Abstract
Objective:
Functional Living Index Emesis (FLIE) is developed to evaluate the relationship between emesis and it’s effects on patient’s daily life and is far more relevant to detect the effectiveness of antiemetic treatment compared with self-diary reports. In this study, the efficacy of oral neurokinin-1 antagonist aprepitant on the prevention of chemotherapy-induced nausea and vomiting and quality of life is evaluated with FLIE.
Study Design:
Cross sectional study.
Material and Methods:
Sixty patients with Non-Small Cell Lung Cancer (NSCLC) receiving a chemotherapy regimen consisting of Cisplatin and Docetaxel were evaluated. The patients were prospectively randomized to two groups before the first cycle of chemotherapy. Patients in Group A (31 patients) received 3 daily doses of aprepitant along with oral ondansetron and dexamethasone. The patients in group B (29 patients) received only ondansetron and dexamathasone. The efficacy of both regimens was evaluated by a modified Turkish version of FLIE scale consisting of 18 questions.
Results:
The number of patients with complete response was 31 in the whole group. Of these 18 patients (58%) were in Group A (Aprepitant) and 13 patients in group B (42%). Median FLIE score in group A was 24.97 (±12.45) while it was 38.1 (±26.987) in group B and the difference was statistically significant (p=0.022). Total score >20 was seen in only 5 of 31 patients in aprepitant group (16%) showing the significant efficiency of aprepitant on quality of life, while in group B, 13 of 29 patients (44%) had total scores >20 (p=0.02).
Conclusion:
Regarding these findings, it is certain to state that aprepitant in combination with other drugs optimizes protection against both nausea and vomiting compared to the prior standard of care, and must be recommended as first-line therapy for patients who are treated with moderately or highly emetogenic chemotherapy.
Keywords: Aprepitant, emezis, quality-of life, FLIE, chemotherapy
Introduction
Approxiamately 70–80% of patients receiving chemotherapy experience nausea and/or vomiting. However, emesis, nausea and vomiting significantly affect patients quality of life and can lead to poor compliance with further treatment. They can also cause metabolic imbalances, nutrient depletion, anorexia, decline of the patient’s performance status and even withdrawal from curative anticancer treatment. There are several factors affecting the severity and incidence of emesis and vomiting, including type of chemotherapy, dosage, schedule and even individual patient variability (1–3).
Vomiting is triggered by impulses to the vomiting center from the chemoreceptor trigger zone, pharynx, gastrointestinal tract and cerebral kortex. The principal neuroreceptors involved in emetic response are dopamine and the seratonin receptors. The others are, corticosteroid, histamine, cannabinoid, acetylcholine and neurokinin-1 (NK-1) receptors. Anti-emetics can block different neuronal pathways and exert their effects at different points during emesis course (4).
Aprepitant (AP) is a selective NK-1 receptor blocker that blocks the binding of substance -P at the NK-1 receptor in the central nervous system. It differs from the other antiemetics by augmenting the antiemetic activity of the 5-HT3-receptor anatagonists and dexamethasone to inhibit both acute and delayed cisplatin-induced emesis. However, most of the studies, including two phase III studies evaluated the efficieny of aprepitant by self-diary reports and reported only the incidence and severity of emesis. It is also commonly claimed that the nausea and vomiting accompanying cytotoxic chemotherapy have a negative impact on quality of life but there is little empirical data demonstrating that the failure to control postchemotherapy emesis affects aspects of quality of life other than directly related physical symptoms (5, 6).
Functional Living Index Emesis (FLIE) is developed to evaluate the relationship between emesis and it’s effects on patient’s daily life and is far more relevant to detect the effectiveness of antimetic treatment compared with self-diary reports (7, 8). Therefore, our study analyzing the efficieny of aprepitant on quality of life with FLIE has an important role in the literature. Another aim of this prospective analyze was also to detect the complete response rate in patients receiving cisplatin-based, high-emetic chemotherapy since there are some confusing results in the literature about the response rates.
Material and Methods
Sixty patients with Non-Small Cell Lung Cancer (NSCLC) were evaluated. Fifty-six patients (93%) were male and 4 patients were female. Median age was 58 years (38–72 years) with no significant difference between the groups. All patients received the same chemautherapetic regimen consisting of cisplatin (75 mg/m2, day 1) and docetaxel (75 mg/m2, day 1). The patients were prospectively randomized to two groups (Group A and B) before the first cycle of chemotherapy. Patients in Group A (31 patients) received 3 daily doses of aprepitant (125 mg on day 1, 80 mg on days 2 and 3) along with oral ondansetron- 4 mg (4 days, begining on day 1 of chemotherapy) and dexamethasone (8 mg on day 1, 4 mg on days 2 and 3). The patients in group B (29 patients) received only ondansetron and dexamathasone same as group A.
The efficacy of both regimens was evaluated by a modified Turkish version of Functional Living Index Emesis (FLIE) scale which is a validated, nausea - and vomiting-specific, patient-reported quality-of life questionnare consisting of 18 questions. It is a validated nausea- and vomiting-specific patient-reported outcome (PRO) instrument comprised of 2 domains (vomiting and nausea) with 9 identical items in each domain. The first item in each domain asks the patient to rate how much nausea (vomiting) he/she experienced over the past 5 days. The remaining 8 items assess the impact of nausea [vomiting] on ability to enjoy meals/liquids, prepare meals/do household tasks, perform daily functions, perform usual recreation/leisure activities, willingness to spend time with family and friends, and the extent to which the side-effect has caused personal hardship and hardship on others. The score of the FLIE is determined by summing the responses to the 18 questions on a 7 point analogue scale and, therefore, the range of total scores possible is between 18 and 126 (7, 8). In our modified scale each item is answered using a 100 mm (1–7 points) visual analogue scale (VAS) with “1 point” corresponding to “none”/“not at all” and 6–7 points” corresponding to “a great deal”. Examples of 2 questions in the scale are shown below. The questionare was completed on day 5 following chemotherapy by each patient.
All statistical analyses was performed with SPSS 13.
e.g: 1. How much nausea have you had in the past 3 days?

e.g: 2. Has vomiting affected your ability to maintain usual recreation or leisure activities during the past 3 days?
Results
The number of patients with complete response (no emesis and no rescue medication) was 31 in the whole group. Of these 18 patients (58%) were in Group A (Aprepitant) and 13 patients in group B (42%) (Table 1). Median FLIE score in group A was 24.97 (±12.45) while it was 38.1 (±26.987) in group B and the difference was statistically significant (p=0.022). Total FLIE score >50 showing a moderate loss in quality of life was obtained in 10 patients and of these 9 patients (90%) was in group B which did not receive aprepitant (Figure 1). Moreover, total score >20 was seen in only 5 of 31 patients in aprepitant group (16%) showing the significant efficiency of aprepitant on quality of life, while in group B, 13 of 29 patients (44%) had total scores >20 (p=0.02) (Figure 2). Combination treatment was well tolerated, with no unexpected events.
Table 1.
Complete response rates
| No. of patients with complete response | % | |
|---|---|---|
| Group 1 (Aprepitant) | 18/31 patients | 58% |
| Group 2 (control) | 13/29 patients | 42% |
Figure 1.

Number of patients with FLIE score >50
Figure 2.
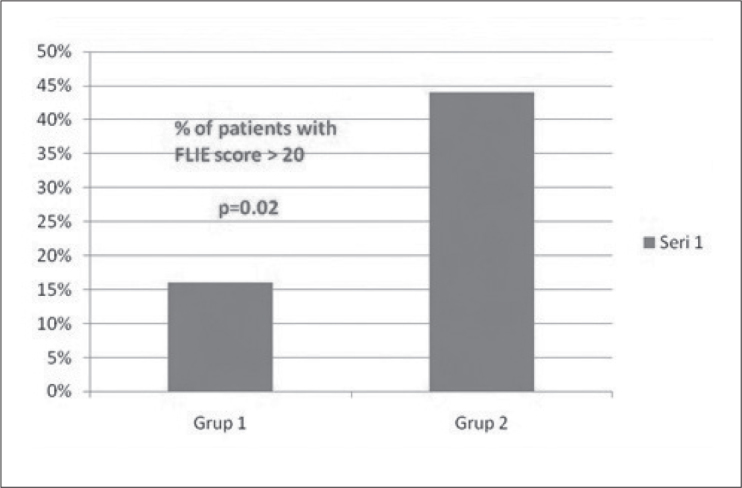
% of patients with FLIE score >20
Discussion
Most of the studies in the literature about the efficiancy of aprepitant is based on patient-self reported diaries and mainly evaluates the presence and amount of emesis. However, the effect of emesis in patients quality of life is really evaluated in only a few trials. Functional Living Index Emesis (FLIE) is developed to evaluate the relationship between emesis and it’s effects on patient’s daily life and is far more relevant to detect the effectiveness of antiemetic treatment compared with self-diary reports. Therefore, our study that anayzes the affect of aprepitant on quality of life with FLIE has an important role in the literature.
The efficieny of Aprepitant (AP) was tested in two large randomized trials. However both of these studies had some limitations. At first hand, both trials had analyses depending on self-reports, as mentioned above. Secondly, the second randomized trial included patients with moderately emesing chemotherapy (non-cisplatin based). The first trial was reported by Hesketh et al. (5) and included patients receiving cisplatin ≥70 mg/m2. For antiemesis treatment, the first group received standard therapy (ondansetron and dexamethasone on day 1; dexamethasone on days 2 to 4) and the second group received an aprepitant regimen (aprepitant plus ondansetron and dexamethasone on day 1; aprepitant and dexamethasone on days 2 to 3; dexamethasone on day 4). Nausea and vomiting episodes were recorded in a diary by patients. Similar to our findings, their results showed that, the percentage of patients with complete response was significantly higher in the aprepitant group (72.7% vs. 52.3%) (7).
In the second randomized trial Warr et al. (6) analyzed the effect of aprepitant after one cycle of moderately emetogenic chemotherapy. The patients were randomized to either an Aprepitant Regimen (day 1: AP 125 mg, ondansetron (OND) 8 mg, and dexamethasone (dex) 12mg before chemotherapy and OND 8mg 8 hours later; days 2–3: AP 80 qd) or a standard Regimen (day 1: OND 8 mg and dex 20 mg before chemotherapy and OND 8 mg 8 hours later; Days 2–3: OND 8 mg bid). Data on nausea, vomiting, and use of rescue medication were collected with a self-report diary. Overall complete response was greater with the aprepitant regimen than with the standard regimen (50.8% vs. 42.5%, p=0.015). More patients taking aprepitant reported no vomiting (75.7% vs. 58.7%, p<0.001). Reports of no use of rescue therapy were similar (58.7% vs. 56.2%) (8).
Both these results were evaluated in a metanalysis by De Wit et al. (9) and the authors stated that aprepitant was useful in then prevention of emesis for patients receiving moderately emetogenic chemotherapy plus high-dose cisplatin.
In 2004, Tremont-Lukats IW et al. (10) reported another meta-analysis of neurokinin-1 receptor antagonists (NK-1 RA) for chemotherapy-induced nausea and vomiting and they also showed that NK1 RAs added to standard therapy significantly increased complete response rates from delayed nausea and vomiting, with no significant effect on acute phase.
Also a few small studies evaluated the the combination regimens with aprepitant. In one of these studies that included 58 patients, Grote et al. (11) reported a complete response rate of 78% with palonosetron and aprepitant combination. The authors stated that more than 90% of patients during all time intervals had no emetic episodes, and between 57% and 71% of patients reported no nausea during each of the 5 days post chemotherapy.
Recently in 2011, an update of consensus recommendations for the prevention of vomiting and nausea following high-emetic risk chemotherapy was published and it was stated that a three-drug combination of a 5-hydroxytryptamine type 3 receptor (5-HT(3)) receptor antagonist, dexamethasone, and aprepitant beginning before chemotherapy and continuing for up to 4 days remains the standard of care (12).
Our results are also in accordance with the literature showing a higher complete response rate in AP group (58% vs. 42%) and a better quality of life documented with FLIE.
Regarding these findings, it is certain to state that aprepitant in combination with other drugs optimizes protection against both nausea and vomiting compared to the prior standard of care, and must be recommended as first-line therapy for patients who are treated with moderately or highly emetogenic chemotherapy. Its role in improving quality of life is also an important fact that must be clearly underlined.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study.
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - G.A.; Design - G.A.; Supervision -G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.; Resource - G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.; Materials - G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.; Data Collection&/or Processing - G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.; Analysis&/or Interpretation - G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.; Literature Search - G.A.; Writing - G.A.; Critical Reviews - G.A., İ.D., F.E., S.Y.Ş., F.H.A., S.T., D.C., K.U.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: No financial disclosure was declared by the authors.
References
- 1.Richardson JL, Marks G, Levine A. The influence of symptoms of disease and side effects of treatment on compliance with cancer therapy. J Clin Oncol. 1988;5:1746–52. doi: 10.1200/JCO.1988.6.11.1746. [DOI] [PubMed] [Google Scholar]
- 2.Lazslo J. Emesis as limiting toxicity in cancer chemotherapy. In: Lazslo J, editor. Antiemetics and cancer chemotherapy. Baltimore, Maryland: Williams & Wilkins; 1983. pp. 1–5. [Google Scholar]
- 3.Borison HL, Wang S. Physiology and pharmacology of vomiting. Pharmacol Rev. 1953;5:193–230. [PubMed] [Google Scholar]
- 4.Siegel LJ, Longo DL. The control of chemotherapy induced emesis. Ann Intern Med. 1981;95:352–9. doi: 10.7326/0003-4819-95-3-352. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting:a multinational, randomizedi double-blind, placebo-controlled trial in patients receiving high-dose cisplatin-The Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 6.Warr DG, Eisenberg P, Hesketh PJ, et al. Effect of aprepitant for the prevention of nausea and vomiting after one cycle of moderately emetogenic chemotherapy:A randomized double-blind trial in 866 patients. ASCO annual meeting Proceedings (post-meeting edition) J Clin Oncol. 2004;22:8007. [Google Scholar]
- 7.Decker GM, DeMeyer ES, Kisko DL. Measuring the maintenance of daily life activities using the functional living index-emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J Support Oncol. 2006;4:35–52. [PubMed] [Google Scholar]
- 8.Martin AR, Carides AD, Pearson JD, Horgan K, Elmer M, Schmidt C, et al. Functional relevance of antiemetic control:experience using the FLIE questionnaire in a randomized study of the NK-1 antagonist aprepitant. Eur J Cancer. 2003;39:1395–401. doi: 10.1016/s0959-8049(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 9.de Wit R, Herrstedt J, Rapoport B, Carides AD, Guoguang-Ma J, Elmer M, et al. The oral NK(1) antagonist aprepitant, given with standart antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy:a combined analysis of two randomized, placebo controlled phase III clinical trials. Eur J Cancer. 2004;40:403–10. [PubMed] [Google Scholar]
- 10.Tremont-Lukats IW, Gonzalez-Barboteo J, Bruera E, et al. Meta-analysis of neurokinin-1 receptor antagonists (NK-1 RA) for chemotherapy-induced nausea and vomiting (CINV). ASCO Annual Meeting Proceedings (post-meeting edition) J Clin Oncol. 2004;22:8047. [Google Scholar]
- 11.Grote T, Hajdenberg J, Cartmell A, Ferguson S, Ginkel A, Charu V. Combination therapy for chemotherapy-induced nausea and vomiting in patients receiving moderately emetogenic chemotherapy:palonosetron, dexamethasone and aprepitant. J Support Oncol. 2006;4:403–8. [PubMed] [Google Scholar]
- 12.Kris MG, Tonato M, Bria E, Ballatori E, Espersen B, Herrstedt J, et al. Consensus recommendations for the prevention of vomiting and nausea following high-emetic-risk chemotherapy. Support Care Cancer. 2011;19(Suppl 1):S25–32. doi: 10.1007/s00520-010-0976-9. [DOI] [PubMed] [Google Scholar]


