Abstract
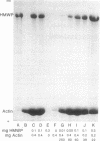
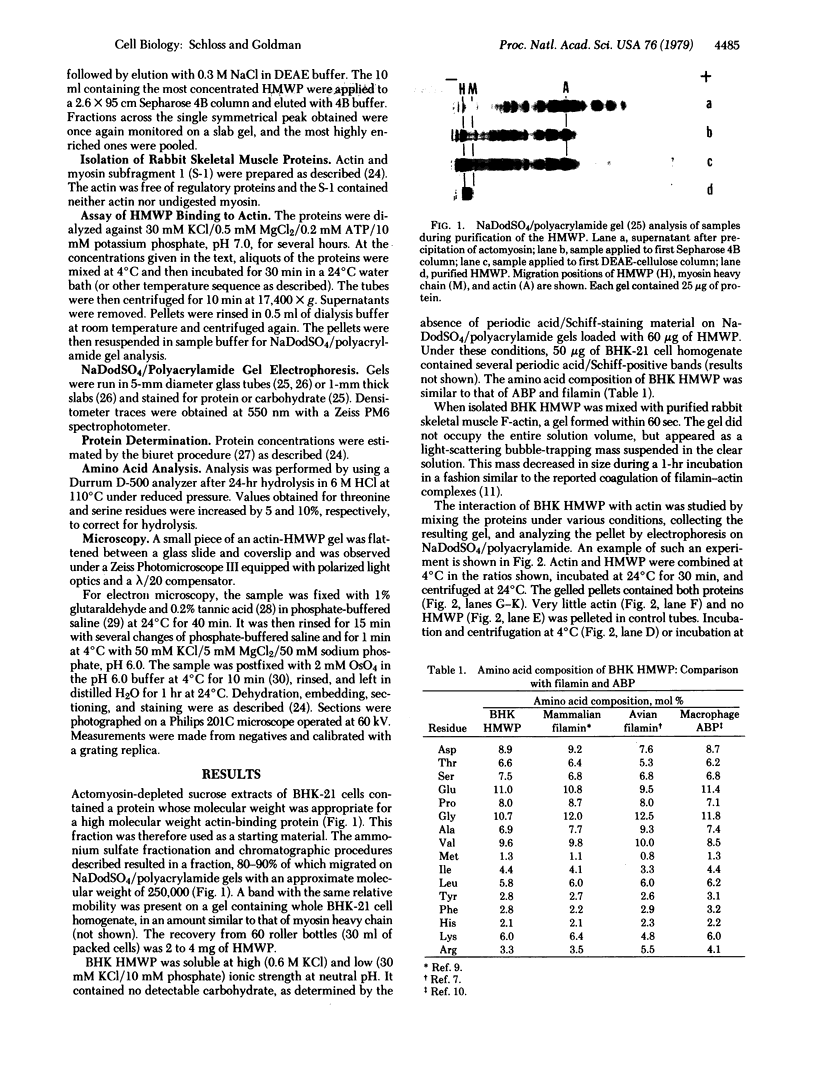
A high molecular weight protein (HMWP) with properties similar to those of both actin-binding protein (ABP) and filamin has been isolated from cultured baby hamster kidney (BHK-21) cells. The protein was present in an actomyosin-depleted sucrose extract of the cells and was eluted, upon gel chromatography on Sepharose 4B, near the void volume. The subunit migration on sodium dodecyl sulfate/polyacrylamide gels and the amino acid composition of HMWP were similar to those of ABP and filamin. HMWP bound to and crosslinked F-actin from rabbit muscle, as shown by the formation of a gel that was sedimented with low-speed centrifugation. This interaction was insensitive to temperature and low concentrations of calcium ions, although it may depend on the presence of myosin. Observation of thin sections of the actin-HMWP gel revealed crosslinked complexes of laterally aggregated actin filaments. The axial period of the dense crosslinks was 34 nm. The HMWP may be involved in regulation of microfilament organization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Begg D. A., Rodewald R., Rebhun L. I. The visualization of actin filament polarity in thin sections. Evidence for the uniform polarity of membrane-associated filaments. J Cell Biol. 1978 Dec;79(3):846–852. doi: 10.1083/jcb.79.3.846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Stossel T. P. Interactions of actin, myosin, and an actin-binding protein of chronic myelogenous leukemia leukocytes. J Clin Invest. 1976 Apr;57(4):964–976. doi: 10.1172/JCI108373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J., Kane R. E. Separation and interaction of the major components of sea urchin actin gel. J Mol Biol. 1978 Oct 25;125(2):207–224. doi: 10.1016/0022-2836(78)90345-5. [DOI] [PubMed] [Google Scholar]
- Clark T. G., Merriam R. W. Actin in Xenopus oocytes. J Cell Biol. 1978 May;77(2):427–438. doi: 10.1083/jcb.77.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. S., Taylor D. L. The contractile basis of amoeboid movement. V. The control of gelation, solation, and contraction in extracts from Dictyostelium discoideum. J Cell Biol. 1977 Sep;74(3):901–927. doi: 10.1083/jcb.74.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P. J., Wallach D., Willingham M. C., Pastan I., Yamaguchi M., Robson R. M. Filamin-actin interaction. Dissociation of binding from gelation by Ca2+-activated proteolysis. J Biol Chem. 1978 Jun 10;253(11):4036–4042. [PubMed] [Google Scholar]
- Davies P., Bechtel P., Pastan I. Filamin inhibits actin activation of heavy meromyosin ATPase. FEBS Lett. 1977 May 15;77(2):228–232. doi: 10.1016/0014-5793(77)80240-8. [DOI] [PubMed] [Google Scholar]
- Davies P., Shizuta Y., Olden K., Gallo M., Pastan I. Phosphorylation of filamin and other proteins in cultured fibroblasts. Biochem Biophys Res Commun. 1977 Jan 10;74(1):300–307. doi: 10.1016/0006-291x(77)91408-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hartwig J. H., Stossel T. P. Isolation and properties of actin, myosin, and a new actinbinding protein in rabbit alveolar macrophages. J Biol Chem. 1975 Jul 25;250(14):5696–5705. [PubMed] [Google Scholar]
- Heggeness M. H., Wang K., Singer S. J. Intracellular distributions of mechanochemical proteins in cultured fibroblasts. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3883–3887. doi: 10.1073/pnas.74.9.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Kane R. E. Preparation and purification of polymerized actin from sea urchin egg extracts. J Cell Biol. 1975 Aug;66(2):305–315. doi: 10.1083/jcb.66.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruta H., Korn E. D. Purification from Acanthamoeba castellanii of proteins that induce gelation and syneresis of F-actin. J Biol Chem. 1977 Jan 10;252(1):399–402. [PubMed] [Google Scholar]
- Maupin-Szamier P., Pollard T. D. Actin filament destruction by osmium tetroxide. J Cell Biol. 1978 Jun;77(3):837–852. doi: 10.1083/jcb.77.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura N., Asano A. Actin-related gelation of Ehrlich tumour cell extracts is reversibly inhibited by low concentrations of Ca2+. Nature. 1978 Mar 16;272(5650):273–276. doi: 10.1038/272273a0. [DOI] [PubMed] [Google Scholar]
- Moore P. B., Carraway K. L. Proteolytic enhancement of gelation of ascites tumor cell extracts. Relationship to actin binding protein. Biochem Biophys Res Commun. 1978 Feb 14;80(3):560–567. doi: 10.1016/0006-291x(78)91605-4. [DOI] [PubMed] [Google Scholar]
- Pollard T. D. The role of actin in the temperature-dependent gelation and contraction of extracts of Acanthamoeba. J Cell Biol. 1976 Mar;68(3):579–601. doi: 10.1083/jcb.68.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss J. A., Milsted A., Goldman R. D. Myosin subfragment binding for the localization of actin-like microfilaments in cultured cells. A light and electron microscope study. J Cell Biol. 1977 Sep;74(3):794–815. doi: 10.1083/jcb.74.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizuta Y., Shizuta H., Gallo M., Davies P., Pastan I. Purification and properties of filamin, and actin binding protein from chicken gizzard. J Biol Chem. 1976 Nov 10;251(21):6562–6567. [PubMed] [Google Scholar]
- Stossel T. P., Hartwig J. H. Interactions of actin, myosin, and a new actin-binding protein of rabbit pulmonary macrophages. II. Role in cytoplasmic movement and phagocytosis. J Cell Biol. 1976 Mar;68(3):602–619. doi: 10.1083/jcb.68.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K. Filamin, a new high-molecular-weight protein found in smooth muscle and nonmuscle cells. Purification and properties of chicken gizzard filamin. Biochemistry. 1977 May 3;16(9):1857–1865. doi: 10.1021/bi00628a015. [DOI] [PubMed] [Google Scholar]
- Wang K., Singer S. J. Interaction of filamin with f-actin in solution. Proc Natl Acad Sci U S A. 1977 May;74(5):2021–2025. doi: 10.1073/pnas.74.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing R. R. Cytochalasin B inhibits actin-related gelation of HeLa cell extracts. J Cell Biol. 1976 Oct;71(1):303–307. doi: 10.1083/jcb.71.1.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihing R. R. Effects of myosin and heavy meromyosin on actin-related gelation of HeLa cell extracts. J Cell Biol. 1977 Oct;75(1):95–103. doi: 10.1083/jcb.75.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerna M. J., Aksoy M. O., Hartshorne D. J., Goldman R. D. BHK21 myosin: isolation, biochemical characterization and intracellular localization. J Cell Sci. 1978 Jun;31:411–429. doi: 10.1242/jcs.31.1.411. [DOI] [PubMed] [Google Scholar]