Abstract
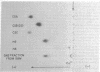
Glycosaminoglycans were isolated from purified fractions of glomerular basement membranes and partially characterized by chemical analysis and cellulose acetate electrophoresis. Basement membranes were prepared by detergent treatment of rat glomeruli and subjected to digestion with papain and Pronase. Glycosaminoglycans were isolated from the digests by precipitation with cetyl pyridinium chloride and ethanol. Results of cellulose acetate electrophoresis of the isolated glycosaminoglycan fraction revealed the presence of one major and one minor spot. The major spot was identified as heparan sulfate because it comigrated with the heparan sulfate standard and was sensitive to heparinase and to nitrous acid oxidation but insensitive to chondroitinase ABC and to testicular or leech hyaluronidase. The minor spot was tentatively identified as hyaluronic acid based on its migratory behavior and sensitivity to leech and testicular hyaluronidase. The chemical composition of the isolated glycosaminoglycan was typical of that of heparan sulfate (high carbazole/orcinol ratio, high sulfate content, absence of galactosamine). The data support and confirm the cytochemical data obtained previously [Kanwar, Y. S. & Farquhar, M. G. (1979) Proc. Natl. Acad. Sci. USA 76, 1303-1307] demonstrating that heparan sulfate is the only sulfated glycosaminoglycan detectable in the glomerular basement membrane. The present results suggest that in addition to sulfated glycosaminoglycan some nonsulfated glycosaminoglycan (hyaluronic acid) may also be present in the glomerular basement membrane.
Keywords: rat kidney, anionic sites, cellulose acetate electrophoresis
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLALOUF D., BER A., SHARON N. ACID MUCOPOLYSACCHARIDES IN RAT KIDNEYS. Biochim Biophys Acta. 1964 Nov 1;83:278–287. doi: 10.1016/0926-6526(64)90005-9. [DOI] [PubMed] [Google Scholar]
- Allalouf D., Ber A., Rechnic J., Sharon N. Anticoagulant properties of an acid mucopolysaccharide preparation isolated from rat kidney. Thromb Diath Haemorrh. 1967 Feb 28;17(1-2):264–272. [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Barry D. N., Bowness J. M. Identification and turnover of glycosaminoglycans in rat kidneys. Can J Biochem. 1975 Jun;53(6):713–720. doi: 10.1139/o75-098. [DOI] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. An assay for total hexosamine and a differential assay for glucosamine and galactosamine. Clin Biochem. 1976 Dec;9(6):269–274. doi: 10.1016/s0009-9120(76)80075-6. [DOI] [PubMed] [Google Scholar]
- Brody J. S., Vaccaro C. Postnatal formation of alveoli: interstitial events and physiologic consequences. Fed Proc. 1979 Feb;38(2):215–223. [PubMed] [Google Scholar]
- Carlson E. C., Brendel K., Hjelle J. T., Meezan E. Ultrastructural and biochemical analyses of isolated basement membranes from kidney glomeruli and tubules and brain and retinal microvessels. J Ultrastruct Res. 1978 Jan;62(1):26–53. doi: 10.1016/s0022-5320(78)80028-8. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Greene J. A. Regional distribution of acid mucopolysaccharides in the kidney. J Clin Invest. 1968 Sep;47(9):2125–2132. doi: 10.1172/JCI105898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Distribution of annionic sites in glomerular basement membranes: their possible role in filtration and attachment. Proc Natl Acad Sci U S A. 1976 May;73(5):1646–1650. doi: 10.1073/pnas.73.5.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. P., Farquhar M. G. Loss of anionic sites from the glomerular basement membrane in aminonucleoside nephrosis. Lab Invest. 1978 Nov;39(5):505–512. [PubMed] [Google Scholar]
- Cohn R. H., Banerjee S. D., Bernfield M. R. Basal lamina of embryonic salivary epithelia. Nature of glycosaminoglycan and organization of extracellular materials. J Cell Biol. 1977 May;73(2):464–478. doi: 10.1083/jcb.73.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comper W. D., Laurent T. C. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978 Jan;58(1):255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A spectrophotometric method for the microdetermination of hexosamines. J Biol Chem. 1950 Jun;184(2):517–522. [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich C. P., Sampaio L. O., Toledo O. M., Cássaro C. M. Cell recognition and adhesiveness: a possible biological role for the sulfated mucopolysaccharides. Biochem Biophys Res Commun. 1977 Mar 21;75(2):329–336. doi: 10.1016/0006-291x(77)91046-4. [DOI] [PubMed] [Google Scholar]
- Hay E. D., Meier S. Glycosaminoglycan synthesis by embryonic inductors: neural tube, notochord, and lens. J Cell Biol. 1974 Sep;62(3):889–898. doi: 10.1083/jcb.62.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRAKOWER C. A., GREENSPON S. A. Localization of the nephrotoxic antigen within the isolated renal glomerulus. AMA Arch Pathol. 1951 Jun;51(6):629–639. [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Anionic sites in the glomerular basement membrane. In vivo and in vitro localization to the laminae rarae by cationic probes. J Cell Biol. 1979 Apr;81(1):137–153. doi: 10.1083/jcb.81.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A., Denduchis B. Structural components of epithelial and endothelial basement membranes. Biochemistry. 1969 Nov;8(11):4613–4621. doi: 10.1021/bi00839a057. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. II. Acid-soluble and -precipitable species of different cell lines. Biochemistry. 1971 Apr 13;10(8):1445–1451. doi: 10.1021/bi00784a027. [DOI] [PubMed] [Google Scholar]
- Lindahl U., Hök M. Glycosaminoglycans and their binding to biological macromolecules. Annu Rev Biochem. 1978;47:385–417. doi: 10.1146/annurev.bi.47.070178.002125. [DOI] [PubMed] [Google Scholar]
- Linker A., Hovingh P. The heparitin sulfates (heparan sulfates). Carbohydr Res. 1973 Jul;29(1):41–62. doi: 10.1016/s0008-6215(00)82069-8. [DOI] [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Murata K. Acidic glycosaminoglycans in human kidney tissue. Clin Chim Acta. 1975 Sep 1;63(2):157–169. doi: 10.1016/0009-8981(75)90158-8. [DOI] [PubMed] [Google Scholar]
- Murata K., Horiuchi Y. Age-dependent distribution of acidic glycosaminoglycans in human kidney tissue. Nephron. 1978;20(2):111–118. doi: 10.1159/000181203. [DOI] [PubMed] [Google Scholar]
- Murata K. Polydisperse distribution of acidic glycosaminoglycans in bovine kidney tissue. Connect Tissue Res. 1976;4(2):131–140. doi: 10.3109/03008207609152208. [DOI] [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Seno N., Ariizumi K., Nagase S., Anno K. Isolation and characterization of heparan sulfate from rat kidney. J Biochem. 1972 Aug;72(2):479–481. doi: 10.1093/oxfordjournals.jbchem.a129924. [DOI] [PubMed] [Google Scholar]
- Spiro R. G. Studies on the renal glomerular basement membrane. Preparation and chemical composition. J Biol Chem. 1967 Apr 25;242(8):1915–1922. [PubMed] [Google Scholar]
- Terho T. T., Hartiala K. Method for determination of the sulfate content of glycosaminoglycans. Anal Biochem. 1971 Jun;41(2):471–476. doi: 10.1016/0003-2697(71)90167-9. [DOI] [PubMed] [Google Scholar]
- Trelstad R. L., Hayashi K., Toole B. P. Epithelial collagens and glycosaminoglycans in the embryonic cornea. Macromolecular order and morphogenesis in the basement membrane. J Cell Biol. 1974 Sep;62(3):815–830. doi: 10.1083/jcb.62.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag D., Stone A. L., Richter A. J., Farber S. J. Compsoition of glycosaminoglycans (Mucopolysaccharides) in rabbit kidney. II. Renal cortex. Biochim Biophys Acta. 1972 Jun 26;273(1):149–156. doi: 10.1016/0304-4165(72)90202-4. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]