Abstract
Background:
Methylenetetrahydrofolate reductase (MTHFR) polymorphisms have recently raised the interest as a possible thrombophilic factors.
Aims:
We aimed to assess the frequency of the methylenetetrahydrofolate reductase (MTHFR) C677T and A1298C polymorphisms in idiopathic venous thromboembolism (VTE) in a Romanian population and the associated risk of VTE.
Study Design:
We performed a case-control transversal study including 90 patients diagnosed with VTE and 75 sex- and age-matched controls.
Methods:
MTHFR C677T and A1298C polymorphisms were detected using PCR-RFLP method.
Results:
The homozygous MTHFR 677TT genotype, present in 18.8% of patients with VTE versus 6.6% of controls, was significantly associated with VTE (p= 0.021, OR= 3.26, 95%CI (1.141–9.313)). The heterozygous MTHFR A1298C genotype, presenting the highest prevalence in the VTE group (34.4%) as well as in controls (37.3%), was not associated with VTE (p=0.7). No associations were found for heterozygous MTHFR C677T (with a frequency of 32.2% in VTE and 37.3% in controls, p=0.492), respective homozygous MTHFR A1298C genotype (with a frequency of 1.1% in VTE and 2.6% in controls, p=0.456).
Conclusion:
Among MTHFR polymorphisms, only homozygosity for MTHFR 677TT may be considered a risk factor for VTE; the MTHFR A1298C polymorphism is not significantly associated with an increased risk of VTE.
Keywords: Methylenetetrahydrofolate reductase C677T polymorphism, methylenetetrahydrofolate reductase A1298C polymorphism, venous thromboembolism, thrombophilia
Introduction
Venous thromboembolism (VTE) is a multifactorial disease, usually the result of the interaction between a genetic background predisposing to hypercoagulability and acquired risk factors. Although considered idiopathic in 25–50% of cases, investigations may detect a cause in over 80% of the patients with VTE (1). Previous studies have shown that an underlying thrombophilia could be found in about half of patients with a first episode of idiopathic VTE (1, 2). Among the “traditional” thrombophilia factors (such as protein C, protein S or antithrombin III deficiency, Factor V Leiden or Prothrombin G20210A mutations), methylenetetrahydrofolate reductase (MTHFR) polymorphisms associated with hyperhomocysteinemia have recently raised interest. The MTHFR gene encodes the enzyme MTHFR, which regulates the reduction of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the substrate for remethylation of homocysteine to methionine. The MTHFR gene is located on the short (p) arm of chromosome 1 at position 36.3. There are two common polymorphisms of the MTHFR gene: the MTHFR 677 C→T polymorphism resulting in the substitution of alanine to valine at codon 222 (Ala→Val) and the MTHFR 1298 A→C polymorphism, resulting in a glutamine to alanine substitution (Glu→Ala). The MTHFR C677T polymorphism, especially in the homozygous state, leads to decreased enzyme activity and hyperhomocysteinemia, whereas the MTHFR A1298C polymorphism presents a less well-defined effect, with a lesser decrease of the enzyme function.
The prevalence of the T allele varies among races and ethnic groups: it is more common in Caucasians and Asians (30%) compared to African Americans (11%) (3, 4). In Europe, the lowest frequency is found in Finland (25.1%) and the Netherlands (27.4%) and the highest in Italy (45%; 47.3% in Sicily), France (34%–36%), Hungary (33.7%) and Spain (33%) (5–7). The incidence of the MTHFR C677T polymorphism in the general population is about 45% and 15% for heterozygous and homozygous genotypes, respectively. The prevalence of homozygotes for the MTHFR 677TT polymorphism in Europe varies between 5 and 15%; the heterozygous MTHFR C677T genotype presents the highest prevalence in Italy (44%) and the lowest in Norway (28%) (3, 4, 8, 9).
The role of MTHFR polymorphisms in VTE is controversial: some authors have shown an association between the MTHFR C677T polymorphism and VTE (10–14), whereas others have proven the contrary (15–17). Considering the insufficient evidence, the professional guidelines do not recommend testing for MTHFR polymorphisms when evaluating the aetiology of VTE (College of American Pathologists, American College of Medical Genetics, The British Committee for Standards in Haematology and the British Society for Haematology); however, hyperhomocysteinemia should be measured in those found to be positive for factor V Leiden (18–20). The MTHFR C677T polymorphism is not associated with recurrences of VTE; among the carriers of genetic thrombophilic defects, only subjects presenting a positive family history of VTE are predisposed to recurrent thrombosis (21, 22).
Several studies regarding MTHFR polymorphisms and VTE were also performed in our geographic area, the Balkans and Eastern Europe (10, 23–26), but as far as we know, no Romanian data have been previously reported.
We aimed to detect the frequency of MTHFR C677T and A1298C polymorphisms in Romanian patients with idiopathic VTE and assess the association with the risk of VTE.
Material and Methods
We performed a case-control transversal study, including 165 patients admitted to Medicala II Department of the University of Medicine and Pharmacy “Iuliu Hatieganu” Cluj-Napoca, Romania. The patients were divided into 2 groups: Group 1, consisting of 90 patients diagnosed with idiopathic VTE, and Group 2: 75 sex- and age-matched healthy controls. The patients were enrolled after written informed consent. The study protocol was approved by the Ethic Committee of the University. The diagnosis of VTE was based upon Wells score, Doppler ultrasound and/or venography (for deep venous thrombosis) and pulmonary scintigraphy or CT angiography (for pulmonary embolism).
Idiopathic (unprovoked) VTE was defined as VTE occurring in the absence of major triggering risk factors (plaster cast immobilisation and/or fracture of a lower limb or surgery under general anaesthesia lasting for more than 30 minutes, bed rest for more than three days, occurring in three previous months or active cancer in the two preceding years) or in the absence of moderate/minor risk factors (pregnancy/post-partum, oral contraception/hormone replacement therapy in the last year, a journey lasting more than 6h) (27). Patients who presented at least one of the risk factors mentioned above or other medical conditions predisposing to VTE (congestive heart failure, acute myocardial infarction, stroke, inflammatory bowel diseases, acute infection, obesity, varicose veins) were excluded from the study. Recurrent VTE was defined by the presence of another episode of VTE in the medical history of the patients confirmed by medical documents.
The MTHFR C677T and A1298C polymorphisms were detected using PCR-RFLP method. The genomic DNA was extracted from 300 μL of peripheral blood collected in an EDTA tube, using the commercial kit Wizard Genomic DNA Purification Kit (Promega®, USA).
MTHFR polymorphisms were genotyped as previously described. All PCR reactions were performed in 25 μL reaction volume, containing 12.5 μL 2X PCR Master Mix (Fermentas, Lithuania), 1 μL BSA solution (2 mg/mL Bovine Serum Albumin, Fermentas, Lithuania), 6–10 pmol of each primer (Eurogentec, Belgium) and 100 ng genomic DNA, in a thermocycler, Mastercycler Personal (Eppendorf, Germany). The amplification products were digested with specific restriction enzymes (Fermentas, Lithuania), HinfI for MTHFR C677T, and MboII for MTHFR A1298C. For the study of each mutation we used 10 μL of amplification products for digestion; digestion was performed at 37°C, for 8–12 hours. The digestion products were then electrophoresed using a 2% agarose gel for MTHFR C677T, and a 3% gel for MTHFR A1298C (Agarose LE, Analytical Grade, Promega, USA). DNA fragments were stained with ethidium bromide 10 mg/mL (Ethidium Bromide, Promega, USA) and then the gels were read with a UV transillumination system coupled with a photo camera (Vilber Lourmat Imaging System®, France).
The MTHFR C677T polymorphism creates a restriction site for enzyme HinfI. Thus, after the digestion of 265bp amplicons with the enzyme HinfI, the homozygous normal genotype presents the full fragment of 265bp, whereas the heterozygous genotype MTHFR C677T presents 3 fragments of 265, 171 and 94bp; the homozygous genotype MTHFR 677TT presents 2 fragments of 171 and 94bp, respectively (Figure 1).
Figure 1.
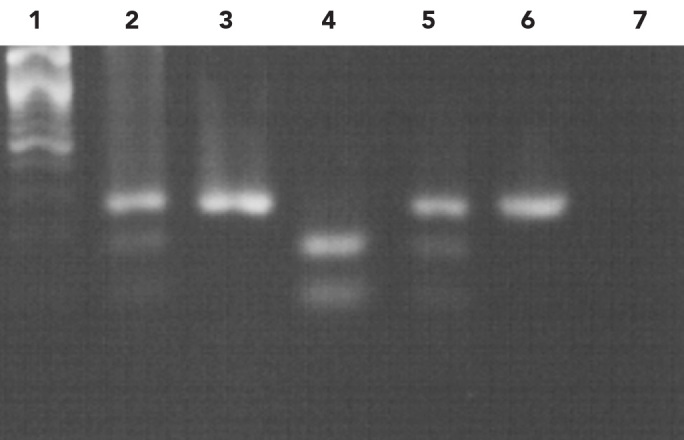
- DNA marker 100bp
- Heterozygous normal allele (265, 171, 94bp)
- Homozygous normal allele (265bp)
- Positive control homozygote mutant allele (171, 94bp)
- Positive control heterozygote mutant allele (265, 171, 94bp)
- Undigested amplification product (265bp)
- Control
The MTHFR A1298C polymorphism abolishes a restriction site of the enzyme MboII. Thus, after digestion of the 163bp amplicon with the enzyme MboII, homozygous individuals for the normal allele presented 5 fragments of 56, 30, 31, 28 and 18bp, whereas heterozygous individuals would present 6 fragments of 84, 56, 30, 31, 28 and 18bp; patients who were homozygous for the mutant allele would present 4 fragments of 84, 31, 30 and 18bp, respectively. In this case, only the fragments of 84 and 56bp were clearly evident in a 3%agarose gel, but this was sufficient for the molecular diagnosis (Figure 2).
Figure 2.
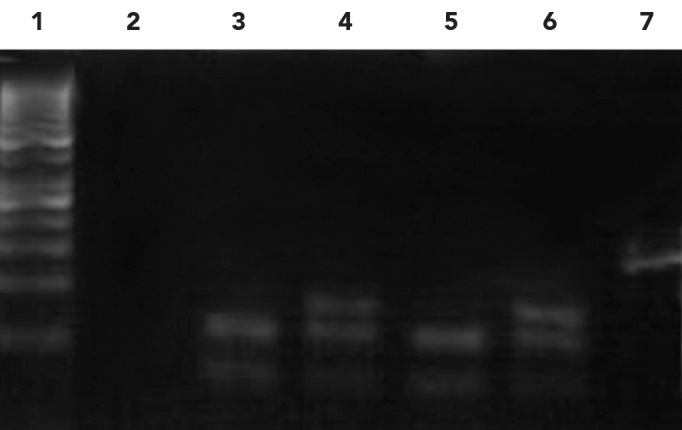
- 1. DNA marker 50bp
- 2. Control
- 3 & 5. Homozygous normal allele (56, 31/30bp)
- 4. Heterozygous normal allele (84, 56, 31/30bp)
- 6. Positive control mutant heterozygote (84, 56, 31/30bp)
- 7. Undigested amplification product (163bp)
We evaluated also the presence of the most common genetic risk factors for VTE, associated with “gain-of-function”: factor V Leiden and prothrombin G20210A, using the PCR-RFLP method; the amplicons were digested with the enzyme HindIII for prothrombin, and MnI for factor V Leiden.
Fasting homocysteinemia was measured with reverse-phase high-performance liquid chromatography (RP-HPLC); the upper limit of the normal laboratory values is 12 μmol/L.
Descriptive statistics were used to determine the polymorphisms’ prevalence and bivariate inferential statistics were used for the assessment of the association with VTE. The frequency of the genotypes in patients and controls was compared using a two-by-two contingency table, analysed with chi-square χ2 with Yates’ correction test, and Fisher’s exact test when the frequency was 5 or under in at least one cell. Phi coefficient was used for the measure of the degree of association between the frequencies of genotypes in VTE, respective in controls. The association between genotype and the risk of VTE was expressed as odds ratio (OR) with 95% confidence intervals (CI). For the assessment of the independent association of MTHFR 677TT genotype with VTE, a predictive model was used, applying binary logistic regression that included VTE as the dependent variable and the MTHFR 677TT genotype, hyperhomocysteinemia, as covariates. The B coefficient showed the independent effect of the MTHFR 677TT genotype on VTE after adjusting for hyperhomocysteinemia. The Wald test and the associated p-value (Sig.) evaluated whether or not the logistic B coefficients of covariates were different from zero in the model. The Statistical Package for the Social Sciences (SPSS) 16.0 for Windows was used for the analysis. The criterion for statistical significance was p<0.05.
Results
The mean age was 55.25 in VTE patients and 56.46 years in controls. The VTE group included 52 men and 38 women, with a sex ratio of 1.36; the control group included 44 men and 31 women, and the sex ratio was 1.41 (p=1.00, no significant difference between groups).
DVT involving the lower limbs was seen in the majority of cases (93.33%); only 6 patients (6.66%) presented an unusual location (in the upper limbs in 4 cases, and cerebral veins in 2 cases). Proximal DVT was present in 55 cases (61.11%) and distal DVT in 29 patients (32.22%). Pulmonary embolism (PE) complicated DVT in 6 patients (6.66%).
The presence of at least one mutant allele T was found in 46 VTE patients (51.1%) and in 33 controls (36.66%), showing no significant association with VTE (p=0.362, OR=1.33, 95%CI [0.838–1.608]). The distribution of the MTFHR C677T polymorphism showed a similar proportion in both sexes, without a significant difference between VTE and controls (p=0.8214: 25 men (54.34%) and 21 women (45.66%), and 19 men (57.57%) and 14 women (42.42%), in patients with VTE and controls, respectively).
The homozygous MTHFR 677TT genotype was predominantly found in VTE patients: 17 patients (19%) versus 5 controls (7%); there was a significant association with VTE (p=0.021, OR=3.26, 95%CI (1.141–9.313), chi-square χ2=5.288, Phi=0.179).
The heterozygous MTHFR C677T genotype was identified in 29 VTE patients (32%) and in 28 controls (37%), showing no association with VTE: p=0.492, OR=0.798, 95%CI (0.410–1.519), chi-square χ2=0.437, Phi=−0.054.
MTHFR 677TT homozygotes presented a 2.9-fold increased risk of thrombotic recurrence; at least 2 episodes of documented VTE were found in 8 patients with this genotype: p=0.037, OR=2.939, 95%CI [1.038–8.317], chi-square χ2=4.357, Phi=0.201. There was no association between heterozygous MTHFR C677T genotype and the recurrence of VTE: p=0.056, chi-square χ2=3.971, Phi (−1; 1)= −0.167.
We identified the MTHFR A1298C polymorphism in 32 VTE patients (35.5%) and in 30 controls (40%); no significant association with VTE was found (p=0.559, OR=0.827, 95%CI (0.439–1.557), chi-square χ2=0.34, Phi (−1; 1)= −0.05). The homozygous genotype was found only in one patient with VTE, whereas the heterozygous genotype was present in 31 patients (34%), showing the highest prevalence among MTHFR polymorphisms in VTE.
The sex distribution showed the presence of the MTFHR A1298C polymorphism in 22 men and in 10 women in the VTE group, and in 16 men and 14 women in the control group, without a significant difference between groups, p=0.363.
The homozygous MTFHR 1298CC genotype was not associated with VTE (p=0.456, OR=0.41, 95%CI (0.036–4.614), chi-square χ2=0.555, Phi (−1; 1)= −0.058). Also, no association with VTE was found for the heterozygous genotype MTFHR A1298C: p=0.7, 95%CI (0.466–1.67), chi-square χ2=0.148, Phi (−1; 1)= −0.3.
Patients with the MTHFR A1298C polymorphism did not present an increased risk for VTE recurrences (p=0.088, chi-square χ2=1.29, Phi (−1; 1)=0.528).
None of the patients or controls carried the double homozygous genotype (MTHFR 677TT/1298CC). The combined double heterozygous genotype MTHFR C677T/A1298C was found only in 4 patients with VTE and 2 controls, showing no significant association with VTE (p=0.689, OR=0.589, 95%CI (0.1049–3.3088), Phi (−1; 1)= −0.05).
The data regarding the frequency of the abnormal MTHFR genotypes and the associated risk of VTE are shown in Table 1.
Table 1.
The frequency of abnormal MTHFR genotypes in VTE and the association with VTE
| Genotype |
Number of cases in VTE
|
χ2 | Phi | p | Odds Ratio |
CI 95%
|
|
|---|---|---|---|---|---|---|---|
| Frequency in VTE % | Lower limit | Upper limit | |||||
| MTHFR 677TT Homozygous | 17 18.88% |
5.288* | 0.179 | 0.021 | 3.260 | 1.141 | 9.313 |
| MTHFR C677T Heterozygous | 29 32.22% |
0.437 | −0.054 | 0.492 | 0.798 | 0.419 | 1.519 |
| MTHFR 1298CC Homozygous | 1 1.11% |
0.555 | −0.058 | 0.456 | 0.410 | 0.036 | 4.614 |
| MTHFR A1298C Heterozygous | 31 34.44% |
0.148 | −0.300 | 0.700 | 0.882 | 0.466 | 1.6701 |
χ2=chi square; Phi= Phi coefficient; CI 95%= 95% confidence interval
The unusual location of deep venous thrombosis (other than at the lower limbs) was detected in 6 cases with at least one mutant allele (T and/or C) and in 2 patients with a normal genotype, showing no significant association with MTHFR polymorphisms (p=0.994, OR=1.06, 95%CI (0.206–5.463)).
Although pulmonary embolism was diagnosed in 6 VTE patients, all with at least one MTHFR polymorphism, the association was not statistically significant: p=0.205, chi-square χ2=1.61.
Homocysteinemia presented a mean value of 17.39 μmol/L in VTE patients, which was significantly different to that found in controls (14.15 μmol/L): p=0.025, 95%CI (0.3846–5.5603). However, the binary logistic regression did not show hyperhomocysteinemia as an independent risk factor: p=0.558, B=0.012, Wald=0.343, OR=1.012, 95%CI (0.971–1.055); only the homozygous MTHFR 677TT genotype was independently associated with VTE: p (Sig.)=0.029, B=1.168, Wald=4.747, OR=3.217, 95%CI (1.125–9.201).
Among the other polymorphisms studied, only factor V Leiden, which was present in 13.33% of VTE cases and 2.66% of controls, was significantly associated with VTE. The concomitant presence of factor V Leiden and the MTHFR C677T polymorphism (found in 7.77% of VTE and 1.33% of controls) led to a 6.2-fold higher risk of VTE, corresponding to a 10.71% increased risk compared to the effect of only factor V Leiden. The frequency and the associated VTE risk of these factors are shown in Table 2.
Table 2.
The frequency of Factor V Leiden, prothrombin G21210A polymorphism in VTE and the associated thrombotic risk
|
Polymorphism
|
N= cases in VTE (frequency %) | χ2 | Phi | p | Odds Ratio |
CI 95%
|
|
|---|---|---|---|---|---|---|---|
| Genotype | Lower limit | Upper limit | |||||
| Factor V Leiden | 12(13.33%) | 5.99 | 0.19 | 0.014 | 5.615 | 1.215 | 25.949 |
| Homozygous | 2 (2.22%) | 1.687 | 0.101 | 0.194 | 1.023 | 0.991 | 1.055 |
| Heterozygous | 10 (11.11%) | 4.326 | 0.162 | 0.038 | 4.563 | 0.967 | 21.517 |
| Prothrombin G21210A | 3 (3.33%) | 1.73 | −0.1 | 0.189 | 0.396 | 0.095 | 1.643 |
| Homozygous | 0 | 2.429 | −0.12 | 0.119 | 0.973 | 0.938 | 1.01 |
| Heterozygous | 3 (3.33%) | 0.43 | −0.04 | 0.526 | 0.612 | 0.133 | 2.825 |
| Compound Factor V /MTHFR C677T | 7 (7.77%) | 3.68 | 0.15 | 0.056 | 6.241 | 0.7501 | 51.92 |
Discussion
Our study shows a T allele frequency of 51.1% in VTE patients, which is similar to studies performed in South and Eastern European countries on the same pathology: 54% in Croatia, 52.6% in Russia, and 42.5% in Macedonia (23–25). It is well known that the frequency of the T allele varies in the general population as well as in VTE, concordant to geographical and ethnic factors.
The prevalence of MTHFR 677TT homozygosity in East European and Balkan populations are 7%, 7.5%, 8%, 11% for Russia, Ukraine, Balkan immigrants in Turkey, and Hungary, respectively (5, 26, 28). This pattern of distribution is according to a geographical south to north decreased gradient of MTHFR C677T polymorphism frequency, reflected in the highest prevalence of the homozygous genotype in VTE in Italy, 25.6%, and a lower frequency in Germany of 10.6% (29, 30).
The frequency of the homozygous MTHFR 677TT genotype in our patients with VTE was 19%, which is concordant with other studies performed in the South East Europe: 20% in Serbia, among women with DVT during pregnancy or puerperium, 17.8% in Greece, and 16.4% in Macedonia (25, 31, 32). A similar frequency of the homozygous genotype in VTE was found in Turkey (23.8%), Spain (23.7%), in Israel (22.8%) and France (21.8%) (10, 33–35), concordant with the frequency peak in Southern regions. A peculiar situation appears in Croatia, with a 9% prevalence of the MTHFR 677TT genotype in VTE (23).
We found that the homozygous MTHFR 677TT genotype was significantly associated with VTE: p=0.021, OR=3.26. The literature shows controversial data: several studies revealed the homozygous MTHFR 677TT genotype as a risk factor for VTE with OR of 3.11, 2.9, 2.1, 1.7, 1.2, respectively (24, 29, 33, 35, 36) and a 20% increased risk of VTE (37), whereas other studies failed to reveal any significant association (25, 38–40).
Previous Romanian studies regarding thrombophilia in obstetric cases detected frequencies of the homozygous MTHFR 677TT genotype of 19.4% and 19.56% in pregnant patients with haemorrhagic complications and in patients with repetitive spontaneous abortions; no significant associations were found, except for a slightly increased risk for repetitive abortions in the presence of this genotype (41, 42). Our result regarding the prevalence of MTHFR 677TT in VTE cases is consistent with these studies.
We detected the heterozygous MTHFR C677T genotype in 32% of VTE patients and in 37% of the controls, without a significant difference. A similar prevalence in VTE was shown in other studies: 36.1%, 34.5% (35, 43). The incidence of heterozygotes in the general population is about 45%, with geographical and ethnic variations (5). The prevalence in Southeastern Europe countries are 45%, 43%, 40%, in Hungary, Ukraine, Russia, respectively (5, 26). Although a study performed in Germany showed a 2.12 increased risk of DVT for individuals with the heterozygous genotype, found in 43.8% of patients, the majority of studies showed no association with VTE, concordant with our results (33, 35–40).
The MTHFR A1298C polymorphism presents a prevalence of 35.5% in VTE in our study, with the predominance of the heterozygous genotype, found in 34% of patients. None of the MTHFR A1298C genotypes, homozygous or heterozygous, were associated with VTE risk. The MTHFR A1298C polymorphism was less well-studied in relation to VTE. A high prevalence of heterozygotes was found in the healthy Irish population (46.7%), as well as in Macedonian patients with VTE (44%) (25, 44). Several studies showed similar results to ours, regarding the prevalence of heterozygous MTHFR A1298C genotype in VTE: 36.9%, 37.4%, 37%, respectively (40, 45, 46). A higher frequency of heterozygotes was found in previous Romanian studies regarding thrombophilia in selected groups of patients: 50.1% and 47% in cases with bleeding in the first trimester of pregnancy and repetitive abortions, respectively (41, 42). All of the mentioned studies from the literature show the preponderance of the heterozygous form of the MTHFR A1298C polymorphism versus the homozygous form and an absence of association with VTE.
We detected a 2.9 increased risk of VTE recurrence only in association with the homozygous MTHFR 677TT genotype. Although it is well known that genetic factors predispose to thrombophilia manifested with repetitive thrombotic episodes, the majority of the authors have shown an absence of the risk of recurrence in cases with MTHFR 677TT genotype (21, 22, 47–49); however, a 1.4-fold mildly increased risk was detected in one study (50).
Our results show that the MTHFR polymorphisms (C677T and A1298C) are not particularly associated with an increased risk of PE, which is similar to the results of some studies (13, 38), but in contrast to others (10, 24).
Although inherited thrombophilia may be associated with an unusual location of thrombosis, we did not detect a significant association in cases of MTHFR polymorphisms, concordant with other studies (51–53).
The concomitant presence of factor V Leiden and the MTHFR C677T polymorphism further increases the risk of VTE comparatively to the singular action of factor V Leiden, showing the importance of multiple risk factors for VTE (12, 33).
Our study included a small sample of patients and thus the conclusions should be confirmed by future research in the field. The study of other genetic factors, including “loss-of-function” mutations such as antithrombin, and protein C and S deficiencies (rare but strong risk factors), would provide more information regarding the complexity of gene-gene interactions in VTE.
We detected significant associations between the homozygous MTHFR 677TT genotype and VTE, including recurrent venous thrombosis. The MTHFR A1209C polymorphism does not represent a risk factor for VTE in Romanian patients. Among the MTHFR polymorphisms, heterozygous MTHFR A1298C genotype presents the highest prevalence in our patients with VTE.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – C.H.; Design - C.H.; Supervision -C.H., D.F.; Resource - C.H., A.T., R.P.; Materials - A.T., R.P.; Data Collection&/or Processing - C.H.; Analysis&/or Interpretation - C.H., A.T., R.P.; Literature Search – A.T., R.P.; Writing - C.H.; Critical Reviews - D.F.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Whire RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(Suppl 1):I4–8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107(Suppl 1):I9–16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 3.Lovricevic I, Franjic BD, Tomicic M, Vrkic N, De Syo D, Hudorovic N, et al. 5, 10-Methylenetetrahydrofolate reductase (MTHFR) 677 C --> T genetic polymorphism in 228 Croatian volunteers. Coll Antropol. 2004;28:647–54. [PubMed] [Google Scholar]
- 4.Pepe G, Camacho Vanegas O, Giusti B, Brunelli T, Marcucci R, Attanasio M, et al. Heterogeneity in world distribution of the thermolabile C677T mutation in 5,10-Methylenetetrahydrofolate reductase. Am J Hum Genet. 1998;63:917–20. doi: 10.1086/302015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcken B, Bamforth F, Li Z, Zhu H, Ritvanen A, Redlund M, et al. Geographical and ethnic variation of the 677C>T allele of 5,10 methylenetetrahydrofolate reductase (MTHFR): findings from over 7000 newborns from 16 areas world wide. J Med Genet. 2003;40:619–25. doi: 10.1136/jmg.40.8.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mayor-Olea A, Callejón G, Palomares AR, Jiménez AJ, Gaitán MJ, Rodríguez A, et al. Human genetic selection on the MTHFR 677C>T polymorphism. BMC Med Genet. 2008;9:104. doi: 10.1186/1471-2350-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guéant-Rodriguez RM, Guéant JL, Debard R, Thirion S, Hong LX, Bronowicki JP, et al. Prevalence of methylenetetrahydrofolate reductase 677T and 1298C alleles and folate status: a comparative study in Mexican, West African, and European populations. Am J Clin Nutr. 2006;83:701–7. doi: 10.1093/ajcn.83.3.701. [DOI] [PubMed] [Google Scholar]
- 8.Castro R, Rivera I, Ravasco P, Jakobs C, Blom HJ, Camilo ME, et al. 5,10-Methylenetetrahydrofolate reductase 677C-->T and 1298A-->C mutations are genetic determinants of elevated homocysteine. QJM. 2003;96:297–303. doi: 10.1093/qjmed/hcg039. [DOI] [PubMed] [Google Scholar]
- 9.Chango A, Boisson F, Barbé F, Quilliot D, Droesch S, Pfister M, et al. The effect of 677C-->T and 1298A-->C mutations on plasma homocysteine and 5,10-methylenetetrahydrofolate reductase activity in healthy subjects. Br J Nutr. 2000;83:593–6. doi: 10.1017/s0007114500000751. [DOI] [PubMed] [Google Scholar]
- 10.Kupeli E, Verdi H, Simsek A, Atac FB, Eyuboglu FO. Genetic mutations in Turkish population with pulmonary embolism and deep venous thrombosis. Clin Appl Thromb Hemost. 2011;17:E87–94. doi: 10.1177/1076029610385224. [DOI] [PubMed] [Google Scholar]
- 11.Moheimani F, Jackson DE. Venous thromboembolism: classification, risk factors, diagnosis, and management. ISRN Hematol. 2011;2011:124610. doi: 10.5402/2011/124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Almawi WY, Tamim H, Kreidy R, Timson G, Rahal E, Nabulsi M, et al. A case control study on the contribution of factor V-Leiden, prothrombin G20210A, and MTHFR C677T mutations to the genetic susceptibility of deep venous thrombosis. J Thromb Thrombolysis. 2005;19:189–96. doi: 10.1007/s11239-005-1313-x. [DOI] [PubMed] [Google Scholar]
- 13.Nizankowska-Mogilnicka E, Adamek L, Grzanka P, Domagala TB, Sanak M, Krzanowski M, et al. Genetic polymorphisms associated with acute pulmonary embolism and deep venous thrombosis. Eur Respir J. 2003;21:25–30. doi: 10.1183/09031936.03.00034302. [DOI] [PubMed] [Google Scholar]
- 14.Jang MJ, Jeon YJ, Choi WI, Choi YS, Kim SY, Chong SY, et al. The 677C>T Mutation of the MTHFR Gene Increases the Risk of Venous Thromboembolism in Koreans and a Meta-Analysis From Asian Population. Clin Appl Thromb Hemost. 2012 Feb 12; doi: 10.1177/1076029612436677. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Bezemer ID, Doggen CJ, Vos HL, Rosendaal FR. No association between the common MTHFR 677C->T polymorphism and venous thrombosis: results from the MEGA study. Arch Intern Med. 2007;167:497–501. doi: 10.1001/archinte.167.5.497. [DOI] [PubMed] [Google Scholar]
- 16.Naess IA, Christiansen SC, Romundstad PR, Cannegieter SC, Blom HJ, Rosendaal FR, et al. Prospective study of homocysteine and MTHFR 677TT genotype and risk for venous thrombosis in a general population--results from the HUNT 2 study. Br J Haematol. 2008;141:529–35. doi: 10.1111/j.1365-2141.2008.07073.x. [DOI] [PubMed] [Google Scholar]
- 17.Keijzer MB, Borm GF, Blom HJ, Bos GM, Rosendaal FR, den Heijer M. No interaction between factor V Leiden and hyperhomocysteinemia or MTHFR 677TT genotype in venous thrombosis. Results of a meta-analysis of published studies and a large case-only study. Thromb Haemost. 2007;97:32–7. [PubMed] [Google Scholar]
- 18.Key NS, McGlennen RC. Hyperhomocyst(e)inemia and thrombophilia. Arch Pathol Lab Med. 2002;126:1367–75. doi: 10.5858/2002-126-1367-HAT. [DOI] [PubMed] [Google Scholar]
- 19.Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA, ACMG Factor V. Leiden Working Group American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med. 2001;3:139–48. doi: 10.1097/00125817-200103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baglin T, Gray E, Greaves M, Hunt BJ, Keeling D, Machin S, et al. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol. 2010;149:209–20. doi: 10.1111/j.1365-2141.2009.08022.x. [DOI] [PubMed] [Google Scholar]
- 21.Cushman M. Inherited risk factors for venous thrombosis. Hematology Am Soc Hematol Educ Program. 2005:452–7. doi: 10.1182/asheducation-2005.1.452. [DOI] [PubMed] [Google Scholar]
- 22.Mansilha A, Araujo F, Severo M, Sampaio SM, Toledo T, Albuquerque R. Genetic polymorphisms and risk of recurrent deep venous thrombosis in young people: prospective cohort study. Eur J Vasc Endovasc Surg. 2005;30:545–9. doi: 10.1016/j.ejvs.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 23.Alfirevic Z, Simundic AM, Nikolac N, Sobocan N, Alfirevic I, Stefanovic M, et al. Frequency of factor II G20210A, factor V Leiden, MTHFR C677T and PAI-1 5G/4G polymorphism in patients with venous thromboembolism: Croatian case control study. Biochemia Medica. 2010;20:229–35. [Google Scholar]
- 24.Avdonin PV, Kirienko AI, Kozhevnikova LM, Shostak NA, Babadaeva NM, Leont’ev SG, et al. C677T mutation in methylenetetrahydrofolate reductase gene in patients with venous thromboses from the central region of Russia correlates with a high risk of pulmonary artery thromboembolism. Ter Arkh. 2006;78:70–6. [PubMed] [Google Scholar]
- 25.Spiroski I, Kedev S, Antov S, Arsov T, Krstevska M, Dzhekova-Stojkova S, et al. Association of methylenetetrahydrofolate reductase (MTHFR-677 and MTHFR-1298) genetic polymorphisms with occlusive artery disease and deep venous thrombosis in Macedonians. Croat Med J. 2008;49:39–49. doi: 10.3325/cmj.2008.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tatarskyy P, Kucherenko A, Livshits L. Allelic polymorphism of F2, F5 and MTHFR genes in population of Ukraine. Tsitol Genet. 2010;44:3–8. [PubMed] [Google Scholar]
- 27.Pernod G, Biron-Andreani C, Morange PE, Boehlen F, Constans J, Couturaud F, et al. Recommendations on testing for thrombophilia in venous thromboembolic disease: A French consensus guideline. J Mal Vasc. 2009;34:156–203. doi: 10.1016/j.jmv.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Baytan B, Meral AG, İlçöl YO, Günay U. The prevalence of factor V Leiden (1691G-A)and methylenetetrahydrofolate reductase C677T mutations in healthy newborns in Bursa, Turkey. Turk J Hematol. 2007;24:90–2. [PubMed] [Google Scholar]
- 29.Margaglione M, D’Andrea G, d’Addedda M, Giuliani N, Cappucci G, Iannaccone L, et al. The methylenetetrahydrofolate reductase TT677 genotype is associated with venous thrombosis independently of the coexistence of the FV Leiden and the prothrombin A20210 mutation. Thromb Haemost. 1998;79:907–11. [PubMed] [Google Scholar]
- 30.Koch HG, Nabel P, Junker R, Auberger K, Schobess R, Homberger A, et al. The 677T genotype of the common MTHFR thermolabile variant and fasting homocysteine in childhood venous thrombosis. Eur J Pediatr. 1999;158(Suppl 3):S113–6. doi: 10.1007/pl00014332. [DOI] [PubMed] [Google Scholar]
- 31.Dordević V, Rakićević L, Spasić M, Miković D, Kovać M, Radojković D. Factor V Leiden, FII G20210A, MTHFR C677T mutations as risk factors for venous thrombosis during pregnancy and puerperium. Vojnosanit Pregl. 2005;62:201–5. doi: 10.2298/vsp0503201d. [DOI] [PubMed] [Google Scholar]
- 32.Angelopoulou K, Nicolaides A, Constantinou Deltas C. Prevalence of genetic mutations that predispose to thrombophilia in a Greek Cypriot population. Clin Appl Thromb Hemost. 2000;6:104–7. doi: 10.1177/107602960000600211. [DOI] [PubMed] [Google Scholar]
- 33.Salomon O, Steinberg DM, Zivelin A, Gitel S, Dardik R, Rosenberg N, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19:511–18. doi: 10.1161/01.atv.19.3.511. [DOI] [PubMed] [Google Scholar]
- 34.García-Hernández MC, Romero Casanova A, Marco Vera P. Clinical comments on genetic marker prevalence (factor V Leiden, prothrombin 20210A and homozygous methylenetetrahydrofolate reductase form [Ho-MTHFR]): based on a study conducted in Health Department No. 19 of the Valencian Community. Rev Clin Esp. 2007;207:26–8. doi: 10.1157/13098497. [DOI] [PubMed] [Google Scholar]
- 35.Couturaud F, Oger E, Abalain JH, Chenu E, Guias B, Floch HH, et al. Methylenetetrahydrofolate reductase C677T genotype and venous thromboembolic disease. Respiration. 2000;67:657–61. doi: 10.1159/000056296. [DOI] [PubMed] [Google Scholar]
- 36.Ray JG, Shmorgun D, Chan WS. Common C677T polymorphism of the methylenetetrahydrofolate reductase gene and the risk of venous thromboembolism: meta-analysis of 31 studies. Pathophysiol Haemos Thromb. 2002;32:51–8. doi: 10.1159/000065076. [DOI] [PubMed] [Google Scholar]
- 37.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and the risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3:292–9. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 38.Obeidat NM, Awidi A, Sulaiman NA, Abu-Khader IB. Thrombophilia-related genetic variations in patients with pulmonary embolism in the main teaching hospital in Jordan. Saudi Med J. 2009;30:921–5. [PubMed] [Google Scholar]
- 39.Tsai AW, Cushman M, Tsai MY, Heckbert SR, Rosamond WD, Aleksic N, et al. Serum homocysteine, thermolabile variant of methylene tetrahydrofolate reductase (MTHFR), and venous thromboembolism: Longitudinal Investigation of Thromboembolism Etiology (LITE) Am J Hematol. 2003;72:192–200. doi: 10.1002/ajh.10287. [DOI] [PubMed] [Google Scholar]
- 40.Domagala TB, Adamek L, Nizankowska E, Sanak M, Szczeklik A. Mutations C677T and A1298C of the 5,10-methylenetetrahydrofolate reductase gene and fasting plasma homocysteine levels are not associated with the increased risk of venous thromboembolic disease. Blood Coagul Fibrinolysis. 2002;13:423–31. doi: 10.1097/00001721-200207000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Stamatian F, Caracostea G, Muresan D, Bartok I, Militaru M, Procopciuc L, et al. The evaluation of inherited thrombophilic conditions in patients with bleeding in the first trimester of pregnancy. HVM Bioflux. 2009;1:1–7. [Google Scholar]
- 42.Popp R, Crisan T, Militaru M, Rotar IC, Farcas M, Pop IV. The C677T Variant in the Methylenetetrahydrofolate Reductase Gene and Idiopathic Spontaneous Abortion in a Romanian Population Group. Not Sci Biol. 2012;4:7–11. [Google Scholar]
- 43.Hanta I, Soydas Y, Karatasli M, Koseoglu Z, Satar S, Hasturk S. Plasma homocysteine level and 677C-->T mutation on the MTHFR gene in patients with venous thromboembolism. Bratisl Lek Listy. 2010;111:70–3. [PubMed] [Google Scholar]
- 44.McCarthya C, Ryan F, Vaughan J. Increased frequency of the MTHFR A1298C mutation in an Irish population. Clinical Chemistry. 2004;50:2462–3. doi: 10.1373/clinchem.2004.041517. [DOI] [PubMed] [Google Scholar]
- 45.Tug E, Aydin H, Kaplan E, Dogruer D. Frequency of genetic mutations associated with thromboembolism in the Western Black Sea Region. Intern Med. 2011;50:17–21. doi: 10.2169/internalmedicine.50.4144. [DOI] [PubMed] [Google Scholar]
- 46.Franco RF, Morelli V, Lourenço D, Maffei FH, Tavella MH, Piccinato CE, et al. A second mutation in the methylenetetrahydrofolate reductase gene and the risk of venous thrombotic disease. Brit J Haematol. 1999;105:556–9. [PubMed] [Google Scholar]
- 47.González-Porras JR, García-Sanz R, Alberca I, López ML, Balanzategui A, Gutierrez O, et al. Risk of recurrent venous thrombosis in patients with G20210A mutation in the prothrombin gene or factor V Leiden mutation. Blood Coagul Fibrinolysis. 2006;17:23–8. doi: 10.1097/01.mbc.0000201488.33143.09. [DOI] [PubMed] [Google Scholar]
- 48.Mansilha A, Araújo F, Severo M, Sampaio SM, Toledo T, Albuquer que R. Genetic polymorphisms and risk of recurrent deep venous thrombosis in young people: prospective cohort study. Eur J Vasc Endovasc. 2005;30:545–9. doi: 10.1016/j.ejvs.2005.05.038. [DOI] [PubMed] [Google Scholar]
- 49.Baglin T, Luddington R, Brown K, Baglin C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: prospective cohort study. Lancet. 2003;362:523–6. doi: 10.1016/S0140-6736(03)14111-6. [DOI] [PubMed] [Google Scholar]
- 50.Keijzer MB, den Heijer M, Blom HJ, Bos GM, Willems HP, Gerrits WB, et al. Interaction between hyperhomocysteinemia, mutated methylenetetrahydrofolatereductase (MTHFR) and inherited thrombophilic factors in recurrent venous thrombosis. Thromb Haemost. 2002;88:723–8. [PubMed] [Google Scholar]
- 51.Joffe HV, Goldhaber SZ. Laboratory thrombophilias and venous thromboembolism. Vascular Medicine. 2002;7:93–102. doi: 10.1191/1358863x02vm426ra. [DOI] [PubMed] [Google Scholar]
- 52.Vayá A, Plumé G, Bonet E, Carrasco P, Morales-Suárez-Varela MM. Hyperhomocysteinemia and the methylene tetrahydrofolate reductase C677T mutation in splanchnic vein thrombosis. Eur J Haematol. 2011;86:167–72. doi: 10.1111/j.1600-0609.2010.01551.x. [DOI] [PubMed] [Google Scholar]
- 53.Sottilotta G, Siboni SM, Latella C, Oriana V, Romeo E, Santoro R, et al. Hyperhomocysteinemia and C677T MTHFR genotype in patients with retinal vein thrombosis. Clin Appl Thromb Hemost. 2010;16:549–53. doi: 10.1177/1076029609348644. [DOI] [PubMed] [Google Scholar]


