Abstract
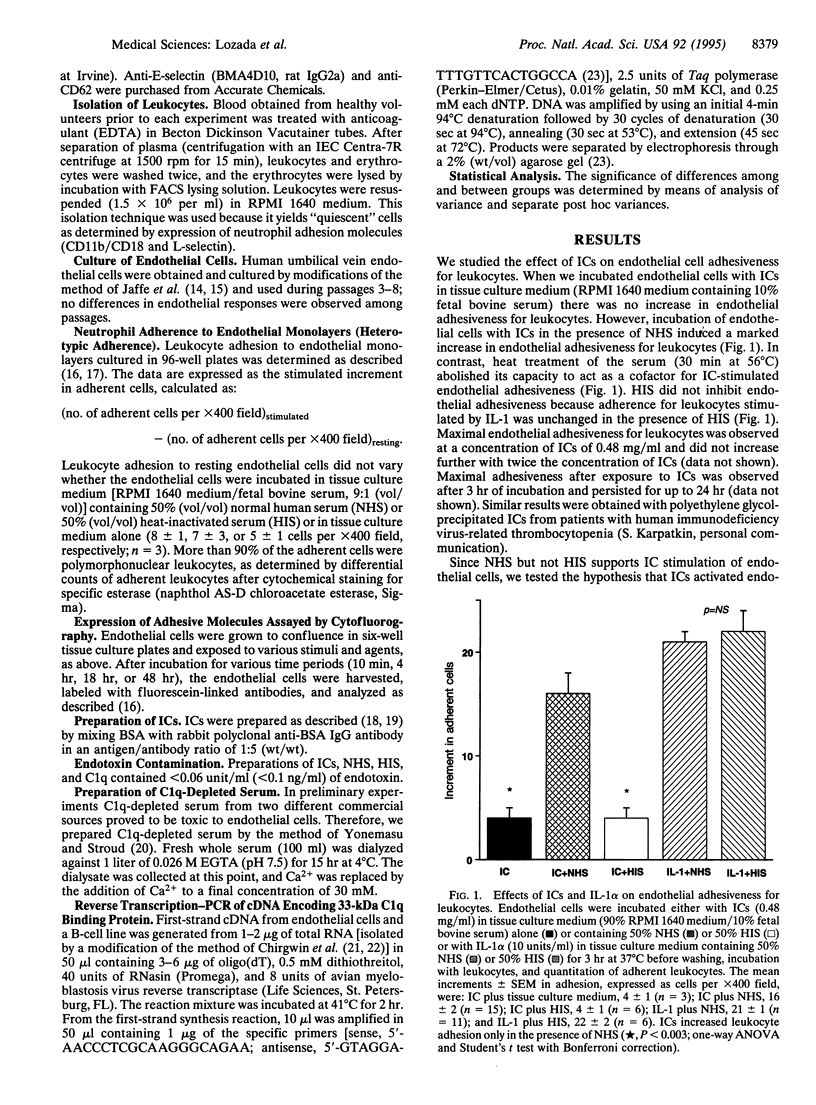
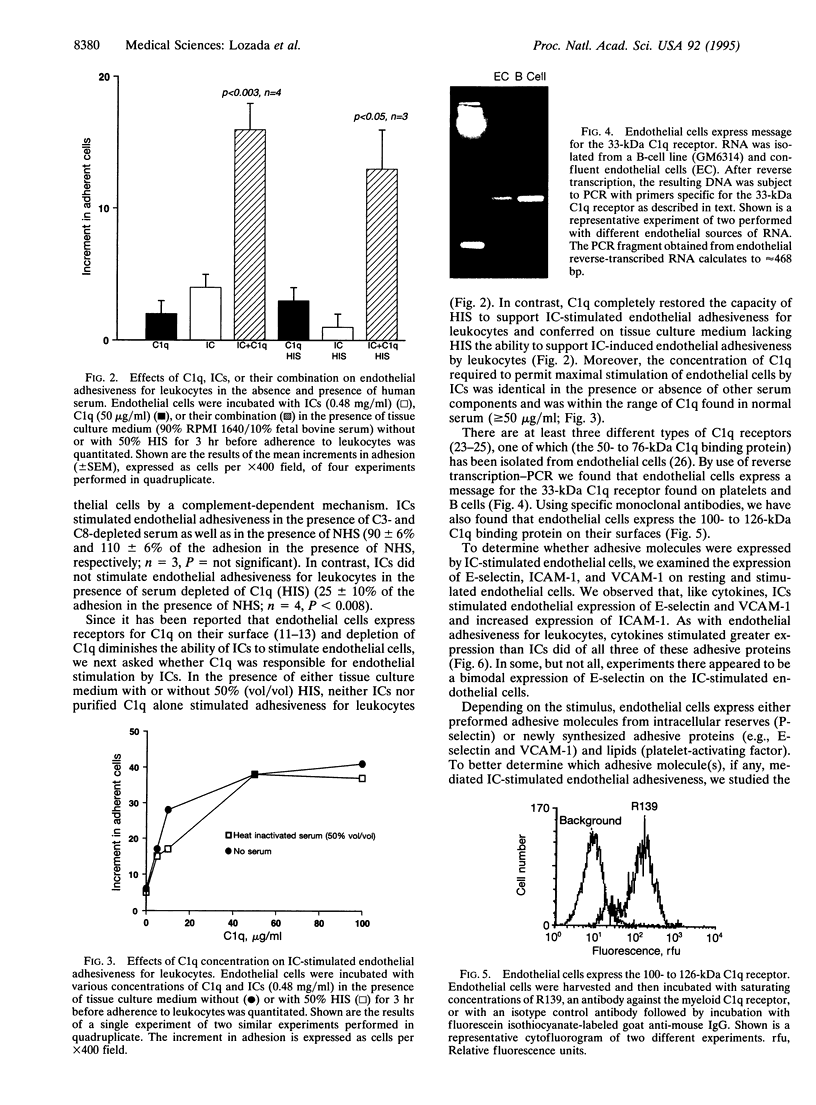
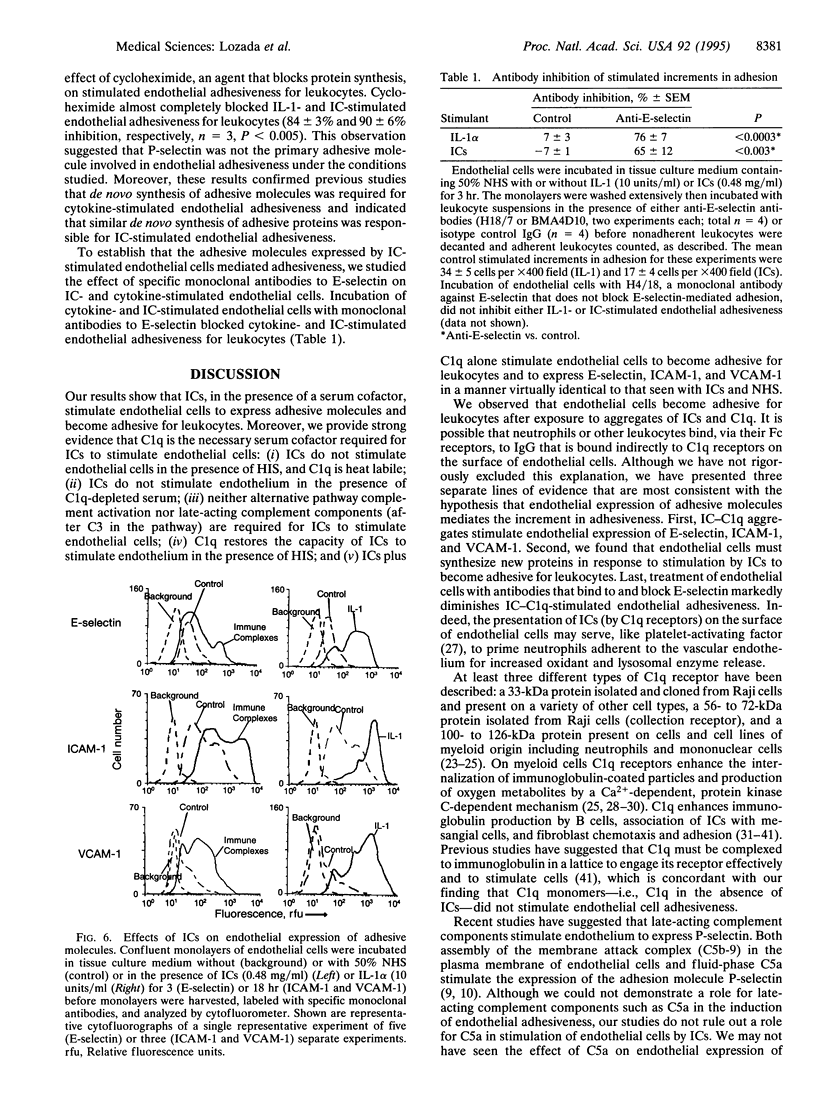
To examine the role of complement components as regulators of the expression of endothelial adhesive molecules in response to immune complexes (ICs), we determined whether ICs stimulate both endothelial adhesiveness for leukocytes and expression of E-selectin and intercellular and vascular cell adhesion molecules 1 (ICAM-1 and VCAM-1). We found that ICs [bovine serum albumin (BSA)-anti-BSA] stimulated endothelial cell adhesiveness for added leukocytes in the presence of complement-sufficient normal human serum (NHS) but not in the presence of heat-inactivated serum (HIS) or in tissue culture medium alone. Depletion of complement component C3 or C8 from serum did not prevent enhanced endothelial adhesiveness stimulated by ICs. In contrast, depletion of complement component C1q markedly inhibited IC-stimulated endothelial adhesiveness for leukocytes. When the heat-labile complement component C1q was added to HIS, the capacity of ICs to stimulate endothelial adhesiveness for leukocytes was completely restored. Further evidence for the possible role of C1q in mediating the effect of ICs on endothelial cells was the discovery of the presence of the 100- to 126-kDa C1q-binding protein on the surface of endothelial cells (by cytofluorography) and of message for the 33-kDa C1q receptor in resting endothelial cells (by reverse transcription-PCR). Inhibition of protein synthesis by cycloheximide blocked endothelial adhesiveness for leukocytes stimulated by either interleukin 1 or ICs in the presence of NHS. After stimulation with ICs in the presence of NHS, endothelial cells expressed increased numbers of adhesion molecules (E-selectin, ICAM-1, and VCAM-1). Endothelial expression of adhesion molecules mediated, at least in part, endothelial adhesiveness for leukocytes, since leukocyte adhesion was blocked by monoclonal antibodies directed against E-selectin. These studies show that ICs stimulate endothelial cells to express adhesive proteins for leukocytes in the presence of a heat-labile serum factor. That factor appears to be C1q.
Full text
PDF

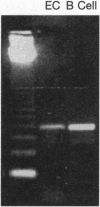
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akamine A., Raghu G., Narayanan A. S. Human lung fibroblast subpopulations with different C1q binding and functional properties. Am J Respir Cell Mol Biol. 1992 Apr;6(4):382–389. doi: 10.1165/ajrcmb/6.4.382. [DOI] [PubMed] [Google Scholar]
- Andrews B. S., Shadforth M., Cunningham P., Davis J. S., 4th Demonstration of a C1q receptor on the surface of human endothelial cells. J Immunol. 1981 Sep;127(3):1075–1080. [PubMed] [Google Scholar]
- Beynon H. L., Davies K. A., Haskard D. O., Walport M. J. Erythrocyte complement receptor type 1 and interactions between immune complexes, neutrophils, and endothelium. J Immunol. 1994 Oct 1;153(7):3160–3167. [PubMed] [Google Scholar]
- Bobak D. A., Gaither T. A., Frank M. M., Tenner A. J. Modulation of FcR function by complement: subcomponent C1q enhances the phagocytosis of IgG-opsonized targets by human monocytes and culture-derived macrophages. J Immunol. 1987 Feb 15;138(4):1150–1156. [PubMed] [Google Scholar]
- Bordin S., Smith M., Ghebrehiwet B., Oda D., Page R. C. Smooth muscle and epithelial cells express specific binding sites for the C1q component of complement. Clin Immunol Immunopathol. 1992 Apr;63(1):51–57. doi: 10.1016/0090-1229(92)90093-4. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cines D. B., Lyss A. P., Bina M., Corkey R., Kefalides N. A., Friedman H. M. Fc and C3 receptors induced by herpes simplex virus on cultured human endothelial cells. J Clin Invest. 1982 Jan;69(1):123–128. doi: 10.1172/JCI110422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Eberle M. A., Gruber H. E., Levin R. I. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2441–2445. doi: 10.1073/pnas.88.6.2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronstein B. N., Weissmann G. The adhesion molecules of inflammation. Arthritis Rheum. 1993 Feb;36(2):147–157. doi: 10.1002/art.1780360204. [DOI] [PubMed] [Google Scholar]
- Daha M. R., Klar N., Hoekzema R., van Es L. A. Enhanced Ig production by human peripheral lymphocytes induced by aggregated C1q. J Immunol. 1990 Feb 15;144(4):1227–1232. [PubMed] [Google Scholar]
- Daha M. R., Miltenburg A. M., Hiemstra P. S., Klar-Mohamad N., Van Es L. A., Van Hinsbergh V. W. The complement subcomponent C1q mediates binding of immune complexes and aggregates to endothelial cells in vitro. Eur J Immunol. 1988 May;18(5):783–787. doi: 10.1002/eji.1830180519. [DOI] [PubMed] [Google Scholar]
- Foreman K. E., Vaporciyan A. A., Bonish B. K., Jones M. L., Johnson K. J., Glovsky M. M., Eddy S. M., Ward P. A. C5a-induced expression of P-selectin in endothelial cells. J Clin Invest. 1994 Sep;94(3):1147–1155. doi: 10.1172/JCI117430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedl H. P., Till G. O., Ryan U. S., Ward P. A. Mediator-induced activation of xanthine oxidase in endothelial cells. FASEB J. 1989 Nov;3(13):2512–2518. doi: 10.1096/fasebj.3.13.2806779. [DOI] [PubMed] [Google Scholar]
- Ghebrehiwet B. Functions associated with the C1q receptor. Behring Inst Mitt. 1989 Jul;(84):204–215. [PubMed] [Google Scholar]
- Ghebrehiwet B., Lim B. L., Peerschke E. I., Willis A. C., Reid K. B. Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular "heads" of C1q. J Exp Med. 1994 Jun 1;179(6):1809–1821. doi: 10.1084/jem.179.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M., Roos D., Kaplan H. B., Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975 Nov;56(5):1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E. B., Tenner A. J. Signal transduction mechanisms of C1q-mediated superoxide production. Evidence for the involvement of temporally distinct staurosporine-insensitive and sensitive pathways. J Immunol. 1992 Jun 15;148(12):3920–3928. [PubMed] [Google Scholar]
- Guan E. N., Burgess W. H., Robinson S. L., Goodman E. B., McTigue K. J., Tenner A. J. Phagocytic cell molecules that bind the collagen-like region of C1q. Involvement in the C1q-mediated enhancement of phagocytosis. J Biol Chem. 1991 Oct 25;266(30):20345–20355. [PubMed] [Google Scholar]
- Guan E., Robinson S. L., Goodman E. B., Tenner A. J. Cell-surface protein identified on phagocytic cells modulates the C1q-mediated enhancement of phagocytosis. J Immunol. 1994 Apr 15;152(8):4005–4016. [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., McEver R. P., Sims P. J. Complement proteins C5b-9 induce secretion of high molecular weight multimers of endothelial von Willebrand factor and translocation of granule membrane protein GMP-140 to the cell surface. J Biol Chem. 1989 May 25;264(15):9053–9060. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Dame M. K., Lane C. L., Smith C. W., Anderson D. C., Ward P. A. Role of endothelial-leukocyte adhesion molecule 1 (ELAM-1) in neutrophil-mediated lung injury in rats. J Clin Invest. 1991 Oct;88(4):1396–1406. doi: 10.1172/JCI115446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy H. S., Shayman J. A., Till G. O., Mahrougui M., Owens C. B., Ryan U. S., Ward P. A. Superoxide responses of endothelial cells to C5a and TNF-alpha: divergent signal transduction pathways. Am J Physiol. 1992 Jul;263(1 Pt 1):L51–L59. doi: 10.1152/ajplung.1992.263.1.L51. [DOI] [PubMed] [Google Scholar]
- Oiki S., Okada Y. C1q induces chemotaxis and K+ conductance activation coupled to increased cytosolic Ca2+ in mouse fibroblasts. J Immunol. 1988 Nov 1;141(9):3177–3185. [PubMed] [Google Scholar]
- Peerschke E. I., Ghebrehiwet B. Platelet C1q receptor interactions with collagen- and C1q-coated surfaces. J Immunol. 1990 Nov 1;145(9):2984–2988. [PubMed] [Google Scholar]
- Peerschke E. I., Ghebrehiwet B. Platelet C1q receptor interactions with collagen- and C1q-coated surfaces. J Immunol. 1990 Nov 1;145(9):2984–2988. [PubMed] [Google Scholar]
- Peerschke E. I., Ghebrehiwet B. Platelet interactions with C1q in whole blood and in the presence of immune complexes or aggregated IgG. Clin Immunol Immunopathol. 1992 Apr;63(1):45–50. doi: 10.1016/0090-1229(92)90092-3. [DOI] [PubMed] [Google Scholar]
- Peerschke E. I., Malhotra R., Ghebrehiwet B., Reid K. B., Willis A. C., Sim R. B. Isolation of a human endothelial cell C1q receptor (C1qR). J Leukoc Biol. 1993 Feb;53(2):179–184. doi: 10.1002/jlb.53.2.179. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Del Vecchio P. J., Ryan J. W. Endothelial cells of bovine pulmonary artery lack receptors for C3b and for the Fc portion of immunoglobulin G. Science. 1980 May 16;208(4445):748–749. doi: 10.1126/science.7367890. [DOI] [PubMed] [Google Scholar]
- Ryan U. S., Schultz D. R., Ruan J. W. Fc and C3b receptors on pulmonary endothelial cells: induction by injury. Science. 1981 Oct 30;214(4520):557–558. doi: 10.1126/science.6270789. [DOI] [PubMed] [Google Scholar]
- Sedmak D. D., Davis D. H., Singh U., van de Winkel J. G., Anderson C. L. Expression of IgG Fc receptor antigens in placenta and on endothelial cells in humans. An immunohistochemical study. Am J Pathol. 1991 Jan;138(1):175–181. [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994 Jan 28;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Identification of types of cells in human peripheral blood that bind C1q. J Immunol. 1981 Mar;126(3):1174–1179. [PubMed] [Google Scholar]
- Tenner A. J., Cooper N. R. Stimulation of a human polymorphonuclear leukocyte oxidative response by the C1q subunit of the first complement component. J Immunol. 1982 Jun;128(6):2547–2552. [PubMed] [Google Scholar]
- Ward P. A., Zvaifler N. J. Quantitative phagocytosis by neutrophils. I. A new method with immune complexes. J Immunol. 1973 Dec;111(6):1771–1776. [PubMed] [Google Scholar]
- Webster R. O., Hong S. R., Johnston R. B., Jr, Henson P. M. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology. 1980 Jun;2(3):201–219. doi: 10.1016/0162-3109(80)90050-8. [DOI] [PubMed] [Google Scholar]
- Yonemasu K., Stroud R. M. Clq: rapid purification method for preparation of monospecific antisera and for biochemical studies. J Immunol. 1971 Feb;106(2):304–313. [PubMed] [Google Scholar]
- Young K. R., Jr, Ambrus J. L., Jr, Malbran A., Fauci A. S., Tenner A. J. Complement subcomponent C1q stimulates Ig production by human B lymphocytes. J Immunol. 1991 May 15;146(10):3356–3364. [PubMed] [Google Scholar]
- van den Dobbelsteen M. E., van der Woude F. J., Schroeijers W. E., Klar-Mohamad N., van Es L. A., Daha M. R. C1Q, a subunit of the first component of complement, enhances the binding of aggregated IgG to rat renal mesangial cells. J Immunol. 1993 Oct 15;151(8):4315–4324. [PubMed] [Google Scholar]