Dear Editor,
The first outbreak of OXA-48-producing Klebsiella pneumoniae isolates was reported in Turkey in 2008 (1). Two different clones were detected among 39 K. pneumoniae isolates in the first published outbreak (1). In addition to the clones of individual hospitals, the plasmid-mediated nature of OXA-48 offers the opportunity for horizontal transfer between strains and genera, resulting in dissemination both on a national and international level (2, 3).
Sixteen carbapenem-resistant strains of K. pneumoniae isolated from patients hospitalised at Başkent University Hospital between the years 2007 and 2011 were included in this study. All strains were isolated from patients in intensive care units. Five of the 16 strains were isolated from solid organ transplant recipients. Carbapenem-resistant K. pneumoniae isolates were screened by PCR amplication of genes encoding a variety of carbapenemases, using a multiplex real-time PCR assay described by Monteiro et al. (4) with minor modifications. The presence of genes encoding blaTEM, blaSHV, and blaCTX-M types of ESBLs was investigated according to the method described by Mostein et al. (5). Pulsed-field gel electrophoresis (PFGE) typing of the K. pneumoniae isolates was performed according to a previously described protocol (6).
A total of eight genetic clones were identified in the PFGE analysis. All strains were found to be positive for the gene encoding blaOXA-48, but none were positive for the IMP, VIM and NDM types of metalloenzymes, or the GES and KPC types of carbapenemases. DNA sequencing confirmed the presence of the OXA-48 gene in all isolates.
A total of 11 isolates were found to be harbouring at least one of the TEM and CTX-M types of ESBL genes and the remaining five were negative for these enzymes in the PCR analysis. The SHV type ESBL gene was not present in any of the strains. The characteristics of the 16 isolates are shown in Figure 1.
Figure 1.
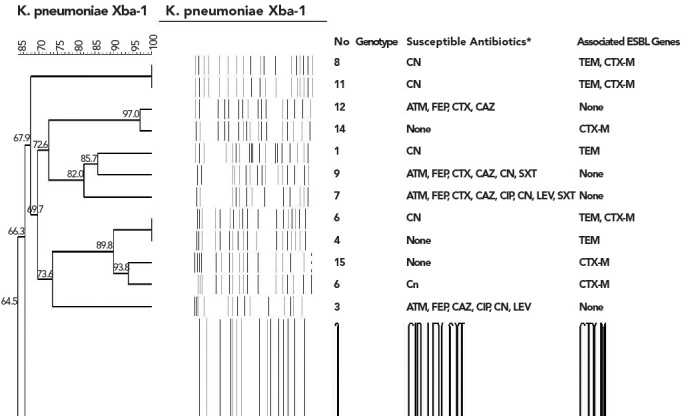
Pulse field gel electrophoresis patterns of K.penumonia strains with OXA-48 like enzymes
In SDS-PAGE analysis, 13 strains were found to be lacking in at least one of the OmpL35 and OmpK36 porin proteins. A representative gel image of the outer membrane protein profiles of the isolates is shown in Figure 2.
Figure 2.
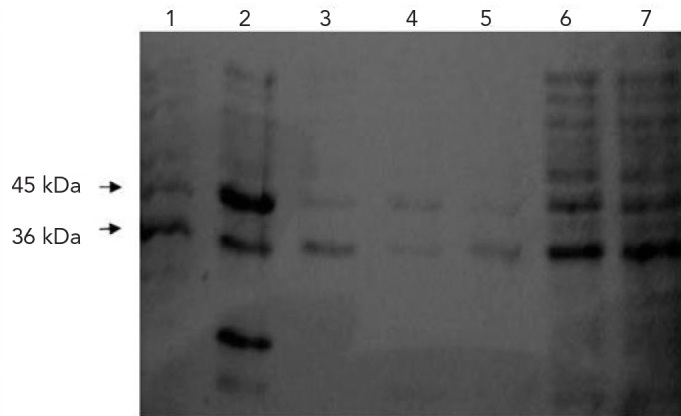
SDS-PAGE image of outer membrane proteins
In vitro susceptibility test showed that all K. pneumonia isolates were susceptible to amikacin and colistin. Only one isolate was resistant to tigecycline (number 7). Eleven strains were positive for ESBL production in the double disc diffusion test, and no isolate was positive for MBL production as tested with E-test strips.
Imipenem and meropenem MIC values generally fall into the ‘susceptible’ category for OXA-48-producing strains, if it is the only resistance mechanism present (7). However, the presence of other resistance mechanisms along with OXA-48, such as outer membrane protein loss, results in high imipenem and meropenem MIC values. All strains in this study had MICs of 32 μg/mL for carbapenems, i.e. they were resistant. This result seems to be related to the presence of accompanying resistance mechanisms, such as outer membrane protein loss. A similar co-presentation, i.e. outer membrane protein loss and OXA-48 positivity, was recently published in Turkey (8).
There seems to be no dominant clone among our isolates. This finding is in accordance with the previously published data emphasising the horizontal dissemination between strains and even species (2, 9). In this study, clonal diversity implicates the plasmid-mediated dissemination of OXA-48-mediated resistance in clonal and non-clonal ways in our country. The accumulation of different resistance mechanisms, such as ESBL production and outer membrane protein loss, makes OXA-48-producing K. pneumoniae strains more resistant to available therapeutic agents.
Footnotes
Ethics Committee Approval: This study was approved by Başkent University Institutional Review Board (Project no: DA12/24)..
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – Ö.K.A.; Design - Ö.K.A, B.O.; Supervision - Y.Y.; Resource - Ö.K.A., B.O., Y.Y.; Materials - B.O., Y.Y.; Data Collection&/or Processing - Ö.K.A, B.O, A.Y.; Analysis&/or Interpretation - Ö.K.A, B.O., Y.Y.; Literature Search - Ö.K.A, A.Y.; Writing - Ö.K.A., Y.Y.; Critical Reviews - Ö.K.A., Y.Y.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Carrer A, Poirel L, Eraksoy H, Çağatay AA, Badur S, Nordmann P. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in İstanbul, Turkey. Antimicrob Agents Che-mother. 2008;52:2950–4. doi: 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. Characterization of Enterobacteriaceae producing OXA-48 like carbapenemases in the UK. J Antimicrob Cheomther. 2012;67:1660–5. doi: 10.1093/jac/dks124. [DOI] [PubMed] [Google Scholar]
- 3.van der Bij A, Pitout JD. The role of international travel in the worldwide spread of multiresistant Enterobacteriaceae. J Antimicrob Chemother. 2012;67:2090–100. doi: 10.1093/jac/dks214. [DOI] [PubMed] [Google Scholar]
- 4.Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S. Rapid detection of carbapenemase genes by multiplex real-time PCR. J Antimicrob Chemother. 2012;67:906–9. doi: 10.1093/jac/dkr563. [DOI] [PubMed] [Google Scholar]
- 5.Monstein HJ, Ostholm-Balkhed A, Nilsson MV, Nilsson M, Dorn-busch K, Nilsson LE. Multiplex PCR amplification assay for the detection of blaSHV, blaTEM and blaCTX-M genes in Enterobacteriaceae. APMIS. 2007;115:1400–8. doi: 10.1111/j.1600-0463.2007.00722.x. [DOI] [PubMed] [Google Scholar]
- 6.Durmaz R, Otlu B, Koksal F, Hosoglu S, Ozturk R, Ersoy Y, et al. The optimization of a rapid pulsed-field gel electrophoresis protocol for the typing of Acinetobacter baumannii, Escherichia coli and Klebsiella spp. Jpn J Infect Dis. 2009;62:372–7. [PubMed] [Google Scholar]
- 7.Mlynarczyk G, Kosykowska E, Walter de Walthoffen SW, Szymanek-Majchrzak K, Sawicka-Grzelak A, Baczkoska T, et al. A threat of the Klebsiella pneumoniae carbapenemase-producing strains among transplant recipients. Transplantation Proceed. 2011;43:3135–6. doi: 10.1016/j.transproceed.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Gülmez D, Woodford N, Palepou MF, Mushtaq S, Metan G, Yakupogullari Y, et al. carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48 like carbapenemases and outer membrane protein loss. Int J Antimicrob Agents. 2008;31:523–6. doi: 10.1016/j.ijantimicag.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Carrer A, Poirel L, Yılmaz M, Akan ÖA,, Cilli F, Cuzon G, et al. Spread of OXA-48-encoding polasmid in Turkey and beyond. Antimicrob Agents Chemother. 2010;54:1369–73. doi: 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


