Abstract
Objective:
Methotrexate (MTX) is known to have deleterious side effects on lung tissue. We aimed to investigate the effects of erythropoietin (EPO) and N-acetyl-cysteine (NAC) on MTX-induced lung injury in rats.
Study Design:
Animal experiment.
Material and Methods:
Twenty-six female Sprague-Dawley rats were divided into 4 groups. Sham group, 0.3 mL saline; MTX group, 5 mg/kg MTX; EPO group, 5mg/kg MTX and 2000 IU/kg EPO; NAC group, 5 mg/kg MTX and 200 mg/kg NAC were administered once daily for 4 consecutive days. Malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) and inflammation and congestion scores in lung tissues were evaluated.
Results:
In MTX group MDA were significantly higher, CAT and SOD were significantly lower than in sham, EPO and NAC groups (p<0.005). In EPO group MDA, CAT, and SOD were higher, but not significant than those in group NAC (p>0.005). In group MTX both scores were significantly higher than in sham (p<0.005). The congestion score of group MTX was significantly higher than those of group EPO and NAC (p<0.005).
Conclusion:
EPO and NAC have significant preventive effects on MTX-induced lung injury in rats. Decreased antioxidant capacity and increased MDA level may cause the oxidative damage in MTX group. Also, higher antioxidant capacity and lower MDA level may be a response to oxidative stress in EPO and NAC groups.
Keywords: Methotrexate, lung, oxidative damage, erythropoietin, N-acetyl-cysteine
Introduction
Many medications used for different therapeutic goals have been associated with pulmonary complications of various types, including interstitial inflammation, fibrosis, bronchospasm, pulmonary edema, and pleural effusions (1). Methotrexate (MTX) is a folic acid antagonist which has been used clinically for more than 40 years and is widely used as an agent for the treatment of several malignancies and various inflammatory diseases. For children with acute lymphoblastic leukemia, it is an important component of chemotherapy (2). MTX has anti-proliferative, anti-inflammatory, and immunomodulating effects (3, 4). In 60 to 93 percent of patients treated with MTX, there have been a number of adverse reactions, including nonproductive cough, dyspnea, fever, pneumonitis, interstitial lung disease, and pulmonary fibrosis (5). Most of these reactions are dose-dependent in nature and are not life-threatening, but up to 30 percent of patients treated with MTX for more than five years discontinue the therapy because of unacceptable toxicity (6).
MTX-induced pneumonitis is a serious side effect and the precise pathological mechanism for this syndrome still remains unclear. Because pulmonary toxicities occur with both low and high doses and by different routes of administration, some MTX-induced side effects are thought to result from idiosyncratic mechanisms unrelated to folate antagonism (7).
The tissue levels of the reactive oxygen species (ROS) are kept in strict control by a complex antioxidant defense system that includes enzymes such as superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) (8, 9). The major enzymatic scavenger of oxygen radicals in lung is SOD which catalyses the dismutation of O2 to H2O2 that is then enzymatically degraded by either the GSH redox cycle or by CAT (10). Oxidative stress is known to appear because of an imbalance between the production and degradation of ROS in tissues. ROS-induced injury is well characterized and includes DNA base oxidation, lipid peroxidation, and protein oxidation (11). Malondialdehyde (MDA) is an indicator of oxidative stress and is produced during lipid peroxidation of tissues (8).
Erythropoietin (EPO) is a glycoprotein cytokine that is produced primarily by the kidney in order to regulate the production of red blood cells. In addition to its traditional hematopoietic role, effects on tissue protection were shown in many studies. The protective effects of EPO are seemed to be related to its anti-apoptotic, anti-oxidative, and anti-inflammatory properties as well as its angiogenic effect (12–15).
N-acetylcysteine (NAC) has been widely used as an anti-oxidant in vivo and in vitro (16–18). NAC plays an important role at protection of cell from oxidative stress by inhibiting the H2O2 formation in vitro. NAC also increases the cellular GSH level by directing cystine into the GSH synthesis pathway (18).
To best of our knowledge, the effects of EPO and NAC on MTX-induced lung injury have not been investigated yet. Therefore, we designed the study to evaluate the protective role of EPO and NAC on MTX induced-lung injury.
Material and Methods
Animals
This study was approved by the Ethics Committee of Kahramanmaraş Sütçü İmam University. Twenty-six female Sprague-Dawley rats weighing 210 to 230 grams were used in the study. The animals were housed under 12 h dark/light period at a stable ambient temperature (21–23°C) with standard diet and water ad libitum. The rats were divided into 4 groups: S group (sham, n=5) animals were administered subcutaneous (SC) injections of 0.3 mL of 0.9% NaCl once daily for 4 consecutive days. MTX group (Control, n=7) were administered SC injections of MTX (5 mg/kg) once daily for 4 consecutive days. EPO group (EPO-treated, n=7) were administered SC injections of MTX (5 mg/kg) and EPO (2000 IU/kg, Recormon, Roche Diagnostics GmbH, Mannheim, Germany) once daily for 4 consecutive days. NAC group (NAC-treated, n=7) were administered SC injections of MTX (5 mg/kg) and NAC (200 mg/kg, n-acetlylcysteine) once daily for 4 consecutive days. On the fifth day, animals in all groups were sacrificed by decapitation and the lungs were removed, then biochemical and histological investigations were done. One halves of removed lungs were stored at −80°C until biochemical analysis and the other halves were fixed in 10% neutral-buffered formaldehyde solution for histological evaluation.
Biochemical Evaluation
All chemicals used in our study were analytical grade and were from the Sigma Chemical Company (St. Louis, MO, USA) with exceptions of NAC which was from Bilim (İstanbul, Turkey) and EPO which was from Janssen Cilag (İstanbul, Turkey).
Preparation of Homogenates: The lung tissues of the rats were rapidly removed, weighed, perfused 1.15% ice-cold KCl, minced, then homogenized in five volumes (w/v) of the same solution, using a Heidolph 50110 R2R0 homogenizer. Antioxidant enzymes and MDA assays were performed on the supernatant preparation in a Sorvall RC-2B centrifugation of the homogenate at 14.000 rpm for 30 min at +4°C. The activities of antioxidant enzyme and MDA levels in supernatant were measured at 37ºC in the UV-260 Shimadzu spectrophotometer.
Determination of MDA: Lipid peroxidation level in the tissue samples was expressed in MDA. It was measured according to procedure of Ohkawa et al. (19). The reaction mixture contained 0.1 mL sample, 0.2 mL of 8.1% sodium dodecyl sulphate (SDS), 1.5 mL of 20% acetic acid and 1.5 mL of 0.8% aques solution of TBA. The mixture pH was adjusted to 3.5 and volume was finally made up to 4.0 mL with distilled water and 5.0 mL of the mixture of n-butanol and pyridine (15:1,v/v) were added. The mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the absorbance of the organic layer was measured at 532 nm.
Determination of SOD: SOD activity in lung tissue was measured according to the method described by Fridovich (20). This method employs xanthine and xanthine oxidase to generate superoxide radicals which react with p-iodonitrotetrazlium violet (INT) to form a red formazan dye which was measured at 505 nm. Assay medium consisted of the 0.01 M phosphate buffer, CAPS (3-cyclohexilamino-1-propanesulfonicacid) buffer solution (50 mM CAPS, 0.94 mM EDTA, satd. NaOH) with pH 10.2, solution of substrate (0.05 mM xanthine, 0.025 mM INT) and 80 UL xanthine oxidase. SOD activity was expressed as U/mg protein.
Determination of CAT: CAT activity in tissues was determined by measuring the decrease in hydrogen peroxide concentration at 230 nm by the method of Beutler (21). Assay medium consisted of 1 M Tris HCI, 5 mM Na2EDTA buffer solution (pH 8.0), 1 M phosphate buffer solution (pH 7.0), and 10 mM H2O2 CAT activity was expressed U/mg protein.
Determination of Protein: The protein concentration of the tissue samples was measured with Spectronic-UV 120 spectrophotometer by the method of Lowry et al. (22).
Histopathologic Evaluation
Lung tissue samples were fixed in 10% neutral buffered formalin solution and embedded in paraffin. Serial sections were cut in 4 μm thick slices, stained with hematoxylin-eosin, and examined under light microscope for the presence of tissue damage. A single pathologist examined and scored the lung sections in a blinded fashion. The presences of alveolar congestion, hemorrhage, inflammation or infiltration of neutrophils and lymphocyte in the alveolus or vessel walls were evaluated. Minimal or negligible, mild, moderate, severe or maximal damage were scored from 0 to 4, respectively (23).
Statistical Analysis
Statistical analyses were performed with the SPSS Program, version15.0 (LEAD Technologies Inc, Chicago, IL USA). Analysis of variance (ANOVA) was carried out on the biochemical data and tissue damage scores to examine differences among groups. When a significant group effect was found, Kruskal-Wallis followed by Mann-Whitney U tests were used. Statistical significance was defined as p<0.05. The data were expressed as mean±SD.
Results
Results of Biochemical Analyses
All animals survived except one in EPO group and two in NAC group, during the test protocol. In these groups diarrhea was seen to be more pronounced and probably they died because of this.
MDA levels and SOD and CAT activities of lung tissue were listed in Table 1. MDA levels in MTX group were significantly higher than those in the S, EPO and NAC groups (p<0.05). In the MTX group, CAT and SOD activities were significantly lower than those in S, EPO, and NAC groups (p<0.05).
Table 1.
Biochemical parameters and tissue damage scores in all groups
| Sham | MTX | EPO | NAC | |
|---|---|---|---|---|
| MDA nmol/mg protein | 2.01±0.51* | 4.07±1.02†,‡ | 2.38±0.29 | 2.26±0.51 |
| CAT units/mg protein | 47.78±12.22* | 16.21±4.76†,‡ | 40.68±12.32 | 36.88±4.43 |
| SOD units/mg protein | 10.72±1.05*,§ | 2.11±0.45†,‡ | 9.29±2.21 | 6.96±1.19 |
| Congestion score | 1.40±0.54* | 3.28±0.75†,‡ | 1.83±0.75 | 1.80±0.44 |
| Inflammation score | 1.20±0.44 | 2.28±0.48 | 1.83±0.75 | 1.60±0.54 |
Data are presented as mean±SD. MDA: Malondialdehyde, CAT: Catalase activities, SOD: Superoxide dismutase activities. Groups were sham, MTX: Methotrexate given control group, EPO: Erythropoietin with MTX given, NAC: N-acetlylcysteine with MTX given
p<0.05 when sham compared with MTX
p<0.05 when MTX compared with EPO
p<0.05 when MTX compared with NAC
p<0.05 when sham compared with NAC
In EPO group MDA levels, CAT, and SOD activities were higher, but not significant than those in NAC group (p>0.05). SOD activities were significantly lower in NAC group than in S group (p<0.05). Although SOD activities in NAC group were significantly lower than S group (p<0.05), the other parameters like tissue MDA levels and CAT and SOD activities in S group were not significantly different from group EPO and group NAC (p>0.05) (Figure 1).
Figure 1.
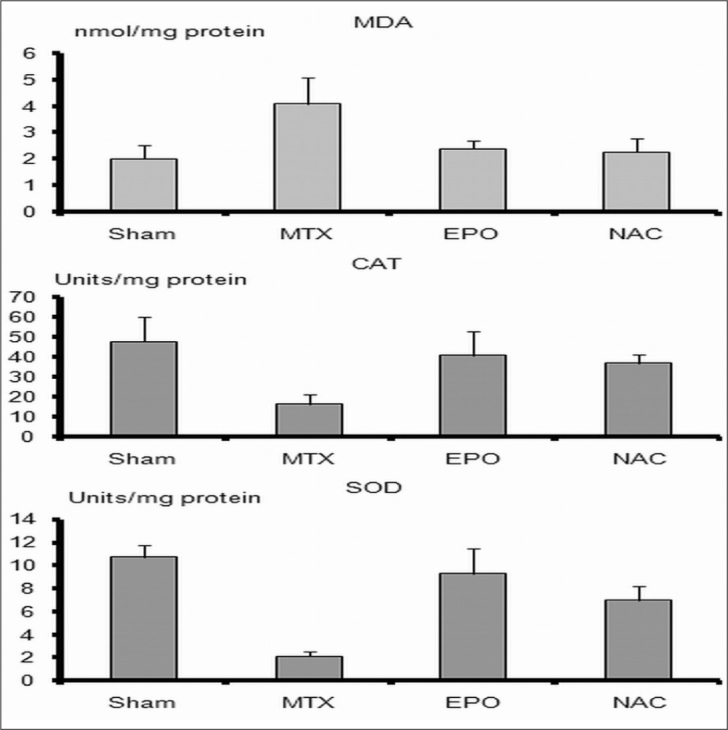
Levels of malondialdehyde (MDA), activities of superoxide dismutase (SOD) and catalase (CAT) in lung tissue. MDA level, and SOD, CAT activities in methotrexate (MTX) group were significantly different than those of the sham, EPO, and NAC groups (p<0.05). MTX, indicates methotrexate given group; EPO indicates both MTX and erythropoietin given group; NAC, indicates both MTX and N-acetylcysteine given group
Histopathological results
We evaluated the presence of tissue congestion and inflammation histopathologically. Congestion scores (CS) and inflammation scores (IS) of all groups were shown in Table 1 and Figure 2.
Figure 2.
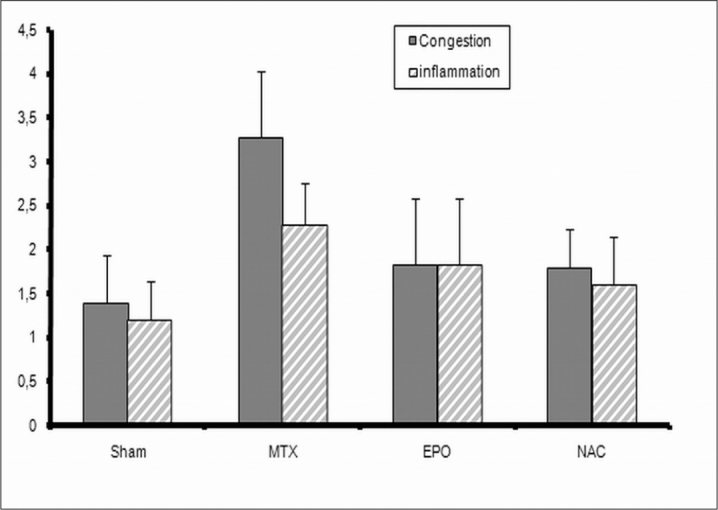
Inflammation and congestion scores were shown. MTX, indicates methotrexate given group; EPO indicates both MTX and erythropoietin given group; NAC, indicates both MTX and N-acetylcysteine given group
In MTX group, CS and IS were significantly higher than those in S group (p<0.05). The CS in MTX group was significantly higher than those in EPO and NAC groups (p<0.05) (Figure 3). The CS in S group was significantly lower than those in NAC group (p<0.05) (Figure 3).
Figure 3.
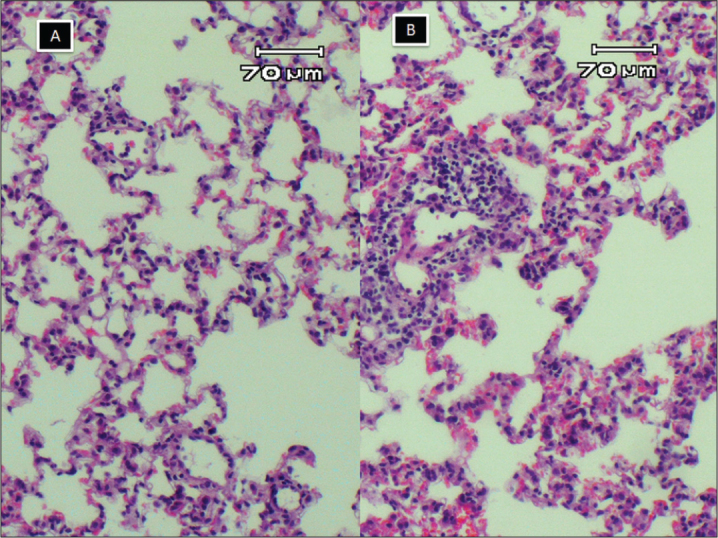
A, normal histology of rat lung, inflammation and congestion scores were zero; B, mild lymphocyte infiltration and congestion, inflammation and congestion scores were 1 (HE X100)
While evaluating the IS, lymphocyte cell infiltrations were detected in all groups (Figure 4, 5). The IS in MTX group was higher but not significantly than those in EPO and NAC groups (p>0.05). When both scores in EPO group were compared to those in NAC group, the difference was not statistically significant (p>0.05).
Figure 4.
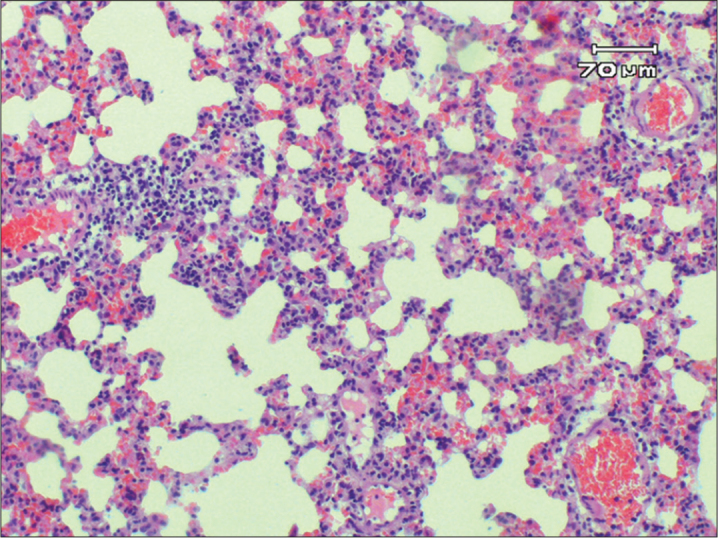
Moderate lymphocyte infiltration and congestion, both scores were 2 in lung tissue (HE X100)
Figure 5.
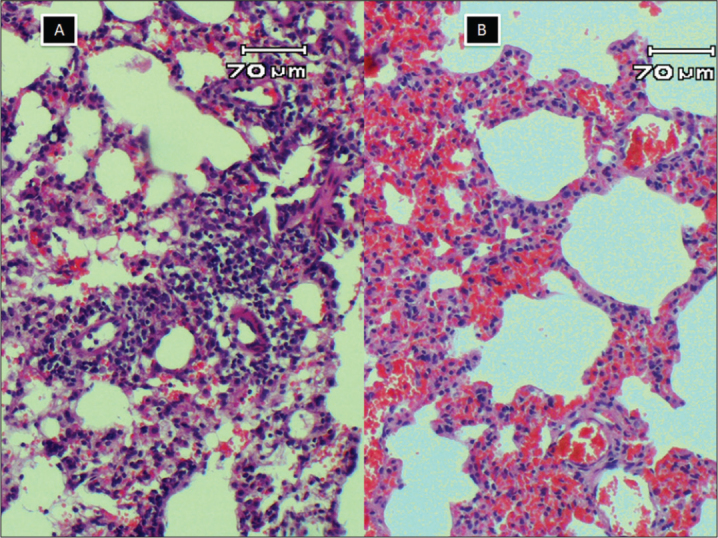
A lymphocyte infiltration was more prominent but congestion was less. Inflammation score was 3 and congestion score was 1; B, lymphocyte infiltration was mild but congestion was more prominent, inflammation score was 1 and congestion score was 3 (HE X100)
Discussion
The present study showed for the first time, biochemically and histopathologically, that early and low dose of MTX increases oxidative damage on lung tissue and also EPO and NAC have protective effects against MTX-induced lung injury in rats. Our result is in agreement with other experimental tissue models showing that oxidative stress plays an important role in MTX-induced damage and antioxidants such as EPO and NAC attenuate the side effects of MTX (24–26).
Methotrexate that is an antineoplastic drug has been used increasingly to treat chronic inflammatory disease and malignancies (2). Pulmonary toxicity has been estimated to occur in 2–7% of patients receiving low-dose MTX therapy (5). The most common pulmonary toxicity associated with the MTX use is hypersensitivity pneumonitis. Other pulmonary toxicities are also determined including acute lung injury with non-cardiogenic pulmonary edema. Most researchers think that MTX generates a type IV delayed hypersensitivity reaction presenting lymphocytic proliferation and alveolitis (27–29). In our study, we determined tissue congestion and lymphocytic infiltration in histopathological examination of rat lung. Increased congestion and lymphocytic infiltration were detected in MTX group. By administration of EPO or NAC to MTX, CS was significantly decreased, and IS was decreased but not significantly. This might be due to EPO and NAC, in the early phase of experiment, showing protective effects on congestion but could not show the protective effects on lymphocytic infiltration. For this reason a longer experimental period may give more accurate results.
Researches in the last decade have shown that EPO and its receptor are expressed in tissues other than those concerned in erythropoiesis. These include the brain, the reproductive tract the heart, the spleen, and the lung (30–32). EPO regarded as a general tissue-protective cytokine, is a strong antioxidant and increases the activity of antioxidant enzymes, such as SOD and CAT, and decreases MDA levels in hypoxic-ischemic organ injuries (33–36). It is also shown that EPO has protective effects associated with acute lung injury model by inhibiting leukocyte accumulation and reducing oxidative stress-associated lipid peroxidation (37). In addition to these studies, it has been also reported that EPO can protect the tracheobronchial epithelia and pulmonary type 2 cells of rats that were exposed to traumatic brain injury (38). In our study SC administration of EPO to MTX received rats decreased the MDA level significantly and increased CAT and SOD activities significantly when compared with MTX group. Our results supported the knowledge of EPO having antioxidant and anti-inflammatory effects.
Erythropoietin with MTX given has been demonstrated to decrease lipid peroxidation levels in hypoxic-ischemic brain injury at a dose of 1000 units/kg (35). Garipardic et al. (26) demonstrated that EPO had protective effect on MTX-induced esophageal damage at a dose of 2000 units/kg/day. Bakan et al. (39) noticed that EPO had protective effect on corrosive esophageal burns at a dose of 1000 units/kg/day. Tascılar et al. (37) reported that in acute lung injury model induced by acute pancreatitis experimentally, EPO administration with a single dose of 1000 units/kg showed cytoprotective effect. Wu et al. (40) demonstrated that pretreatment with a single dose of 3000 units/kg EPO appeared to attenuate ischemiareperfusion-induced lung injury in rats. In our study we used EPO with a dose of 2000 units/kg/day for 4 days.
N-acetyl-cysteine is widely used as a mucolytic agent and shows the major antioxidant effects by reducing extracellular cystine to cysteine, or being a source of thiol metabolites (41). In our study SC administration of NAC to MTX decreased the MDA level significantly and increased the CAT and SOD activities significantly when compared with MTX group. It was determined that MTX-induced changes can be ameliorated by NAC with a dose of 150 mg/kg in small intestine and in liver of rats (24, 25). Maeda et al. (17) determined that NAC in dose of 80 mg/kg attenuated the MTX-induced changes in kidneys of rat. These findings suggested that NAC had also antioxidant and anti-inflammatory effects on MTX-induced lung injury. We compared the EPO group to NAC group by means of the MDA levels, CAT and SOD activities. We did not determine any statistically significant difference between the two groups.
As a result, we have demonstrated that MTX causes an increase in MDA level and decrease in CAT and SOD activities in rat lung. This result showed that the oxidative damage had a role in MTX-induced lung injury. By adding the EPO or NAC to MTX, a decrease in MDA level and an increase in CAT and SOD activities were observed. Both EPO and NAC showed significant protective effects on oxidative damage, both biochemically and histopathologically, in the early stage of MTX-induced lung injury in rats. Additional studies using EPO, NAC, and other antioxidants are needed to confirm our results.
Acknowledgments
The authors thank Prof. Dr. Yakup Gümüşalan, MD, from Sütçüİmam University, Faculty of Medicine, Department of Anatomy and Assoc. Prof. Dr. Ali Çetinkaya, MD, from Sütçüİmam University, Faculty of Medicine, Department of Gastroenterology, for English editing, grammar control, and statistical analysis.
Footnotes
This study was previously presented at the European Respiratory Society Congress, 1–3 September 2012, Vienna, Austria.
Ethics Committee Approval: Ethics committee approval was received from the Ethics Committee of Kahramanmaraş Sütçü İmam University for this study.
Informed Consent: N/A
Peer-review: Externally peer-reviewed.
Author contributions: Concept - H.K., V.B.; Design - V.B., E.K.; Supervision -V.B.; Resource - H.K., .SB.; Materials - H.K., M.T.; Data Collection&/or Processing -H.K., M.T., S.B.; Analysis&/or Interpretation - E.K., B.K., H.C.; Literature Search - H.K., N.K.; Writing - H.K.; Critical Reviews - E.K., N.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Camus P, Bonniaud P, Fanton A, Camus C, Baudaun N, Foucher P. Drug-induced and iatrogenic infiltrative lung disease. Clin Chest Med. 2004;25:479–519. doi: 10.1016/j.ccm.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Salzer WL, Winick NJ, Wacker P, Lu X, Devidas M, Shuster JJ, et al. Plasma methotrexate, red blood cell methotrexate, and red blood cell folate values and outcome in children with precursor B-acute lymphoblastic leukemia:a report from the children’s oncology group. J Pediatr Hematol Oncol. 2012;34:e1–7. doi: 10.1097/MPH.0b013e31820ee239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cronstein BN. Molecular therapeutics. Methotrexate and its mechanism of action. Arthritis Rheum. 1996;39:1951–60. doi: 10.1002/art.1780391203. [DOI] [PubMed] [Google Scholar]
- 4.Kimura E, Nishimura K, Sakata K, Oga S, Kashiwagi K, Igarashi K. Methotrexate differentially affects growth of suspension and adherent cells. Int J Biochem Cell Biol. 2004;36:814–25. doi: 10.1016/j.biocel.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi Y, Okazaki H, Desaki M, Kohyama T, Kawasaki S, Yamamoto K, et al. Methotrexate induces interleukin-8 production by human bronchial and alveolar epithelial cells. Clin Sci. 2004;106:619–25. doi: 10.1042/CS20030262. [DOI] [PubMed] [Google Scholar]
- 6.Hsu DC, Katelaris CH. Long-term management of patients taking immunosuppressive drugs. Aust Prescr. 2009;32:68–7. [Google Scholar]
- 7.Ohbayashi M, Suzuki M, Yashiro Y, Fukuwaka S, Yasuda M, Kohyama N, et al. Induction of pulmonary fibrosis by methotrexate treatment in mice lung in vivo and in vitro. J Toxicol Sci. 2010;35:653–61. doi: 10.2131/jts.35.653. [DOI] [PubMed] [Google Scholar]
- 8.Arikan DC, Bakan V, Kurutas EB, Sayar H, Coskun A. Protective effect of tadalafil on ischemia/reperfusion injury of rat ovary. J Pediatr Surg. 2010;45:2203–9. doi: 10.1016/j.jpedsurg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Eren I, Naziroglu M, Demirdas A. Protective effects of lamotrigine, aripiprazole and escitalopram on depression-induced oxidative stress in rat brain. Neurochem Res. 2007;32:1188–95. doi: 10.1007/s11064-007-9289-x. [DOI] [PubMed] [Google Scholar]
- 10.Beers MF. Oxygen therapy and pulmonary oxygen toxicity. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. Fishman’s Pulmonary Disease and Disorders. 4th ed. . New York: Mc Graw Hill; 2008. pp. 2624–8. [Google Scholar]
- 11.Laskin JD, Black AT, Jan JH, Sinko PJ, Heindel ND, Sunil V, et al. Oxidants and antioxidants in sulfur mustard-induced injury. Ann NY Acad Sci. 2010;1203:92–100. doi: 10.1111/j.1749-6632.2010.05605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erbayraktar S, Lanerolle N, Lotbiniere A, Knisely JP, Erbayraktar Z, Yilmaz O, et al. Carbamylated erythropoietin reduces radiosurgically-induced brain injury. Mol Med. 2006;12:74–80. doi: 10.2119/2006-00042.Erbayraktar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardee ME, Arıcasoy MO, Blackwell KL, Kirkpatrick JP, Dewhirst MW. Erythropoietin biology in cancer. Clin Cancer Res. 2006;12:332–9. doi: 10.1158/1078-0432.CCR-05-1771. [DOI] [PubMed] [Google Scholar]
- 14.Contaldo C, Meier C, Elsherbiny A, Harder Y, Trentz O, Menger MD, et al. Human recombinant erythropoietin protects the striated muscle microcirculation of the dorsal skinfold from postischemic injury in mice. Am J Physiol Heart Circ Physiol. 2007;293:274–83. doi: 10.1152/ajpheart.01031.2006. [DOI] [PubMed] [Google Scholar]
- 15.Cuzzocrea S, Mazzon E, Di Paola R, Patel NS, Genovese T, Muià C, et al. Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther. 2004;311:1272–80. doi: 10.1124/jpet.104.073197. [DOI] [PubMed] [Google Scholar]
- 16.Campos R, Shimizu MH, Volpini RA, de Bragança AC, Andrade L, Lopes FD, et al. N-acetylcysteine prevents pulmonary edema and acute kidney injury in rats with sepsis submitted to mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2012;302:L640–50. doi: 10.1152/ajplung.00097.2011. [DOI] [PubMed] [Google Scholar]
- 17.Maeda T, Miyazono Y, Ito K. Oxidative stress and enhanced para-cellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol. 2010;65:1117–23. doi: 10.1007/s00280-009-1119-1. [DOI] [PubMed] [Google Scholar]
- 18.Srivastava RK, Rahman Q, Kashyap MP, Lohani M, Pant AB. Ameliorative effects of dimetylthiourea and n-acetylcysteine on nanoparticles induced cyto-genotoxicity in human lung cancer cells-A549. PLoS one. 2011;6:e25767. doi: 10.1371/journal.pone.0025767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 20.Fridovich I. Superoxide dismutase. Adv Enzymol Relat Areas Mol Biol. 1974;41:35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- 21.Beutler E. Red Cell Metabolism. 2nd ed. New York: Grune and Stratton Company; 1975. pp. 261–5. [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr A. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 23.Atalay C, Dogan N, Aykan S, Gundogdu C, Keles MS. The efficacy of spironolactone in the treatment of acute respiratory distress syndrome-induced rats. Singapore Med J. 2010;51:501–5. [PubMed] [Google Scholar]
- 24.Ciralik H, Bulbuloglu E, Cetinkaya A, Kurutas EB, Celik M, Polat A. Effects of N-acetylcysteine on methotrexate-induced small intestinal damage in rats. Mt Sinai J Med. 2006;73:1086–92. [PubMed] [Google Scholar]
- 25.Cetinkaya A, Bulbuloglu E, Kurutas EB, Kantarceken B. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med Sci Monit. 2006;12:274–8. [PubMed] [Google Scholar]
- 26.Garipardic M, Bakan V, Davutoğlu M, Sayar H, Kurutaş EB. Oxidative stress and protective effect of erythropoietin on methotrexate-induced esophageal damage. J Pediatr Hematol Oncol. 2010;32:108–12. doi: 10.1097/MPH.0b013e3181ccb678. [DOI] [PubMed] [Google Scholar]
- 27.http://www.uptodate.com/contents/methotrexate-induced-lung-injury?source=search_result&search=mtx+lung&selectedTitle=1%7E150
- 28.Chikura B, Sathi N, Lane S, Dawson JK. Variation of immunological response in methotrexate-induced pneumonitis. Rheumatology. 2008;47:1647–50. doi: 10.1093/rheumatology/ken356. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves MR, Mowat AG, Benson MK. Acute pneumonitis associated with low dose methotrexate treatment for rheumatoid arthritis:report of five cases and review of published reports. Thorax. 1992;47:628–33. doi: 10.1136/thx.47.8.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabie T, Marti HH. Brain Protection by Erythropoietin:A Manifold Task. Physiology. 2008;23:263–74. doi: 10.1152/physiol.00016.2008. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi T, Yanase H, Iwanaga T, Sasaki R, Nagao M. Epididymis is a novel site of erythropoietin production in mouse reproductive organs. Biochem Biophys Res Commun. 2002;296:145–51. doi: 10.1016/s0006-291x(02)00832-x. [DOI] [PubMed] [Google Scholar]
- 32.Fandrey J, Bunn HF. In vivo and in vitro regulation of erythropoietin mRNA:measurement by com-petitive polymerase chain reaction. Blood. 1993;81:617–23. [PubMed] [Google Scholar]
- 33.Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–5. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brines M, Cerami A. Discovering erythropoietin’s extra-hematopoietic functions:biology and clinical promise. Kidney Int. 2006;70:246–50. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 35.Kumral A, Gonenc S, Acikgoz O, Sonmez A, Genc K, Yilmaz O. Erythropoietin increases glutathione peroxidase enzyme activity and decreases lipid peroxidation levels in hypoxicischemic brain injury in neonatal rats. Biol Neonate. 2005;87:15–8. doi: 10.1159/000080490. [DOI] [PubMed] [Google Scholar]
- 36.Guneli E, Cavdar Z, Islekel H, Sarioglu S, Erbayraktar S, Kiray M, et al. Erythropoietin protects the intestine against ischemia/ reperfusion injury in rats. Mol Med. 2007;13:509–17. doi: 10.2119/2007-00032.Guneli. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tascilar O, Cakmak G C, Tekin IO, Tekin IO, Emre AU, Ucan BH, et al. Protective effects of erythropoietin against acute lung injury in a rat model of acute necrotizing pancreatitis. World J Gastroenterol. 2007;46:6172–82. doi: 10.3748/wjg.v13.i46.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yildirim E, Ozisik K, Solaroglu I, Kaptanoglu E, Beskonakli E, Sargon MF, et al. Protective effect of erythropoietin on type II pneumocyte cells after traumatic brain injury in rats. J Trauma. 2005;58:1252–8. doi: 10.1097/01.ta.0000169803.09482.f8. [DOI] [PubMed] [Google Scholar]
- 39.Bakan V, Garipardic M, Okumuş M, Ciralik H, Atli Y, Ozbağ D, et al. The protective effect of erythropoietin on the acute phase of corrosive esophageal burns in a rat model. Pediatr Surg Int. 2010;26:195–201. doi: 10.1007/s00383-009-2480-1. [DOI] [PubMed] [Google Scholar]
- 40.Wu H, Ren B, Zhu J, Dong G, Xu B, Wang C, et al. Pretreatment with recombined human erythropoietin attenuates ischemiareperfusion-induced lung injury in rats. Eur J Cardiothorac Surg. 2006;29:902–7. doi: 10.1016/j.ejcts.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 41.Rahman I, MacNee W. Lung glutathione and oxidative stress:implications in cigarette smoke-induced airway disease. Am J Physiol. 1999;277:1067–88. doi: 10.1152/ajplung.1999.277.6.L1067. [DOI] [PubMed] [Google Scholar]


