Abstract
Background:
Hodgkin’s lymphoma (HL) is a B cell lymphoma characterized by the presence of Reed-Sternberg cells. HL comprises 1% of all cancer cases and 14% of all lymphoma cases.
Aims:
We designed a retrospective study to investigate the clinical features and prognostic factors of HL patients diagnosed at an experienced oncology centre.
Study Design:
Retrospective study.
Methods:
Demographic characteristics, histopathological and clinical features, treatment modalities and response to treatment were obtained from hospital records. Dates of initial diagnosis, remission and relapse, last visit and death were recorded for survival analyses.
Results:
We analysed data of 391 HL patients (61% male, 39% female; mean age 35.7±15.1 years). The most common classical HL histological subtype was nodular sclerosing HL (NSHL) (42.7%). The most common stage was II 50.4%. The most common chemotherapy regimen was doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) (70.6%). Five and 10-year survival rates were 90% and 84%, respectively. Early-stage patients with good prognostic factors had better overall and relapse-free survival rates. The presence of “B” symptoms, albumin level, Eastern Cooperative Oncology Group (ECOG) performance score, and LDH were prognostic factors that affect the survival in both univariate and multivariate analyses.
Conclusion:
This is the first study that demonstrates the demographic, clinical and prognostic features of HL patients in Turkey, and provides a general picture of the HL patients in our country.
Keywords: Hodgkin’s lymphoma, diagnosis, prognosis, treatment
Introduction
Hodgkin’s lymphoma (HL) is a B cell lymphoma characterised by the presence of Reed-Sternberg cells (1). It is a complex of related conditions that is, in part, mediated by genetic susceptibilities, infectious diseases, and immune deficiencies (2). HL comprises 1% of all cancer cases and 14% of all lymphoma cases. Its incidence has been increasing worldwide, albeit varying in different ethnic groups (1, 2). The distribution of ethnic groups varies significantly among Turkish population due to the past multinational empire background of the country; therefore, it would be interesting to study the features of HL in such a country.
The classification of World Health Organisation (WHO) distinguishes two biologically and clinically distinct entities: nodular lymphocytic predominant HL (NLPHL), and classical HL. Classical HL accounts for 95% of all HL cases, and can be further classified into 4 different subtypes; nodular sclerosing HL (NSHL), which is the most common histological subtype, lymphocyte-rich HL (LRHL), mixed cellularity HL (MCHL) and lymphocyte depletion HL (LDHL) (3, 4). However, histological subtypes of HL show wide geographical variation. Three main epidemiological patterns have been described for HL regarding age at presentation, the developmental level of a country, and its association with the Epstein–Barr virus (EBV) (5). Turkey is a developing country, and lacks data on the different aspects of HL.
To date, a number of prognostic factors have been identified for both early- and advanced-stage HL patients. The presence of bulky mediastinal disease, high erythrocyte sedimentation rate (ESR), age, the number of lymph nodes involved, and the presence of extranodal disease are the major prognostic factors (6). However, disease stage and other risk factors rather than histology dictate the prognosis and management of HL. Depending on the stage and risk factor profile, more than 80% of patients with HL can be cured with effective first-line therapy. Today, chemotherapy, often combined with involved field radiotherapy, is the most commonly applied treatment and is standard-of-care for patients with early-stage disease without any additional risk factors. For advanced-stage HL, more aggressive chemotherapy protocols have shown superiority over less intensive regimens (7). There are very few reports from our country on these issues.
It has been estimated that HL accounts for 1% of all cancer cases and 30% of all lymphoma patients in Turkey (1). The annual number of estimated new HL cases in Turkey is 649. Due to the lack of data on many aspects of HL in our country, we designed a retrospective study to investigate the clinical features and prognostic factors of HL patients who were diagnosed at an academic oncology centre.
Material and Methods
Patients
This study retrospectively evaluated the demographic, clinical and pathological features of HL patients followed-up in the Department of Medical Oncology, Cancer Institute, Hacettepe University. We obtained the list of HL patients followed-up in this department since 2003 from the Hacettepe University Cancer Registry by filtering the database according to the International Classification of Disease-10 (ICD-10) coding system. Patients diagnosed before that date were listed according to patient follow-up lists, or daily outpatient lists for follow-up visits.
Demographic data, presenting symptoms, histopathological and clinical characteristics, treatment modalities, and response to treatment were obtained from hospital records. Hospital records were also used for biochemical test results, such as liver and renal function tests, ESR, complete blood count, serum beta-2 microglobulin and serum lactate dehydrogenase (LDH) levels, and results of imaging techniques such as ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI) and gallium scintigraphy. For survival analyses, initial diagnosis dates, remission and relapse dates, last visit dates and death dates for those who died were registered using the hospital records. For patients who had not attended the hospital for more than 6 months, patients or their relatives were contacted by telephone to describe their latest condition.
Definitions
Complete response was defined as complete disappearance of all disease sites lasting for at least 4 weeks after the completion of therapy. The usual Response Evaluation Criteria In Solid Tumors (RECIST) criteria were applied for partial response, stable and progressive disease. Relapse was the reappearance of disease after complete response in the same or a different anatomic site. Overall survival was the time from initial diagnosis to the last control visit or death, whereas relapse-free survival was the time from complete remission to recurrence. Remission duration was defined as the time from remission to recurrence or last control visit date or the date of death.
Statistical Analyses
Data were analysed using the Statistical Package for the Social Sciences (SPSS) version 15.0. Chi-square test was used to demonstrate differences between nominal and ordinal variables. Mann-Whitney U test and Wilcoxon test were used to analyse differences between independent variables, and Student’s-t test was used to evaluate numerical variables with normal distribution. Overall and progression-free survivals were analysed by the Kaplan-Meier. The possible factors identified with univariate analyses were further entered into the Cox regression analysis, with backward selection, to determine the independent predictors of survival. Among the factors correlated with similar effects on survival, only those with clinical significance were included. The proportional hazards assumption and model fit was assessed by means of residual analysis. We gave the hazard ratios for the variables, which were used for statistical modelling. Statistical analyses were two-way, and p<0.05 was considered statistically significant.
Results
Data of 391 patients that followed-up and treated for HL were analysed in this study. Of these patients, 118 (30.1%) were diagnosed prior to 2000. Two-hundred and thirty-nine patients (61.1%) were male and 152 (38.9%) female. The mean patient age was 35.7±15.1 years. The mean age of male patients at initial diagnosis was higher than that of females (37.3±13.2 vs. 32.5±13.7 years, respectively; p<0.001). The overall and gender-specific age distribution curves are shown in Figures 1 and 2, respectively. Although the disease was most common in younger ages for both genders, the age distribution curves were different for each gender. While the disease peaked at around 25 years of age among women, the age distribution was bimodal among men peaking twice at 20 and 40 years.
Figure 1.
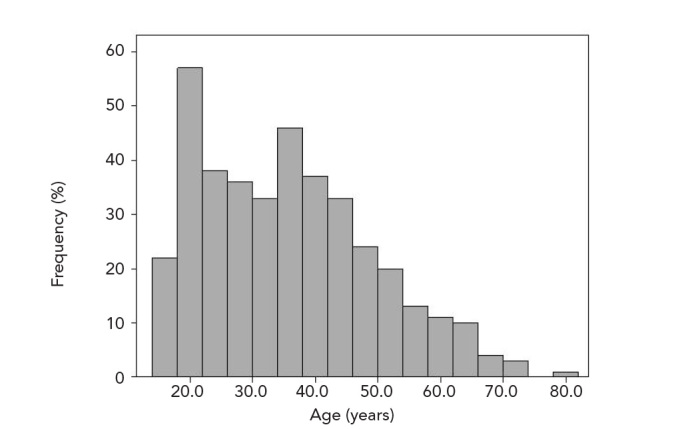
The age distributions of HL patients
Figure 2.
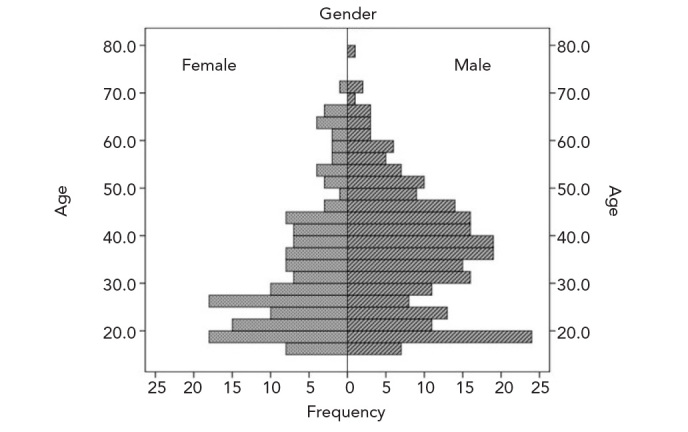
Gender-specific age distributions of HL cases
The most common symptom was painless cervical lymph node enlargement (62.9%; n=246). Of the patients, 10.0% (n=39) had bone marrow involvement at presentation. The median number of involved nodal regions was 4 (range 1–10). The patients’ clinical characteristics are shown in Table 1.
Table 1.
Clinical characteristics of male and female HL patients
| Characteristics | Total n (%) | Male n (%) | Female n (%) | p |
|---|---|---|---|---|
| Stage | 0.007 | |||
| I | 41 (10.5) | 24 (10.0) | 17 (11.2) | |
| II | 197 (50.4) | 109 (45.6) | 88 (57.9) | |
| III | 110 (28.1) | 70 (29.3) | 40 (26.5) | |
| IV | 43 (11.0) | 36 (15.1) | 7 (4.6) | |
| Extranodal disease | 31 (7.9) | 17 (7.8) | 14 (9.2) | 0.610 |
| Spleen | 61 (15.6) | 45 (18.8) | 16 (10.5) | 0.030 |
| Liver | 28 (7.2) | 20 (8.4) | 8 (5.3) | 0.255 |
| Bulky disease | 36 (9.2) | 22 (9.2) | 14 (9.2) | 0.850 |
| “B” symptoms present | 142 (36.3) | 104 (43.5) | 38 (25.0) | 0.019 |
| Histology | 0.036 | |||
| NLPHL | 30 (7.7) | 19 (8.0) | 11 (7.2) | |
| LRHL | 9 (2.3) | 6 (2.5) | 3 (2.0) | |
| MCHL | 158 (40.4) | 111 (46.5) | 47 (30.9) | |
| NSHL | 167 (42.7) | 89 (37.2) | 78 (51.3) | |
| LDHL | 5 (1.3) | 2 (0.8) | 3 (2.0) | |
| Not defined | 22 (5.6) | 12 (5.0) | 10 (6.6) | |
| ECOG Performance score | 0.208 | |||
| 0 | 178 (45.5) | 104 (43.5) | 74 (48.7) | |
| 1 | 151 (38.6) | 91 (38.1) | 60 (39.4) | |
| 2 | 48 (12. 3) | 36 (15.1) | 12 (8.0) | |
| 3 | 14 (3.6) | 8 (3.3) | 6 (3.9) |
HL: Hodgkin’s lymphoma; NLPHL: nodular lymphocyte predominant HL; LRHL: lymphocyte-rich HL; MCHL: mixed cellularity HL; NSHL: nodular sclerosing HL; LDHL: lymphocyte depletion HL; ECOG: Eastern Cooperative Oncology Group
Mean laboratory values of the patients at initial diagnosis are shown in Table 2. Overall, the mean levels for biochemical tests were as follows: haemoglobin 12.3±2.2 g/dL, LDH 442±215 U/L, ESR 54±33 mm/h, albumin 4.1±0.6 g/dL. The level of haemoglobin at diagnosis for female patients was significantly lower than for male cases (p<0.001). While LDH and ESR levels were significantly higher among patients with “B” symptoms, bulky disease and extranodal involvement, haemoglobin and serum albumin levels were lower (p<0.001) compared to those with none of these symptoms.
Table 2.
Mean laboratory values of male and female HL patients at initial diagnosis
| Mean lab values | Total n (%) | Male n (%) | Female n (%) | p |
|---|---|---|---|---|
| Haemoglobin level (g/dL) | 0.001 | |||
| ≤12 | 76 (19.4) | 32 (13.4) | 44 (28.9) | |
| >12 | 315 (80.6) | 207 (86.6) | 108 (71.1) | |
| Albumin level (g/dL) | 0.415 | |||
| ≤3.2 | 45 (11.5) | 30 (12.6) | 15 (9.9) | |
| >3.2 | 346 (88.5) | 209 (87.4) | 137 (90.1) | |
| ESR (mm/h) | 0.021 | |||
| ≤50 | 231 (59.1) | 117 (48.9) | 114 (75.0) | |
| >50 | 160 (40.9) | 122 (51.1) | 38 (25.0) | |
| LDH level (U/L) | 0.760 | |||
| ≤460 | 271 (69.3) | 169 (70.7) | 102 (67.1) | |
| >460 | 120 (30.7) | 70 (29.3) | 50 (32.9) | |
| White blood cell count (per mm3) | 0.985 | |||
| ≤15000 | 357 (91.3) | 217 (90.8) | 140 (92.1) | |
| >15000 | 34 (8.7) | 22 (9.2) | 12 (7.9) | |
| Lymphocyte count (per mm3) | 0.055 | |||
| ≤600 | 15 (3.8) | 4 (1.7) | 11 (7.2) | |
| >600 | 376 (96.2) | 235 (98.3) | 141 (92.8) |
ESR: erythrocyte sedimentation rate; LDH: lactate dehydrogenase; HL: Hodgkin’s lymphoma
Table 3 demonstrates the distribution of HL patients according to the combined stage and prognostic factors. Although patients seemed to have a similar distribution for the 3 categories, the number of patients in advanced stage was higher than those of the other 2 categories.
Table 3.
Distribution of HL patients according to the combined stage and prognostic factors
| Patient distribution | n | % |
|---|---|---|
| Early-stage (I–II) HL with good prognostic factors | 108 | 27.6 |
| Early-stage (I–II) HL with adverse prognostic factors | 131 | 33.5 |
| Advanced stage HL | 151 | 38.6 |
| Unknown | 1 | 0.3 |
HL: Hodgkin’s lymphoma
Treatment modalities used in HL patients are displayed in Table 4. As a primary treatment, the most common chemotherapy regimen was adriamycin, bleomycin, vinblastine, and dacarbazine combination chemotherapy (ABVD) (70.7%), followed by cyclophosphamide, vincristine, procarbazine and prednisone (COPP; 16.1%). During follow-up, 33.2% of the cases (n=130) relapsed (median 25 months; range 6 months–20 years). The most common treatment protocol in relapsing patients was ABVD (36.1%; n=47), especially in patients who previously received COPP. The COPP regimen was used in 15.4% (n=20) of patients who developed relapse. Among patients who relapsed or had primary resistance, 32 were subjected to autologous bone marrow transplantation and 1 to allogeneic bone marrow transplantation. The most commonly used combination chemotherapy as a salvage regimen before bone marrow transplantation was the ifosfamide, mesna, idarubicin, and etoposide combination (IIVP) (n=19; 57.6%). After bone marrow transplantation, 10 patients (30.3%) developed relapse and these patients died. The other patients have survived to date.
Table 4.
Distribution of HL patients according to the combined stage and prognostic factors
| Treatment modalities | n | % |
|---|---|---|
| Radiotherapy only | 33 | 8.4 |
| Combined therapy (chemotherapy and radiotherapy) | 259 | 66.2 |
| Chemotherapy only | 75 | 19.2 |
| No chemotherapy or radiotherapy 6.1 | 24 | |
| Total | 391 | 100.0 |
| Primary Chemotherapy regimen | ||
| ABVD | 236 | 70.7 |
| COPP | 54 | 16.1 |
| COPP/ABVD | 20 | 6.0 |
| Others | 24 | 7.2 |
| Total | 334 | 100.0 |
| Secondary Chemotherapy regimen | ||
| ABVD | 47 | 36.1 |
| COPP | 20 | 15.4 |
| COPP/ABVD | 18 | 13.9 |
| Others | 23 | 17.7 |
| Unknown | 22 | 16.9 |
| 130 | 100.0 | |
| Bone marrow transplantation (dose-escalated chemotherapy + PSCT) | 33 | 8.4 |
ABVD: doxorubicin, bleomycin, vinblastine, and dacarbazine; COPP: cyclophosphamide, vincristine, procarbazine and prednisone; PSCT: peripheral stem cell transplantation
Overall and relapse-free survival curves are shown in Figures 3 and 4, respectively. Five- and 10-year overall survival rates were 90% and 84%, respectively. Five- and 10-year survival rates for patients who received ABVD were 88% and 83%, respectively. While the 10-year survival rate was 95% among stage I patients, it was 62% for stage IV cases. Survival rates according to the combined stage and prognostic factors are depicted in Figures 5 and 6. Early-stage patients with good prognostic factors had better overall and relapse-free survival rates compared to the other 2 groups.
Figure 3.
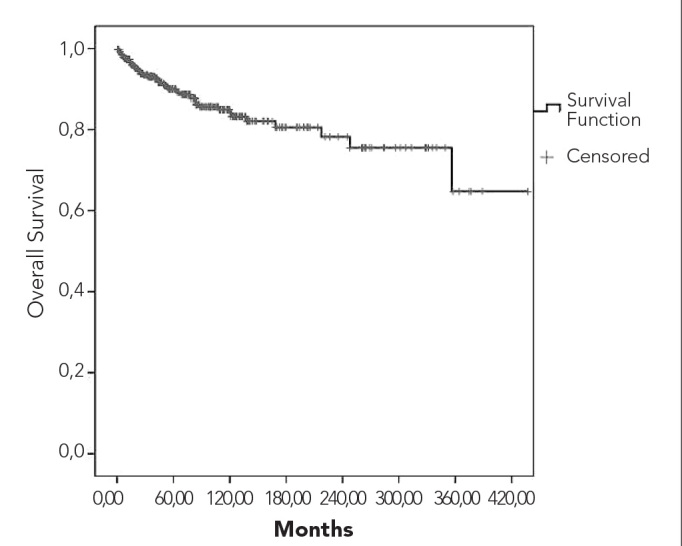
Overall survival curve
Figure 4.
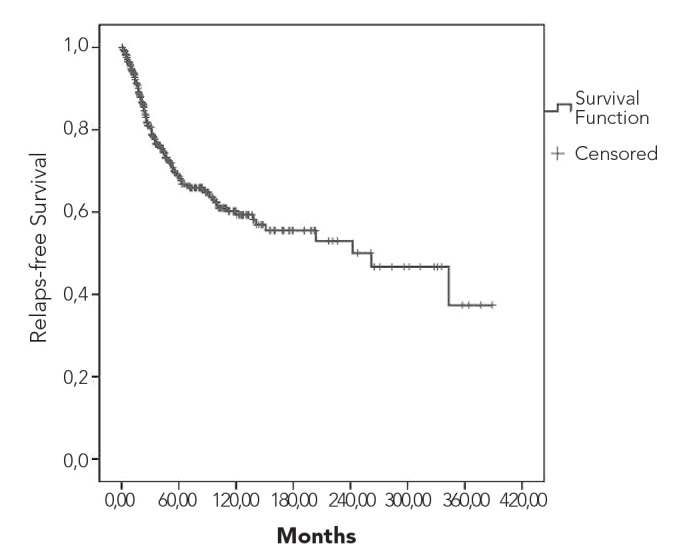
Relapse-free survival curve
Figure 5.
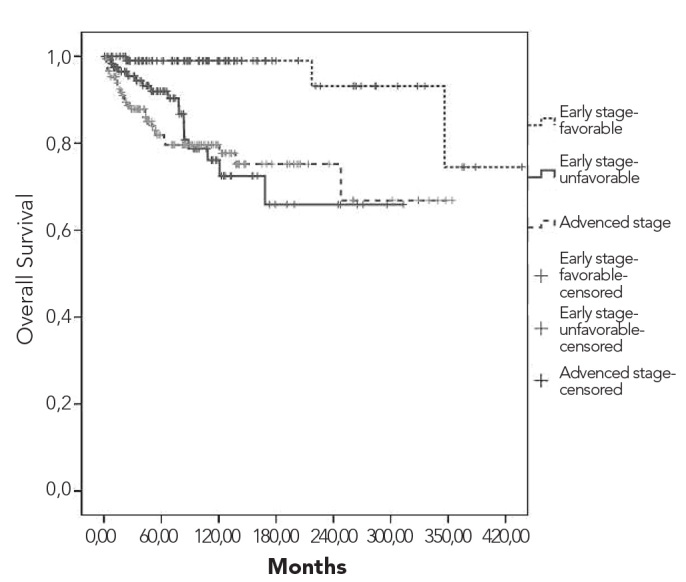
Overall survival curves according to the combined stage and prognostic factors
Figure 6.
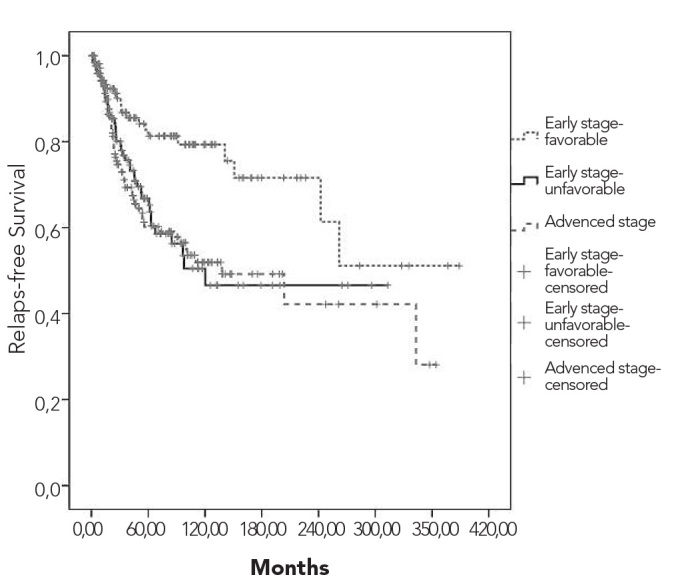
Relapse-free survival curve according to the combined stage and prognostic factors
In univariate analysis, the presence of “B” symptoms, radiotherapy, bulky disease, stage, albumin level, The Eastern Cooperative Oncology Group (ECOG) performance score, ESR, LDH, haemoglobin, bone marrow and extranodal involvement were prognostic factors influencing survival. In multivariate analysis, albumin level, LDH, ECOG performance score and the presence of “B” symptoms were significant prognostic factors. Performance score was the major factor for determining prognosis (Table 5).
Table 5.
Prognostic factors in univariate and multivariate analysis
| p | Exp(B) | 95% CI | ||
|---|---|---|---|---|
| Univariate analysis | ||||
| ECOG Performance score | (0* vs. ≥1) | <0.0001 | ||
| “B” symptoms | (No* vs. yes) | <0.0001 | ||
| Bulky disease | (No* vs. yes) | 0.0028 | ||
| Stage | (I/ II* vs. III/IV) | 0.0001 | ||
| Extranodal involvement | (No* vs. yes) | <0.0001 | ||
| Albumin | (≤3.2* vs. >3.2 g/dL) | <0.0001 | ||
| ESR | (≤50* vs. >50 mm/h) | <0.0001 | ||
| LDH | (≤460* vs. >460 U/L) | <0.0001 | ||
| Haemoglobin | (≤12* vs. >12 gr/dL) | <0.0001 | ||
| Radiotherapy | (No* vs. yes) | 0.0113 | ||
| Multivariate analysis | ||||
| Albumin | >3.2 g/dL (Ref.) | 0.020 | 3.1 | 1.2–7.6 |
| ≤3.2 g/dL) | ||||
| LDH | ≤460 U/L (Ref.) | 0.021 | 5.6 | 1.9–16.9 |
| >460 U/L | ||||
| ECOG Performance score | 0 (Ref.) | <0.001 | 17.5 | 5.7–54.1 |
| ≥1 | ||||
| “B” symptoms | No (Ref.) | 0.003 | 2.8 | 1.4–5.4 |
| Yes |
ESR: erythrocyte sedimentation rate; LDH: lactate dehydrogenase; Ref.: reference;
Reference value for univariate analysis
Discussion
HL cases in developed and developing countries display differences regarding their clinical and histopathological characteristics. This study analysed the clinical and histopathological characteristics as well as factors that have an effect on prognosis among Turkish patients. Epidemiological research showed that HL displayed a bimodal age distribution (8). Socioeconomic status and history of EBV infection seem to play a major role in the aetiology of this disease, particularly among the young (9). EBV incidence could not be investigated due to the retrospective nature of this study.
Our study suggested that the overall HL incidence increased during the second decade of life. However, the gender-specific age distribution of HL cases was different. While the distribution of female patients was similar to the overall distribution, in male patients the disease peaked twice in the 2nd and 4th decades of life. This is similar to the distribution seen in developed countries. The overall peak of the disease in the 2nd decade of life may be attributed to the lack of bimodal distribution among female cases and the smaller number of male cases compared to females. Another significant issue regarding age is that the disease occurs significantly earlier in women than in men.
Similar to other international reports, our study suggested that LDHL and NLPHL subtypes were much less common than other histological types. As mentioned above, the MCHL histological subtype is more common in developing or underdeveloped countries. On the other hand, the NSHL histological sub-type accounts for more than half of all cases in developed parts of the world (4). In addition, NSHL is much more frequent among females. The results of our study indicated that the MCHL sub-type comprised 40% of all HL cases while NSHL accounted for 43%. In addition, the rate of NSHL was more than 50% among females, whereas MCHL was more common among males. In another study from Turkey, MCHL was the most common histological subtype among early-stage HL cases (10). Regarding the distribution of age and the histological subtype, our results seem to be similar to those found in developing countries.
The most common cause of seeking medical care among HL patients is lymph node enlargement, particularly of the cervical lymph nodes (11). More than half of the patients in our study presented with cervical swelling. The frequency of “B” symptoms among HL cases, which has been reported to be 25–30% in the literature, was slightly higher (36%) in our study (12, 13). Extralymphatic involvement was low in our study compared with the results of the relevant literature. Bulky disease was present in 9% of the cases in our study, mostly with NSHL subtype with mediastinal involvement.
The “International Prognostic Factors Project on Advanced Hodgkin’s Disease” assessed the primary care treatment results and prognostic factors in 5141 advanced-stage HL cases from 23 centres (14). This study suggested that 7 parameters had prognostic significance in this patient group. These were age, sex, stage IV disease, low albumin level (<4.0 g/dL), anaemia (<10.5 g/dL), leukocytosis (>15000/mm3) and lymphopenia (<600/mm3).
A number of prognostic factors were defined for early- and advanced-stage HL cases, with bulky disease, ESR, LDH, haemoglobin and serum albumin levels, presence of “B” symptoms, age and extralymphatic involvement being the most significant (6, 14). Treatment for early- and advanced-stage HL cases is designed considering these factors. In our study, stage I and II cases accounted for 60% of all HL cases. Moreover, serum LDH and ESR levels were high among patients with poor prognostic factors such as the “B” symptoms, bulky disease and extranodal involvement. In addition, other factors with prognostic significance such as serum albumin and haemoglobin were less common among those same patients with poor prognostic factors.
The results of our study suggested that up to 8% of our patients were treated only with radiotherapy. The most common chemotherapy regimen was ABVD (71%) and 20% of the patients were treated with COPP or alternatively with COPP/ABVD. Overall, 8% received dose-escalated chemotherapy followed by peripheral stem cell transplantation (PSCT). Although ABVD was intensively used after the 1990s, the most common treatment regimen was COPP in previous years. The standard treatment regimen is ABVD in early-stage patients and bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone (BEACOPP) or dose-escalated BEACOPP for advanced-stage disease (15–20).
HL has become a more curable disease recently due to the novel diagnostic methods and potent treatment regimens. Compared to non-Hodgkin’s lymphoma, HL displays better survival rates (21). Five-year survival rates in HL cases is 60–80% (21–23), increasing up to 90% in early-stage cases (21, 23). Survival rates in our study were similar to those reported in other studies (95% in early-stage patients of our study) (21, 23), but survival decreases with progressing stage. Analysis of survival rates regarding prognostic factors and disease stages revealed that relapse-free survival rates were comparable for early-stage patients with adverse prognostic factors and advanced-stage patients; in fact, patients with advanced-stage disease had non-significant but somewhat better survival rates.
Two studies from Turkey reported treatment results for early stage HL patients. Coskun et al. (10) reported 90% and 55% for 5-year overall and relapse-free survival rates, respectively. In the prospective study by Yildiz et al. (24), 5-year overall and relapse-free survival rates for early-stage HL patients were 98% and 95%, respectively. The survival rates in both studies were comparable to those reported for early-stage (stage I and II) HL cases in our study. In another study, which compared the results of advanced-stage HL patients treated with alternating COPP/ABVD or ABVD regimens with those who were previously treated with COPP in the same medical centre revealed that the overall and disease-free survival rates with COPP/ABDV were similar to those with COPP (25). We showed that patients who had received COPP and relapsed were successfully treated with the ABDV regimen.
The major disadvantages of retrospective studies are survival bias and time factor. Considering that the patients enrolled in this study had been diagnosed more than 10 years ago and were included because they managed to survive until the study initiation, we can conclude that only those patients with a favourable outcome were assessed. The fact that only those patients who had been diagnosed at or before 1990 and had achieved remission were included in this study, the lack of a patient list for that time frame and patients who died or were lost to follow-up might affect the survival rates significantly. The subgroup analysis revealed that patients treated with COPP had similar or even better survival rates compared to those who received ABDV clearly supports this opinion, because better results with conventional treatment regimens compared to those with current standard treatment with ABDV are contradictory to the findings in the literature. We think that the similar survival rates in patients treated with ABVD and COPP regimens are associated with survival bias.
In the overall analysis including early- and advanced-stage HL cases in our study, stage, presence of “B” symptoms, radiotherapy, serum albumin level, ECOG performance score, ESR, serum LDH level, anaemia, bone marrow involvement and extralymphatic disease were factors influencing survival. Reassessment with multivariate analysis of factors determined to be of prognostic significance in the univariate analysis revealed that low serum albumin level, high serum LDH level, ECOG performance score 2 or higher and the presence of “B” symptoms retained their significance as independent adverse prognostic factors. Despite the retrospective nature of our study, these results were similar to those of other studies (6, 14).
This study revealed the demographic, clinical and prognostic features of HL patients treated in our hospital. Although the data obtained from one medical centre cannot be generalised countrywide, considering that the patients of our hospital come from all the regions of Turkey, this study provides a general picture of HL patients in the country. Given the size of the patient group, which is the largest to date, the results are significant and meaningful.
Footnotes
Ethics Committee Approval: N/A.
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – S.K., I.B., G.T.; Design - S.K., G.T.; Supervision - S.K., U.S., A.K.; Resource S.K., I.B., S.U.; Materials - S.K., S.G.; Data Collection&/or Processing - S.K., S.G.; Analysis&/or Interpretation - S.K., I.B., F.Y.; Literature Search - S.K., S.G.; Writing - S.K., I.B., U.S.; Critical Reviews - S.K., I.B., I.C., Y.O.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Ferlay J, Bray F, Pisani P, et al., editors. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. Lyon: IARC Press; 2004. [Google Scholar]
- 2.Pahwa P, Karunanayake CP, Spinelli JJ, Dosman JA, McDuffie HH. Ethnicity and incidence of Hodgkin’s lymphoma in Canadian population. BMC Cancer. 2009;9:141–9. doi: 10.1186/1471-2407-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yung L, Linch D. Hodgkin’s lymphoma. Lancet. 2003;361:943–51. doi: 10.1016/S0140-6736(03)12777-8. [DOI] [PubMed] [Google Scholar]
- 4.MacLennan KA, Bennett MH, Tu A, Hudson BV, Easterling MJ, Hudson GV, et al. Relationship of histopathologic features to survival and relapse in nodular sclerosing Hodgkin’s disease. A study of 1659 patients. Cancer. 1989;64:1686–93. doi: 10.1002/1097-0142(19891015)64:8<1686::aid-cncr2820640822>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Al-Salam S, John A, Daoud S, Chong SM, Castella A. Expression of Epstein-Barr virus in Hodgkin’s lymphoma in a population of United Arab Emirates nationals. Leuk Lymphoma. 2008;49:1769–77. doi: 10.1080/10428190802270894. [DOI] [PubMed] [Google Scholar]
- 6.Connors JM. State-of-the-art therapeutics: Hodgkin’s lymphoma. J Clin Oncol. 2005;23:6400–8. doi: 10.1200/JCO.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Fong D, Steurer M, Greil R, Gunsilius E, Spizzo G, Gastl G, et al. Hodgkin’s lymphoma in Tyrol-a population-based study. Ann Hematol. 2009;88:449–56. doi: 10.1007/s00277-008-0618-1. [DOI] [PubMed] [Google Scholar]
- 8.McMahon B. Epidemiological evidence on the nature of Hodgkin’s disease. Cancer. 1957;10:1045–54. doi: 10.1002/1097-0142(195709/10)10:5<1045::aid-cncr2820100527>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Gutensohn NM, Shapiro DS. Social class risk factors among children with Hodgkin’s disease. Int J Cancer. 1982;30:433–5. doi: 10.1002/ijc.2910300409. [DOI] [PubMed] [Google Scholar]
- 10.Coskun HS, Er O, Eser B, et al. Early stage Hodgkin’s disease: Experiences of Erciyes University. Erciyes Med J. 2002;24:120–5. [Google Scholar]
- 11.Hodgson DC, Gospodarowicz MK. Clinical evaluation and staging of Hodgkin’s lymphoma. In: Hoppe RT, Mauch PM, Armitage JO, Diehl V, Weiss LM, editors. Hodgkin’s lymphoma. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 123–32. [Google Scholar]
- 12.Hoppe RT, Hanlon AL, Hanks GE, Owen JB. Progress in the treatment of Hodgkin’s disease in the United States, 1973 versus 1983. The Patterns of Care Study. Cancer. 1994;74:3198–203. doi: 10.1002/1097-0142(19941215)74:12<3198::aid-cncr2820741219>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy BJ, Loeb V, Jr, Peterson VM, Donegan WL, Natarajan N, Mettlin C. National survey of patterns of care for Hodgkin’s disease. Cancer. 1985;56:2547–56. doi: 10.1002/1097-0142(19851201)56:11<2547::aid-cncr2820561102>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 14.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s Disease. N Engl J Med. 1998;339:1506–14. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 15.Cosset JM, Henry-Amar M, Noordijk P, et al. The EORTC trials for adult patients with early stage Hodgkin’s disease. A 1997 update; 39th Ann Sci Meet ASTRO; Orlando, FL. 1997. [Google Scholar]
- 16.Klimm BD, Engert A, Brillant C, et al. Comparison of BEACOPP and ABVD chemotherapy in intermediate stage Hodgkin’s lymphoma: Results of the fourth interim analysis of the HD 11 trial of GHSG. Proc Am Soc Clin Oncol. 2005;23:561. [Google Scholar]
- 17.Diehl V, Brilliant C, Engert A, et al. Reduction of combined modality treatment intensity in early stage Hodgkin’s lymphoma, interim analysis of HD10 trial of GHSG. Eur J Haematol. 2004;73:37. [Google Scholar]
- 18.Diehl V. Dose-escalation study for the treatment of Hodgkin’s disease. The German Hodgkin’s Lymphoma Study Group (GHSG) Ann Hematol. 1993;66:139–40. doi: 10.1007/BF01697624. [DOI] [PubMed] [Google Scholar]
- 19.Diehl V, Franklin J, Pfreundschuh M, Lathan B, Paulus U, Hasenclever D, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348:2386–95. doi: 10.1056/NEJMoa022473. [DOI] [PubMed] [Google Scholar]
- 20.Sieber M, Bredenfeld H, Josting A, Reineke T, Rueffer U, Koch T, et al. 14-day variant of the bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone regimen in advanced-stage Hodgkin’s lymphoma: results of a pilot study of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol. 2003;21:1734–9. doi: 10.1200/JCO.2003.06.028. [DOI] [PubMed] [Google Scholar]
- 21.Storm HH, Klint A, Tryggvadóttir L, Gislum M, Engholm G, Bray F, et al. Trends in the survival of patients diagnosed with malignant neoplasms of lymphoid, haematopoietic, and related tissue in the Nordic countries 1964–2003 followed up to the end of 2006. Acta Oncol. 2010;49:694–712. doi: 10.3109/02841861003631495. [DOI] [PubMed] [Google Scholar]
- 22.Sant M, Capocaccia R, Coleman MP, et al. EUROCARE Working Group. Cancer survival increases in Europe, but international differences remain wide. Eur J Cancer. 2001;37:1659–67. doi: 10.1016/s0959-8049(01)00206-4. [DOI] [PubMed] [Google Scholar]
- 23.http://seer.cancer.gov/publications/survival/surv_hodgkin.pdf (accessed date: 12 July 2011)
- 24.Yildiz F, Zengin N, Engin H, Güllü I, Barista I, Caglar M, et al. Prospective study of combined modality treatment or radiotherapy alone in the management of early-stage adult Hodgkin’s disease. Int J Radiat Oncol Biol Phys. 2004;60:839–46. doi: 10.1016/j.ijrobp.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Barista I, Tekuzman G, Baltali E, et al. COPP/ABVD regimens in patients with advance stage Hodgkin’s disease. Turk Hematoloji-Onkoloji Dergisi. 1991;1:40–7. [Google Scholar]


