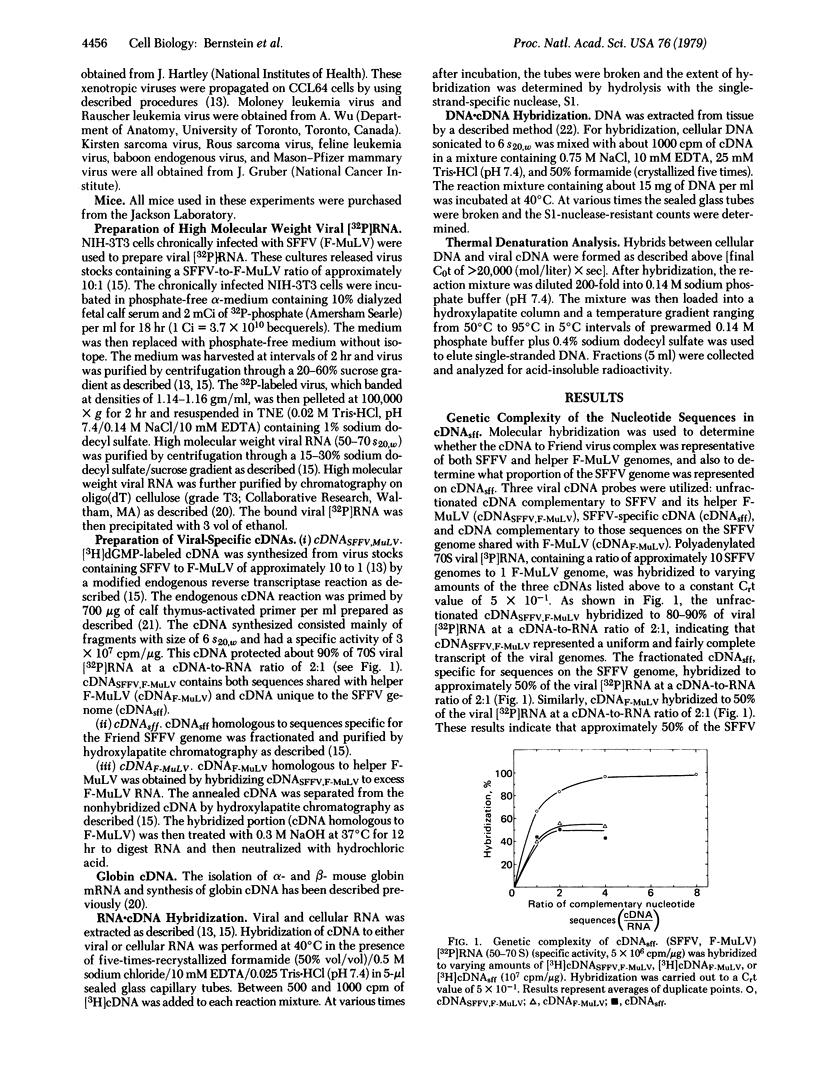
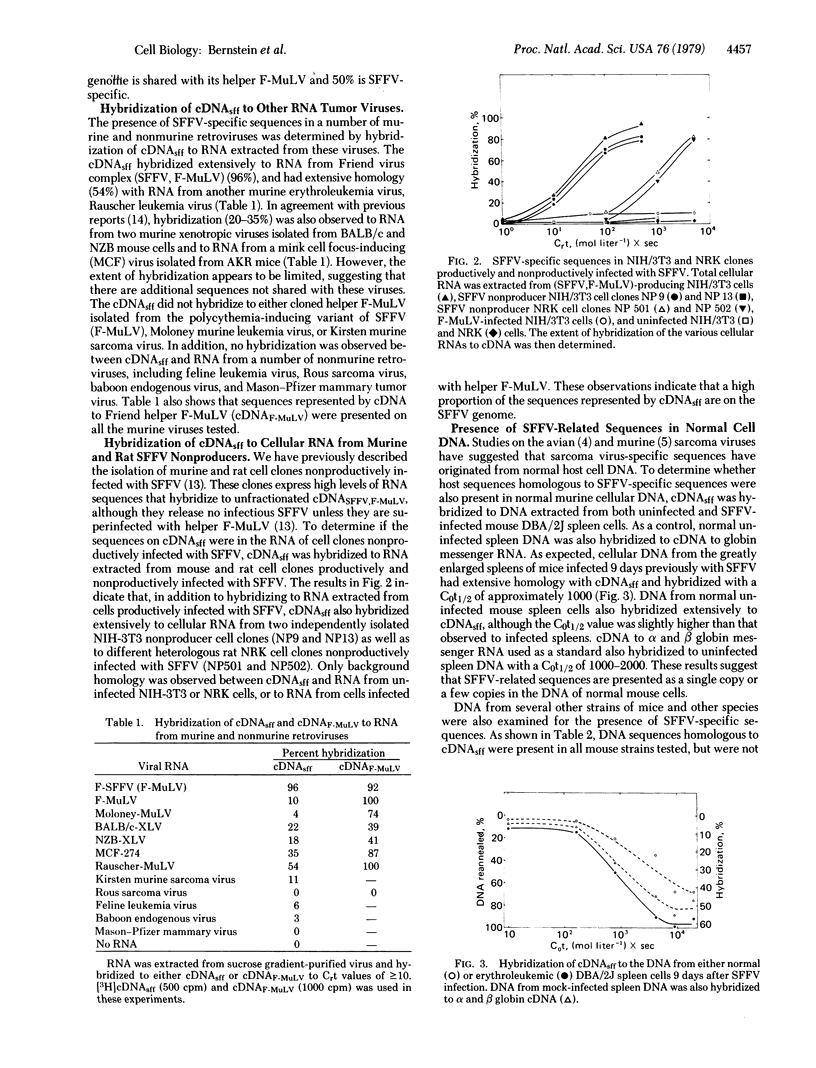
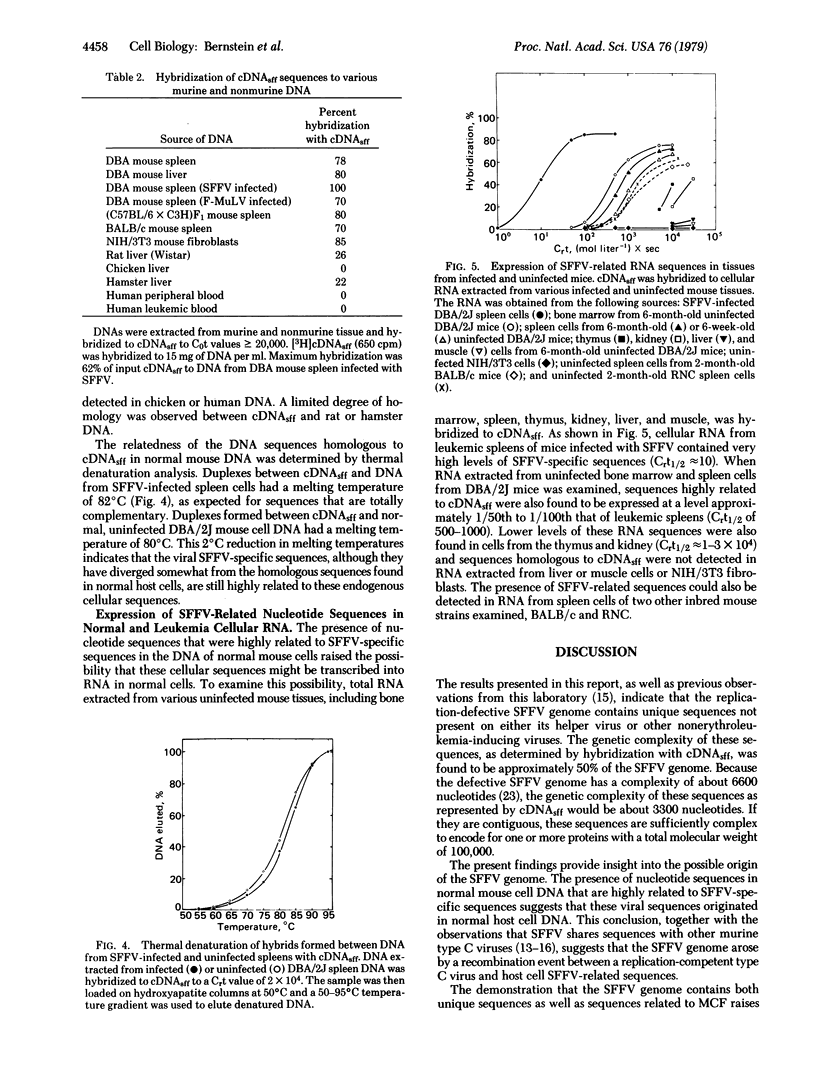
Abstract
The nature and distribution of sequences related to the murine erythroleukemia virus, Friend spleen focus-forming virus (SFFV), have been analyzed by using a radioactive cDNA probe specific for the SFFV genome (cDNAsff). From the proportion of high molecular weight viral [32P]RNA which hybridized to cDNAsff, it was estimated that these sequences represent about 50% of the SFFV genome, indicating a genetic complexity of about 3300 nucleotides. cDNAsff hybridized extensively (80-95%) to SFFV virion RNA and to cellular RNA from murine and rat cells productively or nonproductively infected with SFFV. Only background homology was detected between cDNAsff and viral RNA from a number of murine [Friend murine leukemia virus (MuLV), Moloney-MuLV, and Kirsten sarcoma virus] and nonmurine (Rous sarcoma virus, feline leukemia virus, baboon endogenous virus, and Mason—Pfizer mammary tumor virus) retroviruses. Limited homology was also detected to a number of murine xenotropic and mink cell focus-inducing viruses (20-35%) as well as Rauscher leukemia virus (50%). Nucleotide sequences homologous to cDNAsff were also detected in the DNA of normal cells of several mouse strains as single or a few copies per cell. Thermal denaturation analysis indicated that duplexes formed between cDNAsff and normal DBA/2J cellular DNA have a reduction in melting temperature of 2°C when compared with the dissociation of hybrids between cDNAsff and homologous sequences in SFFV-infected mouse spleen cell DNA. Examination of cellular RNA from uninfected mouse cells indicated that SFFV-related RNA sequences were also expressed in varying degrees in different tissues of adult DBA/2J mice. The highest amounts were observed in cells from bone marrow and spleen, whereas considerably lower amounts were found in cells from the thymus and kidney. No SFFV-related sequences could be detected in RNA extracted from liver, muscle, or fibroblasts. The presence of these SFFV-related sequences in normal, uninfected mouse cell DNA and their differential expression in hematopoietic tissues suggest that these sequences may be an integral part of the program of both normal and leukemic hematopoietic cell differentiation.
Keywords: RNA tumor viruses, Friend spleen focus-forming virus, mechanism of transformation, differentiation, gene expression
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELRAD A. A., STEEVES R. A. ASSAY FOR FRIEND LEUKEMIA VIRUS: RAPID QUANTITATIVE METHOD BASED ON ENUMERATION OF MACROSCOPIC SPLEEN FOCI IN MICE. Virology. 1964 Nov;24:513–518. doi: 10.1016/0042-6822(64)90199-0. [DOI] [PubMed] [Google Scholar]
- Abelson H. T., Rabstein L. S. Influence of prednisolone on Moloney leukemogenic virus in BALB-c mice. Cancer Res. 1970 Aug;30(8):2208–2212. [PubMed] [Google Scholar]
- Barbacid M., Troxler D. H., Scolnick E. M., Aaronson S. A. Analysis of translational products of Friend strain of spleen focus-forming virus. J Virol. 1978 Sep;27(3):826–830. doi: 10.1128/jvi.27.3.826-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M., Steeves R. A., Cudkowicz G., Mirand E. A., Russell L. B. Mutant Sl alleles of mice affect susceptibility to Friend spleen focus-forming virus. Science. 1968 Nov 1;162(3853):564–565. doi: 10.1126/science.162.3853.564. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Hunt D. M., Crichley V., Mak T. W. Induction by ouabain of hemoglobin synthesis in cultured Friend erythroleukemic cells. Cell. 1976 Nov;9(3):375–381. doi: 10.1016/0092-8674(76)90082-9. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Mak T. W., Stephenson J. R. The Friend virus genome: evidence for the stable association of MuLV sequences and sequences involved in erythroleukemic transformation. Cell. 1977 Sep;12(1):287–294. doi: 10.1016/0092-8674(77)90206-9. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Brugge J. S., Erikson R. L. Characterization of a normal avian cell protein related to the avian sarcoma virus transforming gene product. Cell. 1978 Dec;15(4):1363–1369. doi: 10.1016/0092-8674(78)90061-2. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Gautsch J. W., Jensen F. C., Lerner R. A., Hartley J. W., Rowe W. P. Biochemical evidence that MCF murine leukemia viruses are envelope (env) gene recombinants. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4676–4680. doi: 10.1073/pnas.74.10.4676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRIEND C. Cell-free transmission in adult Swiss mice of a disease having the character of a leukemia. J Exp Med. 1957 Apr 1;105(4):307–318. doi: 10.1084/jem.105.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. E., Fischinger P. J. Nucleotide sequences in mouse DNA and RNA specific for Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3705–3709. doi: 10.1073/pnas.73.10.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Wolford N. K., Old L. J., Rowe W. P. A new class of murine leukemia virus associated with development of spontaneous lymphomas. Proc Natl Acad Sci U S A. 1977 Feb;74(2):789–792. doi: 10.1073/pnas.74.2.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. R., Metcalf D. Pure and mixed erythroid colony formation in vitro stimulated by spleen conditioned medium with no detectable erythropoietin. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3879–3882. doi: 10.1073/pnas.74.9.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak T. W., Penrose D., Gamble C., Bernstein A. The Friend spleen focus-forming virus (SFFV) genome: fractionation and analysis of SFFV and helper virus-related sequences. Virology. 1978 Jun 1;87(1):73–80. doi: 10.1016/0042-6822(78)90159-9. [DOI] [PubMed] [Google Scholar]
- McCool D., Mak T. W., Bernstein A. Cellular regulation in Friend virus induced erythroleukemia. Studies with anemic mice of genotype Sl/Sld. J Exp Med. 1979 Apr 1;149(4):837–846. doi: 10.1084/jem.149.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R. Friend erythroleukemia antigen. A viral antigen specified by spleen focus-forming virus and differentiation antigen controlled by the Fv-2 locus. J Exp Med. 1979 May 1;149(5):1152–1167. doi: 10.1084/jem.149.5.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Stockert E., Old L. J. Abelson antigen: a viral tumor antigen that is also a differentiation antigen of BALB/c mice. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3918–3922. doi: 10.1073/pnas.75.8.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelaere J., Faller D. V., Hopkins N. Characterization and mapping of RNase T1-resistant oligonucleotides derived from the genomes of Akv and MCF murine leukemia viruses. Proc Natl Acad Sci U S A. 1978 Jan;75(1):495–499. doi: 10.1073/pnas.75.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M., Howk R. S., Anisowicz A., Peebles P. T., Scher C. D., Parks W. P. Separation of sarcoma virus-specific and leukemia virus-specific genetic sequences of Moloney sarcoma virus. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4650–4654. doi: 10.1073/pnas.72.11.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Fanshier L., Bishop J. M. Identification of nucleotide sequences which may encode the oncogenic capacity of avian retrovirus MC29. J Virol. 1978 Nov;28(2):600–610. doi: 10.1128/jvi.28.2.600-610.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Baker B., Varmus H. E., Bishop J. M. Characteristics of cellular RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):381–386. doi: 10.1016/0092-8674(78)90206-4. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Smith K., Padgett T., McCombe P., Roulland-Dussoix D., Moscovici C., Varmus H. E., Bishop J. M. Uninfected avian cells contain RNA related to the transforming gene of avian sarcoma viruses. Cell. 1978 Feb;13(2):371–379. doi: 10.1016/0092-8674(78)90205-2. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Steeves R. A., Bennett M., Mirand E. A., Cudkowicz G. Genetic control by the W locus of susceptibility to (Friend) spleen focus-forming virus. Nature. 1968 Apr 27;218(5139):372–374. doi: 10.1038/218372a0. [DOI] [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Guntaka R. V., Varmus H. E., Bishop J. M. Purification of DNA complementary to nucleotide sequences required for neoplastic transformation of fibroblasts by avian sarcoma viruses. J Mol Biol. 1976 Mar 5;101(3):349–365. doi: 10.1016/0022-2836(76)90152-2. [DOI] [PubMed] [Google Scholar]
- Stehelin D., Varmus H. E., Bishop J. M., Vogt P. K. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976 Mar 11;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Vogt P. K., Bishop J. M. Integration of deoxyribonucleic acid specific for Rous sarcoma virus after infection of permissive and nonpermissive hosts. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3067–3071. doi: 10.1073/pnas.70.11.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]


