Abstract
Objective:
Ivermectin and sulphadiazine were tested individually to determine their in vitro effects on Toxoplasma gondii grown in human epidermoid larynx carcinoma (Hep-2) cell culture.
Study Design:
In-vitro study.
Material and Methods:
Toxoplasma growth was quantities by an enzyme immunoassay performed directly on the fixed cultures, using a rabbit anti-T. gondii immunoglobulin G as the first antibody and a phosphatase-labeled anti-rabbit immunoglobulin G as the second antibody. For each drug, regression models were used to quantify the relationship between optical density values and antimicrobial agent concentrations in the cultures.
Results:
The 50% inhibitory concentrations (IC50) of ivermectin and sulphadiazine were found to be 0.2 μg/mL and 7.3 μg/mL after 48 h of exposure, respectively. None of the concentrations tested for each drugs demonstrated toxicity to Hep-2 cells after 72 h of incubation.
Conclusion:
These results indicate that ivermectin significantly inhibited replication of the tachyzoites of T. gondii RH strain.
Keywords: Toxoplasma gondii, ivermectin, sulphadiazine, in vitro, Hep-2
Introduction
Toxoplasma gondii is a protozoan parasite that infects up to a third of the world’s population. In most individuals, acute infection with T. gondii is asymptomatic or causes mild symptoms. However, toxoplasmosis can cause severe diseases in fetuses if a seronegative pregnant woman is infected. Toxoplasmosis can also be fatal in immunodeficiency or immuno-compromised individuals (1, 2).
The combination of sulphadiazine (SDZ) and pyrimethamine is frequently used for the treatment of T. gondii infections. These drugs have a remarkable synergistic activity against the replicating form of T. gondii through the sequential inhibition of parasite dihydropteroate synthase (DHPS) and dihydrofolate reductase (DHFR) (3). However, treatment fails to eliminate the encysted form of the parasite. Furthermore, treatment with these agents is associated with frequent and severe adverse reactions, especially in patients with acquired immunodeficiency syndrome (4). The development of new alternatives to sulphadiazine and pyrimethamine therapy requires better knowledge of the effects of antimicrobial agents on T. gondii.
Recently, Merli et al. (5) developed an enzyme immunoassay for evaluation of Toxoplasma growth in tissue culture and suggested its application to study the in vitro activity of anti-T. gondii compounds. With this method, the relationship between the inhibitory effect of a drug and its concentration in the culture medium can be determined by regression analysis (6).
The aim of this study was to investigate the in vitro anti-Toxoplasma activities of antiparasitic ivermectin and sulphadiazine, currently one of the most effective available therapeutic agents, using tachyzoit of T. gondii RH strain infected Hep-2 epithelial cells. For this purpose, Hep-2 cells in tissue culture plates were infected with tachyzoites isolated from peritoneal fluid of mice infected with T. gondii. Subsequently, ivermectin or sulphadiazine was added. Inhibitory activity was assessed after 24 h, 48 h and 72 h incubations using invert microscopy and at 48 h by enzyme-linked immunosorbent assay (ELISA). The toxicities of ivermectin, sulfadiazine and tachyzoit to Hep-2 cells were determined with Neutral Red Uptake (NRU) assay.
Material and Methods
Cultures of T. gondii
(i). Cells
Hep-2 (An1-92041501; Institute of Foot and Mounts Diseases, Virology Laboratory, Ankara, Turkey) were maintained in Minimum Essential Medium Eagle (MEM) (Sigma, Steinheim, Germany) containing penicillin (100 IU/mL), streptomycin (0.1 mg/mL) (Biochrom, Leonorenstr, Berlin), and 5% heat-inactivated fetal bovine serum (FBS) (Biochrom, Leonorenstr, Berlin). For the assays, Hep-2 cultures were prepared in 96-well cell culture plates (TPP, Switzerland). The cells were seeded onto each well (2.0×104 cells per well) and grown to confluence at 37°C in a moist 5% CO2 −95% air atmosphere.
(ii). Parasites
Tachyzoites of the virulent TR-RH strain maintained through serial intraperitoneal of mice passages were used. For experimental infections, tachyzoites were harvested from the peritoneal fluid of mice infected intraperitoneally 2–4 days earlier and purified by centrifugation. The parasites were washed with MEM. They were counted and adjusted to a concentration of 5.0×104 parasites per mL.
(iii). Challenging the cultures
For the assays with antimicrobial agents, confluent monolayers were inoculated with 100 μL of the parasite suspension, i.e., 5000 trophozoites. This inoculum dose was determined in a preliminary experiment in which various parasite inputs concentrations (2.0×105, 1.0×105, 5.0×104, 2.0×104, 1.0×104, 5.0×103, 2.0×103 and 1.0×103 parasites per well) were compared. The inoculums of 5.0×103 parasites were found to be optimum for long incubation times. The cultures could be maintained up to 72 h after challenge, and infected monolayers were fully preserved for ELISA.
Three controls were included in each culture plate: (i) un-infected monolayers as a negative control, (ii) infected monolayers in which the replication of T. gondii was inhibited by the addition of 50 μL of 0.25% sodium azide (Serva, Heidelberg Carl-Benz-Str) per well at 4 h after challenge (inoculum’s controls), and (iii) infected monolayers with culture medium alone as a positive control. After development of the coloration, spectrophotometric readings were performed at a λ of 450 nm using blanks (negative control) in addition to all two controls. The results were expressed as optical density values. Experiments were repeated two times and were duplicated for each drug dose.
Quantitation of T. gondii growth in cultures
For quantitation of T. gondii growth in the culture, the supernatant was removed by aspiration. The monolayers were fixed with cold methanol for 15 min and air dried.
Antimicrobial agents
Sulphadiazine (Sigma, Steinheim, Germany) was initially dissolved in 50% methanol-50% acetone (Riedel-de Haën, Germany) at a concentration of 2000 μg/mL. Ivermectin (Sigma, Steinheim, Germany) was dissolved in dimethyl sulfoxide (DMSO) (Sigma, Steinheim, Germany) at a concentration of 1600 μg/mL.
Antimicrobial agents were added to the culture 4 h after challenge with T. gondii. For each antimicrobial agent, serial dilutions were prepared in MEM-FBS so that the addition of a 100 μL amount to the culture yielded the following final concentrations: SDZ, 0.7, 0.15, 0.3, 0.6, 12.5, 25, 50, and 100 μg/mL; ivermectin, 0.035, 0.07, 0.15, 0.3, 0.6, 1.25, 2.5 and 5 μg/mL. Each concentration was tested in eight replicate wells. Preliminary studies indicated that final concentrations of methanol and acetone in dilution of SDZ did not inhibit the growth of T. gondii. Cultures were incubated with antimicrobial agents up to 72 h after challenge and cultures were examined microscopically, and then fixed with methanol, air dried, and stored at +4°C, and ELISA was performed as described.
ELISA
An ELISA test was performed directly on the tissue culture test plates (TPP, Switzerland) by using (i) anti-T. gondii antibodies (immunoglobulin G fraction) (Abcam Inc, Kendall Square Stc, Cambridge/USA) as the first antibody and (ii) anti-rabbit immunoglobulin G (whole molecule) alkaline phosphatase antibody produced in goat (Sigma A3687, Steinheim, Germany) as the second antibody. Wells were postcoated with 200 μL of phosphate-buffered saline (PBS)-0.5% Tween 20–1% bovine serum albumin (Sigma, Steinheim, Germany) (PBS-T-BSA) for 1 h at 37°C to block nonspecific adsorption sites. The plates were washed five times with PBS containing 0.5% Tween 20 and then 100 μL of anti-Toxoplasma antibodies diluted 1/100 in PBS-TBSA was added to the wells. Plates were incubated for 120 min at 37°C and then washed as described above. A 100 μL sample of the conjugate diluted 1/1000 in PBS-T-BSA was added, and the plates were incubated for 60 min at 37°C. After the plates were washed five times, 100 μL of paranitrophenyl phosphate (Sigma, Steinheim, Germany) 1 mg/mL in substrate solution (0.1 M Glisin, 5 M KOH, 1 mM MgCl2, 1 mM ZnCl2, pH:10.4) (Sigma, Steinheim, Germany) was added, and the plates were incubated for 30 min at room temperature. For reading with a Microplate Reader (Rayto, RT-2100c) was used at 450 nm. The negative control wells were used as the blanks. The results were expressed as optical density (OD) values.
To assess the cytopathic effects and the reproduction of intracellular T. gondii tachyzoites and control cells in 24 h, 48 h and 72 h; the coverslips on 24 well tissue culture plates were stained with Giemsa and examined microscopically (Figure 1).
Figure 1.
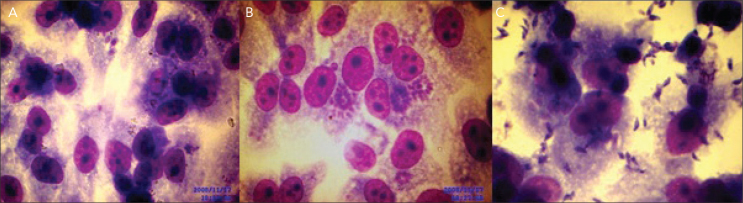
Reproduction of intracellular T. gondii tachyzoites A. one- two tachyzoites in 24 h, B. parasites formed rosettes or large pseudocysts in 48 h, C. free tachyzoites in extracellular fluid in 72 h
The plates prepared with 8 different concentrations of sulphadiazine and ivermectin were examined microscopically after 24 h, 48 h and 72 h.
Statistical analysis
A correlation coefficient was used to quantify the relationship between T. gondii counts and ELISA results. The effect of antimicrobial agents at various concentrations was described by data plotting. Optical density was plotted as a function of the logarithm of the concentration, which suggested models summarizing the concentration effect. Regression analysis was used to quantify the relationship between optical density values and antimicrobial agent concentrations in the cultures (6).
Results
The present study, after the microscopic examination of the plates it was determined that there were no inhibitory effects at all concentrations for ivermectin in 24 h. At 48 h the number of tachyzoites decreased relative to control wells at 0.31, 0.62 and 1.25 μg/mL. An important inhibitory effect was observed at 2.5 and 5 μg/mL. This inhibition of growth was associated with a reduction of the number of parasitized cells and intracellular parasites that were morphologically normal. In 72 h, because of the number of tachyzoites elevated in all wells, the inhibitory effect of ivermectin was not assessed except at 2.5 and 5 μg/mL concentrations.
For sulphadiazine, as for ivermectin, at 24 h there were no inhibitory effects at all concentrations tested. At 48 h, the number of tachyzoites had decreased relative to control wells at 3.12, 6.25, 12.5 and 25 μg/mL. An important inhibitory effect was observed at 50 and 100 μg/mL. In 72 h, because of the number of tachyzoites elevated in all wells, the inhibitory effect of sulphadiazine was not assessed. Therefore, ELISA was performed directly on the culture plates at 48 h. The ELISA did not alter the structure of fibroblasts.
Effect of antimicrobial agents
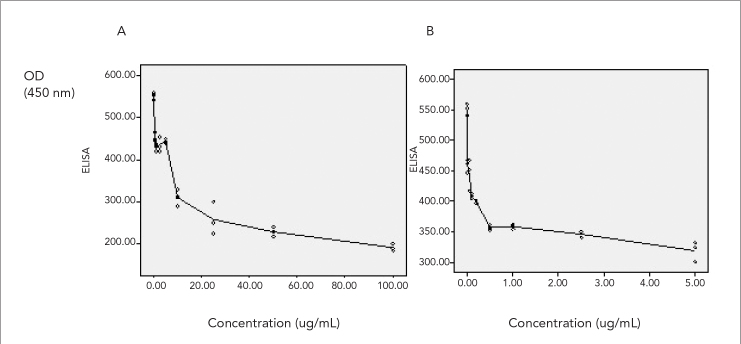
For each antimicrobial agent, experimental OD values were plotted versus concentrations. Regression lines results are shown in Figure 2.
Figure 2.
OD450 values for the ELISA with infected monolayers versus concentrations of antimicrobial agents. y axis, OD450 values; x axis, concentration of antimicrobial agent. A. SDZ, B. Ivermectin
Sulphadiazine
Sulphadiazine in all concentration, except at a concentration of 0.75 and 1.5 μg/mL (p>0.05) showed significantly higher inhibitory activity against tachyzoites after 48 h incubations. The 50% inhibitory concentrations (IC50) of sulphadiazine was estimated to be 7.3 μg/mL [OD=451.75–54.29 in concentration (c)] after 48 h of exposure. None of the concentrations tested for each drug demonstrated toxicity to Hep-2 cells after 72 h of incubation.
Ivermectin
Ivermectin was found to have significant inhibitory activity against tachyzoites after 48 h of incubation at a concentration of 2.5 and 5 μg/mL (p<0.01) and above dose. The 50% IC50 of ivermectin was estimated to be 0.27 μg/mL [OD=358.38–23.35 ln(c)] after 48 h of exposure. None of the concentrations tested for each drugs demonstrated toxicity to Hep-2 cells after 72 h of incubation.
Discussion
An ELISA performed directly on tissue culture was used to quantify the growth of T. gondii in Hep-2 fibroblasts. This assay was performed as described by Merli et al. (5) who initially showed that an ELISA performed on infected cells was a reliable method for studying T. gondii growth kinetics in tissue culture. Therefore, this method was used to evaluate the inhibitory effect of antimicrobial agents. The assessment was based on the relationship between OD values and log concentrations of antimicrobial agents in the cultures as shown by regression analysis in Figure 2.
Diab and El-Bahy (7) determined that the Hep-2 cell line appeared to be the most appropriate cell line for in vitro multiplication of T. gondii (RH strain) in maintenance media with or without fetal calf sera at different temperatures. Therefore, Hep-2 cell line was used in this assay.
With SDZ, we found a significant inhibitory effect for concentrations of 25, 50 and 100 μg/mL (p<0.001); a slight inhibitory effect for 3.12, 6.25, 12.5 μg/mL (p<0.01) and no inhibitory effect for 0.75 and 5 μg/mL (p>0.05) at 48 h incubation. The 50% IC50 of sulphadiazine was estimated to be 7.3 μg/mL [OD=451.75–54.29 ln(c)] after 48 h of exposure.
Similarly, Derouin and Chastang (6) investigated the in vitro inhibitory effect of antimicrobial agents on T. gondii grown on MRC5 fibroblasts. In their study, sulfadoxine was not found inhibitory at concentrations from 2 to 20 μg/mL. An inhibitory effect was observed at 30 μg/mL (p<0.001). Within the interval between 30 and 75 μg/mL, the inhibitory effect could be summarized by a linear function of the concentration [OD=2.35–0.5 ln(c)]. For higher concentrations, the inhibitory effect did not significantly increase.
In a study Meneceuri et al. (8) investigated the in vitro susceptibility of various genotypic strains of T. gondii to pyrimethamine, sulphadiazine and atovaquone. In their study they examined SDZ at 10 concentrations ranging between 0.0005 and 100 mg/liter. SDZ was inhibitory for 13 strains, with IC50 ranging between 3 and 18.9 mg/L.
Derouin and Chastang (9) investigated the in vitro effects of folate inhibitors on T. gondii grown in MRC5 fibroblast tissue culture found that three sulfonamides and four dihydrofolate reductase inhibitors have important inhibitory effects on T. gondii. They studied sulphadiazine at 0.001, 0.01, 0.05, 0.1, 0.2, 0.5, 2, 10, and 20 μg/mL concentrations, the %50 inhibitory concentration estimated from the regression model was 2.5 μg/mL for sulphadiazine.
Ivermectin is a derivative of the avermectins, a family of macrocyclic lactones produced by the filamentous bacterium Streptomyces avermitilis. With ivermectin, we found a significant inhibitory effect for concentrations 2.5 and 5 μg/mL (p<0.001), and a slight inhibitory effect for 0.15, 0.3, 0.6 and 1.25 μg/mL (p<0.01) and no inhibitory effect for 0.035 and 0.07 μg/mL (p>0.3) at 48 h incubation. The 50% IC50 of ivermectin was estimated to be 0.27 μg/mL [OD=358.38–23.35 ln(c)] after 48 h of exposure. Our in vitro results obtained with ivermectin are in agreement with the studies performed by Guzzo et al. (10).
Our results indicated that ivermectin significantly inhibited replication of the tachyzoites of T. gondii RH strain. Therefore, the present study results may be useful for further studies in combination with other drugs and animal models to develop a better treatment model for toxoplasmosis in humans.
Footnotes
Ethics Committee Approval: Ethics committee approval was received from Ondokuz Mayıs University Faculty of Medicine for this study (28.06.2006, HEK 225).
Informed Consent: N/A.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - M.B.; Design - M.B.; Supervision -T.Y.; Resource - M.B.; Materials - M.B.; Data Collection&/or Processing - M.B.; Analysis&/or Interpretation - M.H.; Literature Search - T.Y.; Writing - M.B., T.Y., M.H.; Critical Reviews - M.B., T.Y., M.H.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Montaya JG, Liesenfeld O. Toxoplasmosis. The Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 2.Montoya JG, Kovacs JA, Remington JS. Toxoplasma gondii. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Elsevier Press; 2008. pp. 3170–92. [Google Scholar]
- 3.Wilson M, Jones LJ, McAuley LB. Toxoplasma. In: Murray PR, Baron EJ, Jorgensen JH, Landry ML, Pfaller MA, editors. Manual of Clinical Microbiology. 9th ed. Washington: ASM Press; 2005. pp. 2070–81. [Google Scholar]
- 4.Petersen E. Toxoplasmosis. Seminars in Fetal & Neonatal Medicine. 2007;12:214–23. doi: 10.1016/j.siny.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Merli A, Canessa A, Melioli G. Enzyme immunoassay for evaluation of Toxoplasma gondii growth in tissue culture. J Clin Microbiol. 1985;21:88–91. doi: 10.1128/jcm.21.1.88-91.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derouin F, Chastang C. Enzyme Immunoassay to assess effect of antimicrobial agents on Toxoplasma gondii in tissue cultures. Antimicrob Agents Chemother. 1988;32:303–7. doi: 10.1128/aac.32.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diab MR, El-Bahy MM. Toxoplasma gondii:Virulence of tachyzoites in serum free media at different temperatures. Exp Parasitol. 2008;118:75–9. doi: 10.1016/j.exppara.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Meneceuri P, Bouldouyre MA, Aubert D, Villena I, Menotti J, Sauvage V, et al. Toxoplasma gondii:In vitro susceptibility of various genotypic strains to pyrimethamine, sulphadiazine and atovaquone. Antimicrob Agents Chemother. 2008;52:1269–77. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derouin F, Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob Agents Chemother. 1989;33:1753–9. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guzzo CA, Furtek CI, Chen C, Tipping R, Clineschmidt CM, Sciberras DG, et al. Safety, tolerability, pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J Clin Pharmacol. 2002;42:1122–33. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]