Abstract
Hypertension is common, difficult to diagnose, and poorly controlled among patients with ESRD. However, controversy surrounds the diagnosis and treatment of hypertension. Here, we describe the diagnosis, epidemiology, and management of hypertension in dialysis patients, and examine the data sparking debate over appropriate methods for diagnosing and treating hypertension. Furthermore, we consider the issues uniquely related to hypertension in pediatric dialysis patients. Future clinical trials designed to clarify the controversial results discussed here should lead to the implementation of diagnostic and therapeutic techniques that improve long-term cardiovascular outcomes in patients with ESRD.
Keywords: hypertension, hemodialysis, dialysis
Hypertension is common among patients with ESRD. In this review, we discuss the diagnosis, epidemiology, and management of hypertension among dialysis patients. We also review areas of existing controversies and briefly discuss the issue of hypertension in pediatric dialysis patients.
Epidemiology
The prevalence, treatment, and control of hypertension among people on hemodialysis (HD) have used varying definitions to diagnose hypertension. The epidemiology differs based on how BP is measured: either before and after dialysis or using ambulatory BP recordings.
Epidemiology with Routine BP Measurements
The prevalence of hypertension (defined as 1-week average predialysis systolic BP [SBP] measurements >150 mmHg or diastolic BP [DBP]>85 mmHg or the use of antihypertensive medications) was 86% among 2535 clinically stable adult HD patients participating in a multicenter trial.1 Among hypertensive patients, 12% did not receive antihypertensive drugs, 58% were treated but not controlled, and only 30% were controlled. The use of antihypertensive drugs has been reported to vary from 59% to 83%.2–5 Furthermore, even among children on long-term HD, similar findings have been reported.6 Several studies have confirmed greater antihypertensive drug use to be associated with poorer control.7,8 It should be noted that antihypertensive drug use per se do not lead to worse BP control; in the absence of adequate volume control, increasing antihypertensive drug use may simply reflect difficult-to-control BP.
Epidemiology Using Ambulatory BP Measurements
The prevalence of hypertension (defined by either a 44-hour interdialytic ambulatory BP of ≥135/85 mmHg or the prescription of any antihypertensive agent) was 86% among 369 chronic HD patients.8 Although hypertension was being treated with antihypertensive drugs in 89% of patients, it was adequately controlled only in 38%. The independent determinants of poor control were the use of antihypertensive drugs and an expanded extracellular volume state. If patients were volume overloaded, nearly 80% became hypertensive when medications were withdrawn. Paradoxically, the more medications the patients received, the more likely they were to be hypertensive.
Epidemiology of Hypertension in Peritoneal Dialysis
Some studies suggest that hypertension control in patients on peritoneal dialysis (PD) is superior compared with those on HD.9,10 For example, among 1202 patients participating in the 1995 Peritoneal Dialysis Core Indicators Study, the average BP among PD patients was 139/80 mmHg.11 This is in contrast with the predialysis BP of 152/82 mmHg among 1238 participants in the Hemodialysis study3 or in another study including 414 Italian PD patients, in which the prevalence of hypertension was 88% based on office BP≥140/90 mmHg and 69% based on BP load >30%.12 Some have theorized that better BP control in PD patients could be explained in part by removal of vasopressors and sodium pump inhibitors by PD.13
In another study, the comparison of 22 patients on HD with 24 patients on PD with 44-hour ambulatory BP monitoring showed no differences in daytime and nighttime BP.14 Nonetheless, high-quality head-to-head studies are sparse and the epidemiology of hypertension may be similar to that seen among HD patients.15
Among PD patients, volume excess, as assessed by tracer dilution, was common and was related to DBP and eccentric left ventricular hypertrophy (LVH).16 Volume overload in PD patients may be related to the peritoneal transport characteristics.17 High transporters tend to have a higher BP; ultrafiltration may restore their BP to more normotensive levels.17 In a small study, patients on continuous cyclic PD were reported to have a greater left ventricular mass compared with those on continuous ambulatory PD.18 This was thought to be a result of greater volume overload.18
Diagnosis
The diagnosis of hypertension among patients on HD is challenging and this may lead to overtreatment or undertreatment of hypertension.19–22 Diagnosing hypertension is difficult for several reasons.23 BP in these patients is often measured without attention to technique.24 BP declines during HD with ultrafiltration. This decline in BP can be variable and in part is related to the magnitude and intensity of ultrafiltration.25 For example, those patients who have a large volume removed over a short period of time may have a large decline in BP. These patients may also gain the removed volume back over the interdialytic interval and have a large increase in BP.26 Predialysis BP may therefore be hypertensive and postdialysis BP may be hypotensive. It therefore becomes unclear which BP measurement to use to diagnose hypertension,27 and substantial errors can occur both in detecting hypertension and assessing its severity.28,29 Both predialysis and postdialysis BP measurements are highly variable such that the variability between patients is about the same as variability in an individual patient over time.30 In addition, HD patients have significant seasonal variability in BP; BP is highest during winters and lowest during summers.31 This may be related to temperature-induced vasodilation. Although significant relationships exist between both predialysis and postdialysis BP and interdialytic ambulatory BP,32 a meta-analysis has shown that predialysis and postdialysis BP measurements agree poorly with interdialytic ambulatory BP.33 Accordingly, among HD patients, large errors are possible when using predialysis or postdialysis BP to judge the magnitude of elevation in interdialytic ambulatory BP.
The existing, although somewhat dated, National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines recommend that BP measurements should be <140/90 and <130/80 mmHg before and after HD, respectively.34 Use of predialysis or postdialysis BP measurements to make management decisions in the interdialytic period can be problematic. For example, in a survey in the United Kingdom, centers that achieved better postdialysis BP targets had more intradialytic hypotension.35 What is clear is that interdialytic weight gain increases predialysis BP3,36–39 and provokes the use of antihypertensive therapy.36,37 However, interdialytic weight gain does not correlate with interdialytic ambulatory BP.40,41 Therefore, whether achieving these peridialysis BP targets would cause clinical harm (or benefit) remains unknown.
Using all BP values measured during a mid-week dialysis may serve as a more useful tool to estimate interdialytic ambulatory BP.42 Although the mean intradialytic BP serves as a useful tool to assess hypertension, the calculation of median intradialytic BP is computationally easier than calculating the mean. It may therefore be used as a bedside tool to predict interdialytic ambulatory BP. A mid-week median intradialytic BP of ≥140/90 mmHg has sensitivity and specificity that exceeds predialysis or postdialysis measurements and can serve as a rapid and convenient tool to assess hypertension in long-term HD patients.42 However, this is the method of last resort because better methods are available to evaluate hypertension in HD patients.
Home BP monitoring is a practical way to diagnose and manage hypertension in all patients with kidney disease.43,44 Home BP monitoring is recommended by both the American Heart Association and the European Society of Hypertension for diagnosing and managing hypertension.45,46 Home BP monitoring is especially valuable in diagnosing and managing hypertension for those on HD for the following reasons.47 Home BP correlates more closely with ambulatory BP compared with predialysis or postdialysis BP recordings.48 Home BP can track changes in BP evoked by the reduction in dry weight.49 Home BP, compared with predialysis or postdialysis BP recordings, is much more reproducible from one week to the next.49 Home BP is superior to measurements made in the dialysis unit, even when the dialysis unit measurements are made using recommended techniques, in predicting the presence of target organ damage (echocardiographic LVH)50,51 or long-term outcomes such as cardiovascular events52 or mortality.52–55 The association of BP and outcomes is discussed further in the section on prognosis. A recent trial randomized stable HD patients to home BP-guided therapy or predialysis BP-guided therapy.56 The primary goal was to assess change in interdialytic ambulatory BP at 6 months and change in echocardiographic LVH. There was no change in ambulatory BP at 6 months in the predialysis BP-guided therapy group. A significant decrease in ambulatory SBP (but not DBP) was noted at 6 months in the group treated using home BP recordings. Between-group differences were significant. Given the small number of patients and variability in timing of echocardiographic left ventricular mass measurements, no between-groups differences were noted. Another trial randomized 17 HD patients to usual care and 17 patients to home BP monitoring. Significant improvement in average weekly SBP was seen in the home BP group only.57 These data support the use of home BP measurement to manage HD patients.
Among HD patients, the timing and frequency of home BP monitoring is of particular importance. Home BP increases on average at a rate of 4 mmHg every 10 hours elapsed after dialysis.58 Therefore, measurement soon after dialysis or just before dialysis will underestimate or overestimate the burden of hypertension. Therefore, it is important to measure BP at various intervals after dialysis. Simply obtaining a BP measurement 20 minutes postdialysis may not yield the most representative interdialytic BP.59 We recommend that measurements be made twice daily (on waking up in the morning and just before going to sleep) after a midweek dialysis for 4 days,60 given that interdialytic BP measures may more capably predict LVH and mortality.50–55 These measurements allow an adequate number of measurements for diagnosing and managing hypertension. For long-term follow-up, monthly measurement (over 4 days after a midweek dialysis as noted above) should suffice in most patients. More frequent measurements may be needed in individuals who are clinically unstable.
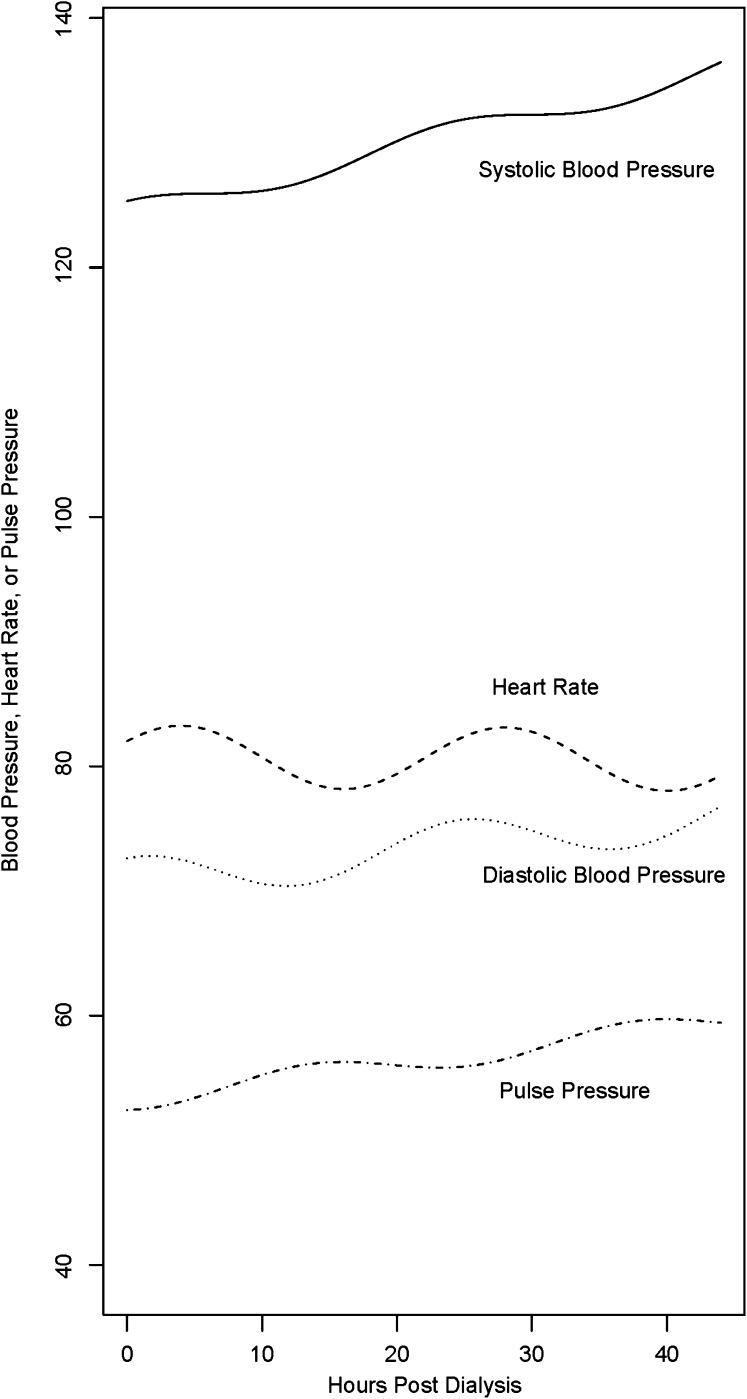
Ambulatory BP monitoring, among HD patients, is held to be the gold standard for diagnosing hypertension.27,61–63 Compared with peridialytic BP recordings, it correlates better with LVH50 and all-cause mortality.64 While using a validated monitor,65 we recommend measuring BP over the entire interdialytic interval (44 hours). We recommend recording BP every 20 minutes from 6 am to 10 pm and every 30 minutes from 10 pm to 6 am66 As in the case of home BP, interdialytic SBP increases, albeit at a slower rate of 2.5 mmHg every 10 hours.67,68 Because a much greater number of measurements during the interdialytic interval are typically available compared with home BP, patterns of BP can be evaluated. Figure 1 illustrates the pattern of BP and heart rate over an interdialytic interval. Among HD patients, ambulatory BP monitoring remains a research technique.
Figure 1.
Modeled trended cosinor BP and pulse rate in HD patients. Notice the linear trend in SBP, DBP, and pulse pressure but the lack thereof in heart rate. Reprinted from reference 67, with permission.
In approximately 10%–15% of patients, instead of decreasing, BP paradoxically increases during dialysis.69 These patients have intradialytic hypertension. Intradialytic hypertension is defined in different ways. These definitions include the following: (1) a discrete change in BP from predialysis to postdialysis in a certain number of dialysis treatments; (2) regression of all intradialytic BP with a slope >0; and (3) a change of >0 mmHg from predialysis to postdialysis. Intradialytic hypertension is associated with greater short-term (6-month) mortality in HD patients.70 In another cohort, an increase in SBP by >10 mmHg during HD occurred in approximately 10% of incident patients. Although this increase in SBP during HD was associated with decreased 2-year survival, these findings were limited to patients with predialysis SBP of <120 mmHg.71 Although the exact mechanism of this relationship is unclear,72,73 a study shows that intradialytic hypertension in HD patients using definition 2 noted above is associated with both volume excess and interdialytic hypertension.74 Another study, using definition 1, confirmed the association between intradialytic hypertension and interdialytic hypertension.75
At least two studies suggest that lowering dry weight may improve interdialytic hypertension. Cirit et al. treated seven hypertensive patients on HD with marked cardiac dilation that experienced paradoxical hypertension during dialysis.76 After probing dry weight, both BP and postdialysis weight was reduced; the BP reduction was 46/22 mmHg and postdialysis weight was reduced by 6.7 kg. The authors concluded that BP may paradoxically rise with ultrafiltration when patients are volume overloaded. Dry weight was reduced progressively in the randomized Dry-Weight Reduction in Hypertensive Hemodialysis Patients (DRIP) trial, as discussed below.77 Those patients with intradialytic hypertension who had additional ultrafiltration and therefore a reduction in dry weight had improvement in both intradialytic and interdialytic hypertension.74 This suggests that an appropriate therapy for this condition would be to further lower dry weight. However, the phenomenon of intradialytic hypertension is complex and incompletely understood; pilot studies suggest that endothelial dysfunction may be operative in its pathogenesis.78
Home BP measurement is a practical way to measure and manage hypertension among HD patients. The targets of therapy using home BP monitoring will need to be defined in future trials. Guidelines of the American Heart Association define hypertension as home BP of at least 135/85 mmHg45; lowering BP in the interdialytic period to at least 140/90 mmHg appears to be a reasonable goal (Table 1).
Table 1.
Diagnosis and epidemiology of hypertension
| Summary Statements |
| 1. Diagnosing hypertension in dialysis patients is challenging. |
| 2. Compared with predialysis and postdialysis BP measurements, diagnosis of hypertension is better made by using home BP recordings or interdialytic ambulatory BP recordings. |
| 3. Hypertension is frequently treated with antihypertensive drugs, but remains adequately controlled in only a minority of the patients. |
| 4. Hypertension in children on dialysis is as prevalent as in adults and given the life-time risk needs to be controlled. |
Nonpharmacologic Treatment
Once an accurate diagnosis is made, the therapy of hypertension among HD patients rests on nonpharmacologic management. Although scarcely studied, one small study lasting 6 months showed a beneficial effect of exercise on BP and medication requirements.79 Exercise consisted of using a stationary bicycle during dialysis.79 Besides this promising strategy, the nonpharmacologic management of hypertension is based on four principles: dietary sodium restriction, individualizing dialysate sodium, the management of dry weight, and providing an adequate duration of dialysis. These principles are further discussed.
Dietary Sodium Restriction
Dietary sodium restriction limits interdialytic weight gain and improves the feasibility of achieving dry weight.80,81 Instead of restricting dietary sodium, patients on HD are sometimes prescribed fluid-restricted diets. With the exception of treating hyponatremia, there is no scientific basis for prescribing a fluid-restricted diet in these patients.82
Recent guidelines suggest that elderly persons and individuals with CKD are most likely to derive the greatest benefits from dietary sodium restriction.83 These guidelines are even stricter on sodium intake than those advocated earlier (2 g/d). Dietary sodium restriction to no more than 1.5 g sodium (or approximately 65 mmol) per day is now recommended.
Although no randomized trials have been performed among patients with ESRD, observational studies among long-term HD patients suggest that restricting dietary sodium and achieving dry weight can improve LVH.84
Individualizing Dialysate Sodium
High sodium dialysis was initially prescribed to provide hemodynamic stability, fewer disequilibrium symptoms, and fewer muscle cramps.85 Early studies found that high sodium dialysate among normotensive patients reduced dialysis-induced hypotension and was not associated with long-term hypertension.86 However, its effect among those with hypertension was less clear.86 A double-blind crossover trial in seven dialysis patients found that compared with dialysate sodium of 135 mEq/L, both dialysate sodium of 143 mEq/L or sodium gradient dialysate of 160–133 mEq/L were associated with greater interdialytic weight gain (2.2, 2.6, and 2.8 kg respectively).87 Another study showed that interdialytic weight gain and thirst can be provoked with the prescription of hypertonic dialysate.88 These findings are now being recognized as important treatment targets.89 The prescription of high sodium dialysate allows increased fluid volume removal and better hemodynamic stability. However, it provokes increased thirst, increased interdialytic weight gain, and more fluid removal with next dialysis. It may therefore provoke hemodynamic instability and prescription of even a higher dialysate sodium, perpetuating a vicious cycle.90 In some patients, worsening of BP control may ensue.86 The vicious cycle can be interrupted by individualizing dialysate sodium concentration,91 which may improve BP control.92 In a pilot study of 16 patients, dialysate sodium was progressively decreased in four phases from 137.8 to 135.6 mmol.93 As a result of this maneuver, the net sodium loss increased nearly 100 mmol from 383 to 480 mmol per treatment; this was associated with reduced interdialytic weight gain and BP.93 Thus, facilitating diffusive sodium losses in addition to convective loss can increase net sodium removal and therefore lower BP. Sodium ramping that is prescribed to offset intradialytic hemodynamic instability is associated with fewer hypotensive episodes on dialysis but greater interdialytic fatigue and thirst, greater interdialytic weight gain, and hypertension.94 Interdialytic 24-hour ambulatory BP increased when the time-averaged concentration of sodium was extremely elevated at 147 mEq/dl.95 Therefore, one sodium prescription may not fit all patients.
In a nonrandomized trial, improvement in nocturnal mean arterial pressure was found among PD patients who were prescribed a low dialysate sodium.96 However, if the low dialysate sodium was not accompanied by a reduction in dry weight, BP did not change. Prescribing both low dialysate sodium and challenging dry weight may improve BP control over and above one strategy alone. In addition, BP increments provoked by higher sodium dialysate can be adequately controlled by the adjustment of dry weight.97
Management of Dry Weight
The management of dry weight poses several challenges. First and foremost, there is no universally agreed-upon definition of dry weight. Sinha and Agarwal define dry weight as the lowest tolerated post dialysis weight achieved via gradual change in postdialysis weight at which there are minimal signs or symptoms of either hypovolemia or hypervolemia.98
Assessment of Dry Weight
The physical examination is notoriously unreliable in excluding volume overload. For example, pedal edema does not correlate with dry weight very well. In a case-control study, Agarwal et al. found that inferior vena cava diameter, blood volume monitoring, plasma volume markers, and inflammation markers were not determinants of edema.99 For the most part, the assessment and achievement of dry weight is an iterative process that often provokes uncomfortable intradialytic symptoms such as hypotension, dizziness, cramps, nausea, and vomiting. These symptoms often lead to interventions such as cessation of ultrafiltration, administration of saline, premature cessation of dialysis, or placing the patient in the head-down (Trendelenburg) position. Interestingly, placing the patient in the head-down position does little to protect the BP and this practice is questionable100; raising the leg passively without lowering the head can, however, be effective to raise ventricular filling pressure.101 Often physicians will respond to these distressing symptoms by raising dry weight, and then adding more antihypertensive medication. Paradoxically, this may make subsequent achievement of dry weight even more difficult. However, if dry weight is reduced gently either by setting the ultrafiltration goal to just a little above the previous achieved postdialysis weight (e.g., by 0.2–0.3 kg in an adult) either without changing the dialysis time or better still by prolonging the dialysis time to allow for slower ultrafiltration with dialysis, dry weight can then be successfully achieved.
Benefits of Probing Dry Weight
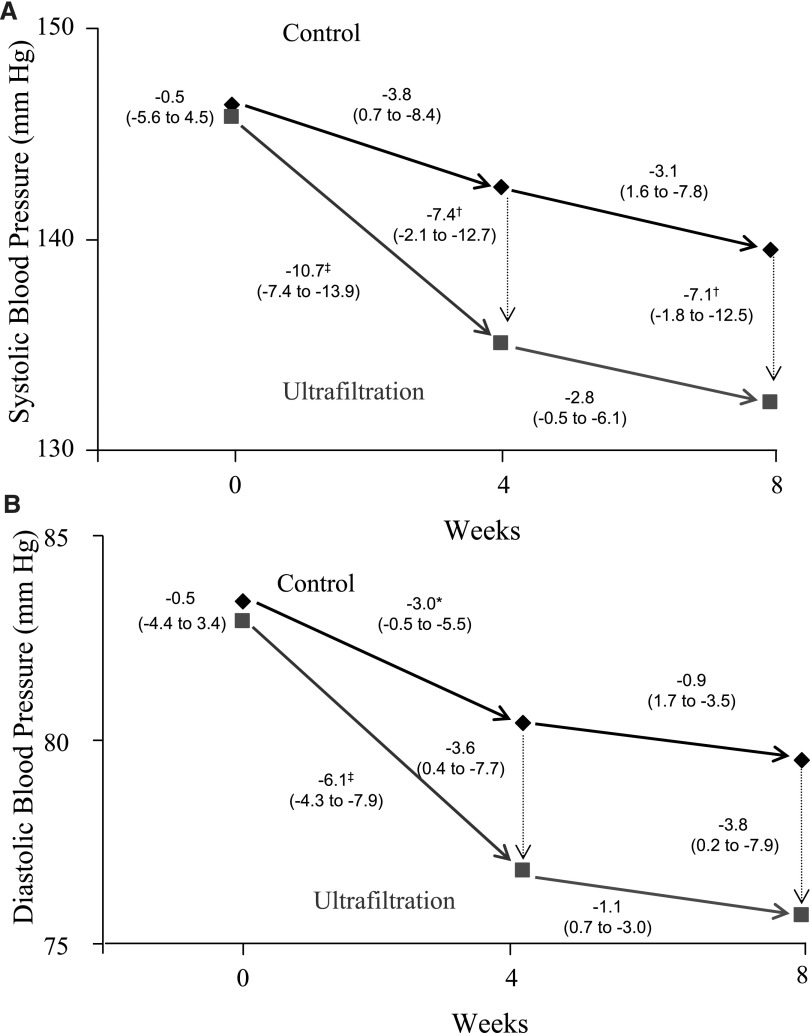
Dry weight was probed without changing the dialysis time in a randomized controlled trial of hypertensive HD patients.77 Notably, in this study, patients with obvious volume overload were excluded. Thus, the study tested the hypothesis that hypertension among HD patients who do not manifest overt signs of volume overload is mediated by excess volume. Interdialytic ambulatory BP monitoring was performed three times (at baseline, 4 weeks, and 8 weeks) in 50 patients randomized to a control group and 100 patients randomized to ultrafiltration group. Ambulatory BP was reduced within 4 weeks by 11/6 mmHg77 (Figure 2). This level of BP reduction was achieved despite stable concurrent use of 2.7 antihypertensive drugs. The magnitude of reduction in BP is therefore much larger than what would be expected by adding an additional antihypertensive agent. Because the control group had a placebo effect, subtracting this effect from the intervention group resulted in still a significant ambulatory BP reduction of 7/3 mmHg. This antihypertensive effect was sustained for 8 weeks of observation. Despite provoking occasional uncomfortable intradialytic symptoms, the quality of life was not impaired. Even in this randomized trial, the presence or absence of edema, which is often taken as a reliable sign of volume overload, had no predictive value in separating the responders from nonresponders. Furthermore, 10% of the patients in the control group developed accelerated hypertension, defined as BP ≥175/105 mmHg by interdialytic ambulatory monitoring. This study provides strong support for the hypothesis that among HD patients, dry weight reduction is an effective strategy for reducing BP.
Figure 2.
The effect of dry weight reduction on interdialytic ambulatory SBP and DBP in hypertensive HD patients. The mean SBPs (A) and DBPs (B) are shown for the baseline control and ultrafiltration groups. The mean changes in BP are shown for weeks 4 and 8 after randomization (solid arrows), and the mean differences in BPs (dotted arrows) between the two groups at each 4-week interval. The numbers next to the dotted lines connecting the data points are the mean changes in BP between groups at 4 and 8 weeks after randomization. The 95% confidence intervals (95% CIs) are given in parentheses. Significant differences between groups or within groups are as indicated as follows: *P<0.05; †P<0.01; ‡P<0.001. The ultrafiltration-attributable change in SBP was −6.9 mmHg (95% CI, −12.4 to −1.3 mmHg; P=0.02) at 4 weeks and −6.6 mmHg (95% CI, −12.2 to −1.0 mmHg; P=0.02) at 8 weeks. The ultrafiltration-attributable change in DBP was −3.1 mmHg (95% CI, −6.2 to −0.02 mmHg; P=0.05) at 4 weeks and −3.3 mmHg (95% CI, −6.4 to −0.2 mmHg; P=0.04) at 8 weeks. Reprinted from reference 77, with permission.
Observational studies also support the practice of probing dry weight. In 1969, Vertes et al. reported that 35 of 40 patients became “normotensive” by achieving dry weight.102 In a more recent report from Turkey, Kayikcioglu et al. compared the benefit of nonpharmacologic therapy versus pharmacologic therapy for control of left ventricular mass among HD patients.103 In a case-control study, patients who had been treated at one center with salt restriction and dry weight reduction were compared with patients at another center where antihypertensive-based therapy was the primary method for management of hypertension. The center using dry weight and salt restriction as a primary strategy had the following benefits: lower antihypertensive drug use (7% versus 42%), lower interdialytic weight gain, lower left ventricular mass, better diastolic and systolic left ventricular function, and fewer episodes of intradialytic hypotension. These observations are important and of clinical relevance; they suggest that probing for dry weight as opposed to adding more antihypertensive drugs perhaps diminishes the risk for cardiac remodeling and mitigates LVH, and preserves systolic and diastolic left ventricular function. Although a case-control study cannot assert causation, the results of this study support the use of nonpharmacologic therapies in the management of patients with ESRD.
Dry Weight and Outcomes
Studies among HD patients in both adults and children suggest that managing intradialytic relative plasma volume (RPV) may reduce the number of hospital admissions caused by fluid overload,104,105 may improve BP control, and may decrease hypotension-associated dialysis symptoms.106 It is possible that the latter benefit is, in part, related to diminished use of antihypertensive medication. Accordingly, monthly monitoring of RPV and home BP may offer an attractive way to assess the adequacy of volume control among HD patients.
To note, the multicenter randomized Crit-Line Intradialytic Monitoring Benefit (CLIMB) trial107 demonstrated that RPV-guided therapy was associated with worse outcomes, contrary to the original hypothesis. The CLIMB trial randomized 227 HD patients to RPV monitoring and 216 to conventional monitoring for 6 months to test the hypothesis that RPV-guided monitoring would result in reduced hospitalization rates. Compared with the conventional group, the adjusted risk ratios (RR) were 1.61 (95% confidence interval [95% CI], 1.15 to 2.25; P=0.01) for nonaccess hospitalization and 1.52 for access-related hospitalization (P=0.04) in the RPV-guided monitoring group. Mortality was 8.7% and 3.3% (95% CI, 1.02 to 2.28; P=0.021) in the RPV-guided monitoring and conventional monitoring groups, respectively. An elaborate protocol was available to guide fluid management based on RPV-guided monitoring; algorithm use was encouraged but not mandated. Furthermore, highly variable implementation of the monitoring and interventional algorithm occurred within and across dialysis units. At baseline, as determined by RPV slope patterns, patients in the conventional group appeared to be more volume overloaded compared with the RPV-guided group. At 6 months, both groups had similar RPV slopes. Thus, the conventional group appeared to have had greater volume challenge than the intervention group. Although this was a randomized trial, the findings should be interpreted with caution for the above reasons.
To study the effect of volume status on mortality, Wizemann et al. followed 269 prevalent HD patients for several years.108 They measured hydration state using a body composition analyzer. If there was >15% excess of extracellular water (2.5 L volume excess), they classified such patients as volume overloaded; 25% of the patients had excess extracellular fluid (ECF) volume. In a multivariate adjusted analysis, they found that excess volume was associated with high mortality. Compared with those without excess ECF volume, the hazard ratio of mortality with excess fluid volume was 2.1 (95% CI, 1.39 to 3.18; P=0.003). Although the study did not examine the effect of reduction in ECF volume on subsequent outcomes, such studies need to be performed in the future.
Inrig et al. compared the change in pulse pressure during dialysis as a risk factor for hospitalization and mortality among prevalent HD patients participating in a randomized controlled trial.109 They found that patients who had the least change in pulse pressure from before to after dialysis had clinical characteristics indicating volume overload. Among these patients, lowering of the pulse pressure from before to after dialysis was associated with lower hospitalization and mortality outcomes. Because pulse pressure is largely driven by SBP, it is likely that lowering of pulse pressure with dialysis reflects more volume loss and a lower ECF volume state, and may provide better cardiovascular outcomes, perhaps through less pressure/volume stress on the heart.
Potential Hazards of Probing Dry Weight
There are potential hazards related to probing dry weight, none of which have been adequately examined.77 These include the following: (1) increased risk of clotted angioaccess, (2) increased rate of attrition in residual renal function, and (3) complications related to intradialytic hypotension. Intradialytic hypotension, besides requiring more nursing interventions, can be complicated by cerebral hypoperfusion, seizures, myocardial dysfunction, and mesenteric ischemia. Furthermore, it has been associated with mortality.110 The relative risks and benefits of probing dry weight need to be examined in long-term randomized trials.
Providing Adequate Duration of Dialysis
The European Best Practice Guidelines recommend that dialysis should be delivered at least three times a week and the total duration should be at least 12 hours per week, unless substantial residual renal function is present.111 An increase in treatment time and or frequency should be considered in patients who experience hemodynamic instability or remain hypertensive despite maximal possible fluid removal.
In the United States, a recent study reported that the average duration of dialysis among 32,065 participants in the ESRD Clinical Performance Measures Project was 217 minutes.112 The interquartile range was 195–240 minutes. This means that one-quarter of the patients were receiving <3 hours and 15 minutes of dialysis and only one-quarter of the patients were receiving >4 hours of dialysis.
Although what constitutes an adequate dialysis is still debated, it is clear that patients who shorten treatment have hypertension that is more difficult to control.5 Patients who dialyze 8 hours three times a week have excellent BP control, minimal requirement for antihypertensive drugs, and excellent long-term survival.41,113 In a randomized crossover trial of 38 patients, the effects of 4-hour dialysis to 5-hour dialysis were evaluated.114 Hemodynamic stability and hypotensive episodes were fewer with longer dialysis, especially among older patients (aged >65 years). However, these data are difficult to generalize because treatment was evaluated only over 2 weeks and those requiring >4 L ultrafiltration were excluded. Longer or more frequent dialysis sessions, in general, are associated with less hemodynamic instability, better achievement of postdialysis dry weight, better control of BP, and the reduced need for antihypertensive drugs.
Frequent Dialysis and Its Effect on BP
Observational studies suggest that conversion of patients from three times a week conventional dialysis to nocturnal dialysis may improve BP and left ventricular mass.115 In a cumulative analysis of 72 patients from nine centers it was noted that predialysis SBP and DBP fell within 1 month of dialysis by 13/7 mmHg from 163/94 mmHg.116 This reduction was accompanied by a 1% decline in postdialysis weight. Although BP did not change after 1 month, the number of antihypertensive agents declined significantly. At baseline, 54% of patients were not taking antihypertensive drugs, whereas at 12 months after switching to daily dialysis, 75% were not taking antihypertensive agents.
Several observations have suggested improvements in BP and left ventricular mass among patients undergoing more frequent dialysis. For example, Chan et al. reported an improvement in both SBP and DBP, a reduction in antihypertensive drugs and doses, and a reduction in left ventricular mass in patients undergoing nocturnal dialysis.115 This group also reported an improvement in pharyngeal size among nocturnally dialyzed patients.117 This may improve sleep apnea and consequently ambulatory BP. Another mechanism of BP reduction with frequent dialysis is suggested to be an increase in arterial compliance and consequently improvement in baroreflex sensitivity.118 Other mechanisms may be better volume and toxin removal.119
A randomized controlled trial assigned 52 HD patients to either frequent dialysis, six nights per week, or conventional three times a week treatment. In the frequent dialysis group, the results showed an improvement in cardiac magnetic resonance–imaged left ventricular mass and a reduction in the need for antihypertensive medications.120 The Frequent Hemodialysis Network (FHN) study randomized HD patients to either conventional dialysis three times weekly or more frequent in-center dialysis; the primary endpoint was an improvement in joint composite end points of either (1) death or LVH or (2) death or physical health composite. The primary end point was met, but perhaps the most notable findings were an improvement in SBP, a reduction in antihypertensive drug use, and an improvement in left ventricular mass.121 These findings suggest better achievement of dry weight in these patients.122 Improvement in SBP was also noted in the companion FHN Nocturnal trial.123 Increasing the treatment duration may improve hemodynamic stability of dialysis and make the procedure more tolerable, but it is not a requirement for improvement in left ventricular mass. Shortening the procedure to tailor dialysis to a minimum Kt/V may provoke intradialytic symptoms, postdialysis fatigue, and nonadherence to therapy; thus, this is not recommended.124 Normotension can be achieved independently of the duration of dialysis if the control of volume is adequate.125 In fact, left ventricular mass index was also improved to a comparable degree in DRIP trial participants, in which the duration of dialysis was not altered but the dry weight was challenged.126
Pharmacologic Treatment
All classes of antihypertensive drugs, except diuretics, are useful for managing hypertension in HD patients.127 Diuretics are generally ineffective at very low GFR. There is no role of loop diuretics even when given in a high dose (e.g., as high as 250 mg intravenously of furosemide) among anuric HD patients.128 Tissue Doppler imaging revealed that central cardiac hemodynamics were unaltered when anuric HD patients were given even such high doses of loop diuretics. Given the ototoxicity associated with high doses of loop diuretics, their use, especially in high doses, is not recommended. Further research is needed to clarify the role of loop diuretics among patients with substantial residual renal function (e.g., patients new to long-term dialysis). Consideration of pharmacokinetics is important when prescribing these drugs.129 In general, if patients are volume overloaded, antihypertensive medications are less effective. Paradoxically, among HD patients, a greater use of antihypertensive medications is associated with a higher BP.130 However, causality must not be assumed. It is more likely that excessive antihypertensive medication may interfere with achievement of dry weight.
Drugs that block the renin-angiotensin system are often recommended as first-line therapy for HD patients because of their tolerability and extrapolated cardiovascular benefits in the general population with heart and kidney disease. Only one prospective trial compared an angiotensin-converting enzyme inhibitor (fosinopril) versus placebo in HD patients, all of which had LVH. Although hypertension was not an inclusion criterion, all patients underwent a single-blind run-in period with 5 mg of fosinopril, and those who experienced symptomatic hypotension or had a SBP <95 mmHg 4–6 hours after test dose were excluded. Subsequently, in the Fosinopril and Dialysis Trial, 400 HD patients received 20 mg of fosinopril versus placebo in an equal ratio. After 4 years of follow-up, there were no differences between the two treatment groups in the primary end point of cardiovascular events that included cardiovascular death.131 Another smaller trial compared candesartan versus placebo in HD patients, but noted a nearly 3-fold reduction in cardiovascular events with active treatment versus placebo.132 These conflicting results indicate the need for larger studies. There are no studies in PD patients, nor are there any studies in HD patients with diabetes.
β-Blockers may be an effective therapeutic strategy in HD patients with reduced ejection fraction (<35%). One study randomized 114 (not necessarily hypertensive) patients with dilated cardiomyopathy to 25 mg of carvedilol twice daily or placebo for 2 years. β-Blocker treatment reduced hospitalizations (RR, 0.44) and all-cause death (RR, 0.51).133
Regrettably, the reduction in all-cause mortality with antihypertensive drug therapy in HD patients has not been realized with adequately powered randomized controlled trials. This may be due to multiple reasons, including the low numbers of patients. Nonetheless, meta-analyses of these trials show improvement in the cardiovascular event rate.134,135 These benefits are especially seen among individuals who have hypertension.135
The recently reported Hypertension in HemoDialysis Patients Treated with Atenolol or Lisinopril (HDPAL) trial randomly assigned 200 patients to either open-label lisinopril (n=100) or atenolol (n=100) each administered three times per week after dialysis. The HDPAL trial aimed to determine whether angiotensin-converting enzyme inhibitor–based antihypertensive therapy causes a greater regression of LVH compared with β-blocker–based antihypertensive therapy among maintenance HD patients with echocardiographic LVH and hypertension.136 Monthly monitored home BP was controlled to <140/90 mmHg with medications, dry weight adjustment, and sodium restriction. The primary outcome was the change in the left ventricular mass index from baseline to 12 months. At baseline, 44-hour ambulatory BP was similar in the atenolol (151.5/87.1 mmHg) and lisinopril groups, and improved similarly over time in both groups. However, monthly measured home BP was consistently higher in the lisinopril group despite needing a greater number of antihypertensive agents and a greater reduction in dry weight. An independent data safety monitoring board recommended early termination of the trial because of cardiovascular safety. Serious cardiovascular events occurred in 16 participants in the atenolol group who had 20 events and in 28 participants in the lisinopril group who had 43 events (incidence rate ratio [IRR], 2.36; 95% confidence interval, 1.36 to 4.23; P=0.001). Combined serious adverse events of myocardial infarction, stroke, hospitalization for heart failure, or cardiovascular death occurred in 10 participants in the atenolol group who had 11 events and in 17 participants in the lisinopril group who had 23 events (IRR, 2.29; 95% CI, 1.07 to 5.21; P=0.02). Hospitalizations for heart failure were worse in the lisinopril group (IRR 3.13; 95% CI, 1.08 to 10.99; P=0.02). All-cause hospitalizations were higher in the lisinopril group (IRR, 1.61; 95% confidence interval, 1.18 to 2.19; P=0.002). The left ventricular mass index improved with time; no difference between drugs was noted. These data appear to suggest that among maintenance dialysis patients with hypertension and LVH, atenolol-based antihypertensive therapy may be superior to lisinopril-based therapy in preventing cardiovascular morbidity and all-cause hospitalizations. Larger multicenter trials should be performed to confirm these provocative data from a single center.
Nonvolume-Dependent Causes of Hypertension in Dialysis Patients
An increase in BP is a well recognized complication of erythropoietin therapy in HD patients.137 Hypertension is common with erythropoietin therapy, with approximately 30% of patients either developing hypertension or requiring an adjustment in antihypertensive medications.138,139 The etiology of hypertension with erythropoietin therapy is not clear. The incidence of erythropoietin-induced hypertension correlates with the erythropoietin dose but appears to be independent of its effect on red blood cell mass and viscosity.140,141 Available data suggest that the most likely mechanisms involve either an increase in production or an enhanced response to the effect of various vasoactive substances.142,143 Several studies have found that erythropoietin-induced hypertension in hemodialyzed patients is associated with either a significantly increased circulating endothelin-1 concentration or enhanced vasoconstrictive response to endothelin-1.144–146 Erythropoietin treatment has also been shown to be associated with an accentuated increase in the BP response to angiotensin II infusion compared with the BP response before erythropoietin therapy.147 This apparent increased sensitivity to angiotensin II correlated with the erythropoietin-induced increase in BP. Studies have also shown noradrenergic hypersensitivity in hemodialyzed patients with erythropoietin-induced hypertension.148
The effect of erythropoietin on BP can be missed because of variability in BP from predialysis to postdialysis and the lack of home or ambulatory BP measurements. Studies that failed to detect increases in BP with erythropoietin therapy may have managed hypertension more aggressively through the prescription of antihypertensive drugs or closer attention to dry weight. Erythropoietin therapy was an independent predictor of hypertension diagnosed by ambulatory BP monitoring.8 Some studies show an association of erythropoietin use with nondipping.149 Increase in BP with erythropoietin occurs more commonly in individuals with preexisting hypertension150,151 or a family history of hypertension.152
Prevention of erythropoietin-induced hypertension, and other complications, is a clinical challenge with several possible management strategies. Recommended strategies, with little good evidence to support these practices, have included the following: changing the route of administration (subcutaneous versus intravenous), reducing the goal hemoglobin level (especially in patients who are unresponsive to erythropoietin therapy), starting with a low erythropoietin dose and increasing the dose slowly, and avoiding the use of erythropoietin altogether.
Sleep apnea is very common in dialysis patients and is often associated with volume overload. Hypopneic spells during the night lead to nocturnal hypoxemia and provoke intense sympathetic arousal and an increase in nocturnal BP.153 Table 2 summarizes the key points in the management of hypertension.
Table 2.
Management of hypertension
| Summary Statements |
| 1. Volume overload is often an overlooked factor in managing hypertension. Erythropoietin-induced hypertension and untreated sleep apnea are other important causes. |
| 2. Volume overload is associated with increased mortality in HD patients. |
| 3. The iterative trial-and-error method of dry weight assessment remains the current clinical standard in assessing volume status. |
| 4. Dietary salt restriction and individualizing dialysate sodium prescription may improve the feasibility of achieving dry weight. |
| 5. Probing dry weight can improve BP among hypertensive HD patients. The long-term risks and benefits of probing dry weight need to be examined in future trials. |
| 6. Delivery of dialysis of at least 4 hours duration three times a week may facilitate volume and hypertension control. |
| 7. Antihypertensive drugs are frequently needed to control hypertension but are an adjunct to facilitate volume control. Diuretics have little to no role in patients with ESRD. β-blockers may be preferred to other agents. |
Hypoxemia that characterizes sleep apnea in patients with ESRD may cause hypertension.154 Patients with ESRD with sleep apnea have shown a 7-fold higher prevalence of resistant hypertension than individuals in the general hypertension population.155 In the recumbent position, the volume overload is redistributed from the legs to the chest and neck areas and may induce a peripharyngeal and upper airway resistance.156 Volume overload and the specific redistribution described in patients with ESRD may be not only a consequence but also an important cause of obstructive sleep apnea.157
Relationship of BP and Mortality
Among HD patients, the relationship of BP with cardiovascular outcomes is a subject of much controversy.158–164 As previously discussed, controversy relates to the specific relationship between the BP measurement times and technique (predialytic, postdialytic, or intradialytic BP measurements or interdialytic ambulatory BP) and morbidity and mortality. Some studies suggest an association of high BP with strokes,165,166 cerebral atrophy,167 cardiovascular events,168 complex cardiac arrhythmias,169 the development of congestive heart failure,161 and all-cause mortality.170 Other studies suggest that low BP measured either predialysis or postdialysis is associated with increased mortality.164,171–173 This association of low BP and mortality is further magnified when BP is considered as a time-dependent covariate.173 High BP measured either before dialysis or after dialysis are either not associated or minimally associated with increased mortality. The phenomenon of lower BP being associated with increased mortality has been labeled as reverse epidemiology of hypertension. This has raised concerns regarding lowering of BP among hypertensive HD patients.174,175 Other studies have demonstrated a direct relationship between BP and mortality.170,176 Consideration of the patient characteristics, dialysis practices, and BP measurement techniques is useful when evaluating these outcomes.
Consideration of the level of illness and the vintage of the patient are also instructive to ascertain the value of hypertension as a risk factor among HD patients. Examining the outcomes of 2770 patients on PD provides such insights.15 These patients were studied between 1997 and 2004 and had been on PD for at least 180 days in England and Wales. In a fully adjusted analysis, greater SBP, DBP, mean arterial, and pulse pressure were associated with decreased mortality among patients who had been on dialysis for <1 year. However, greater SBP and pulse pressure (but not mean arterial pressure and DBP) were associated with increased mortality among patients who had been on PD for ≥6 years. In a subgroup of patients who were placed on the transplant waitlist within 6 months of starting RRT and were presumably healthier, greater SBP, DBP, mean arterial or pulse pressure were not associated with decreased mortality in the first year. Similarly, among 16,959 dialysis patients in the United States, low SBP (<120 mmHg) was associated with increased mortality in years 1 and 2.177 However, high SBP (≥150 mmHg) was associated with increased mortality among patients who survived at least 3 years.177
Regional differences in mortality are unlikely to be caused by patient-specific characteristics alone. For example, a center in Tassin, France, reported a mortality rate of 45 per 1000 patient-years.176 By contrast, Degoulet et al., also from France, reported a mortality rate of 96 per 1000 patient-years.178 Differences in outcomes may be the result of center-specific practices. Patients reported by Charra et al. in Tassin, France, are dialyzed long hours with low sodium dialysate and are given low-sodium bread from the dialysis unit.176 The vast majority of these patients become normotensive without needing antihypertensive drugs.
BP measurement technique is also quite likely to contribute to variation in the relationship between BP and outcomes. For example, Amar et al. were the first to discover the strong relationship between ambulatory BP and mortality.53 These authors reported that nocturnal SBP was directly related to mortality. Agarwal et al. have used ambulatory BP to detect its relationship with mortality. In a cohort of approximately 150 patients, the authors found a direct and statistically significant relationship of both home and ambulatory BP with mortality.54 No such relationship was detectable using predialysis and postdialysis BP recordings. In a larger cohort followed for a longer time, the authors found a W-shaped relationship between both ambulatory BP and home BP and all-cause mortality64 At extremes of BP, mortality was noted to be high. Compared with ambulatory BP, the optimal BP ranges for home BP were approximately 10 mmHg higher.
Hypertension in Pediatric Dialysis Patients
Young adults with childhood-onset ESRD have significantly elevated cardiovascular risk compared with the general population. Data from the Late Effects of Renal Insufficiency in Children cohort study of 249 Dutch adult patients with onset of ESRD between 0 and 14 years of age demonstrated that the overall mortality risk of the patients with ESRD was 31 times that of age-matched Dutch citizens.179 Cardiovascular disease accounted for 41% of all mortalities, with cardiac death becoming the most common cause of mortality after 10 years of receiving RRT.179 Similarly, among 1380 patients with childhood-onset ESRD who died before aged 30 years, 23% of deaths were cardiovascular in origin; the cardiovascular death rate was 1000 times higher among children with ESRD than in the general population, and was 100 times higher among young adults with ESRD than in the general population.180 Although the number of patients with childhood-onset ESRD is small compared with the overall adult ESRD population, they constitute a unique group in whom control of cardiovascular risk factors is key to ensuring long-term survival.
As in adult ESRD patients, hypertension is the most common modifiable cardiovascular risk factor in children on dialysis. Mitsnefes et al.181 reported that approximately 60% of pediatric dialysis patients had uncontrolled hypertension, defined as measured BP≥95th percentile. Young age, recent dialysis initiation, and HD modality were identified as risk factors for having uncontrolled BP. Similar poor control of hypertension using predialysis and postdialysis BP measurements in American dialysis patients has been reported by Chavers et al.6 for HD patients and Halbach et al.182 for both HD and PD patients. In the latter study, demographic factors such as young age and black race and treatment factors such as prescription of antihypertensive medications were also identified as risk factors for poorly controlled hypertension. More recent data from the European Registry for Children on Renal Replacement Therapy on BP control among pediatric ESRD patients in Europe, including patients receiving HD, PD, and postrenal transplant, confirmed the high rate of hypertension in pediatric ESRD. Hypertension was present in 69.4% of HD patients (using BP recordings in the peridialytic period), 68.6% of PD patients, and 66.9% of transplant recipients.183 Among dialysis patients, younger age, recent dialysis initiation, and HD modality were the most important risk factors for hypertension.183 Of note, all of these studies are notable for their reliance upon registry data; thus, there is limited information available on the technique and/or frequency of BP measurement, or the goals of hypertension management.
LVH is the best-studied complication of hypertension in pediatric dialysis patients. A study by Mitsnefes et al. demonstrated increased left ventricular mass at the start of dialysis, and also showed that this increase persisted over a mean follow-up of 10 months.184 Risk factors for LVH included anemia, longer duration of renal disease before start of dialysis, and higher SBP. In a recent multicenter study from the International Pediatric Peritoneal Dialysis Network, LVH was present in 48% of hypertensive PD patients, with fluid overload, high body mass index, and hyperparathyroidism being the primary determinants of LVH.185 Studies comparing the frequency of LVH in children on PD or HD have had variable results. An American study showed that children on HD had LVH more often (85%) than children on PD (68%).186 Similarly, a Finnish study found that 45% of children on PD had LVH; LVH in this study was highly correlated with the severity of hypertension (pressure overload) and with hypervolemia as reflected by the plasma atrial natriuretic peptide level.187 On the contrary, the results from a German study showed similar left ventricular mass index with both modes of treatment.188 It is therefore likely that the prevalence of left ventricular in pediatric dialysis patients is more dependent on the overall control of BP and on volume status than on dialysis modality.
Diagnosis of hypertension and achievement of BP control pose some unique challenges in pediatric dialysis patients. With respect to diagnosis, age-appropriate normative values as published by the National High Blood Pressure Education Program (NHBPEP) must be used, and BP cuffs appropriate for the child’s upper arm must be selected.189 Normal BP in children is defined as a BP value below the 90th percentile for age, sex, and height, and hypertension is defined as BP values repeatedly at or above the 95th percentile.190 Thus, the clinician must consult tables of normative BP values in order to correctly categorize a child’s BP as normal or elevated. It should also be noted that there are no existing consensus recommendations that specifically address the optimal BP treatment goal for children on dialysis. Both the NHBPEP and the Kidney Disease Outcomes Quality Initiative have recommended that hypertensive children with CKD should be treated to a BP below the 90th percentile,189,190 but pediatric dialysis patients were not specifically mentioned in either of these reports. Given that normalization of BP has been shown to lead to regression of LVH in at least one study of pediatric HD patients,191 recommendations to achieve a BP value below the 90th percentile would seem appropriate until further evidence can be generated. Among pediatric patients on dialysis, further studies are needed to define the accuracy of peridialytic BP monitoring in determining interdialytic ambulatory BP.
Strategies to control hypertension in pediatric dialysis patients are similar to those used in adults, and include dietary sodium restriction and control of volume status. Vasodilating antihypertensive medications are generally avoided so as not to compromise fluid removal. A unique and vexing issue in the treatment of hypertensive pediatric dialysis patients is how to accurately assess and achieve dry weight. Infants and young children in particular are expected to demonstrate progressive weight gain and linear growth, a process that does not proceed in a predictable linear manner.192 Thus, it is unreasonable to expect that pediatric patients can achieve and maintain a stable dry weight. Two potential approaches to this problem have been studied: bioimpedance analysis and blood volume monitoring. In a recent single-center study, use of bioimpedance analysis to determine dry weight in a small group of pediatric HD patients was associated with fewer episodes of pulmonary edema and decreased prevalence of LVH compared with a group of historical controls.193 A blood volume monitoring protocol in a multicenter study demonstrated improved control of hypertension with decreased need for antihypertensive medications, although no significant change in postdialysis weight was seen.106 In pediatric PD patients, a plasma atrial natriuretic peptide level >3.0 nmol/L was felt to reflect hypervolemia on one study,187 but this finding has not yet been replicated. Clearly, further refinement of how to establish dry weight in pediatric dialysis patients is required.
Hypertension is an important clinical problem in HD patients. Often medication-directed approaches are utilized due to its perceived simplicity, as in the general population. However, nonpharmacologic and dialytic approaches are more likely to be successful and may target one of the major factors that contribute to the development of congestive heart failure: central pressure/volume overload. Use of antihypertensive drugs may improve cardiovascular outcomes; β-blockers may be a preferred drug class. More clinical trials are needed to evaluate optimal individualized strategies for defining targets for BP and controlling BP using pharmacologic and nonpharmacologic strategies in HD patients.
Disclosures
None.
Acknowledgments
Review of an earlier version of this work by the publication committee of the American Society of Hypertension and the Hypertension Advisory Group of the American Society of Nephrology is gratefully acknowledged. We thank Tia A. Paul, University of Maryland School of Medicine, Baltimore, for expert secretarial support.
This work was supported, in part, by a grant from the National Institutes of Health (2R01-DK6203010) to R.A.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115: 291–297, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Salem MM: Hypertension in the hemodialysis population: A survey of 649 patients. Am J Kidney Dis 26: 461–468, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Rocco MV, Yan G, Heyka RJ, Benz R, Cheung AK, HEMO Study Group : Risk factors for hypertension in chronic hemodialysis patients: Baseline data from the HEMO study. Am J Nephrol 21: 280–288, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Raine AE, Margreiter R, Brunner FP, Ehrich JH, Geerlings W, Landais P, Loirat C, Mallick NP, Selwood NH, Tufveson G: Report on management of renal failure in Europe, XXII, 1991. Nephrol Dial Transplant 7[Suppl 2]: 7–35, 1992 [PubMed] [Google Scholar]
- 5.Rahman M, Fu P, Sehgal AR, Smith MC: Interdialytic weight gain, compliance with dialysis regimen, and age are independent predictors of blood pressure in hemodialysis patients. Am J Kidney Dis 35: 257–265, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Chavers BM, Solid CA, Daniels FX, Chen SC, Collins AJ, Frankenfield DL, Herzog CA: Hypertension in pediatric long-term hemodialysis patients in the United States. Clin J Am Soc Nephrol 4: 1363–1369, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grekas D, Bamichas G, Bacharaki D, Goutzaridis N, Kasimatis E, Tourkantonis A: Hypertension in chronic hemodialysis patients: Current view on pathophysiology and treatment. Clin Nephrol 53: 164–168, 2000 [PubMed] [Google Scholar]
- 8.Agarwal R: Epidemiology of interdialytic ambulatory hypertension and the role of volume excess. Am J Nephrol 34: 381–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudville NC, Cordy P, Millman K, Fairbairn L, Sharma A, Lindsay R, Blake PG: Blood pressure, volume, and sodium control in an automated peritoneal dialysis population. Perit Dial Int 27: 537–543, 2007 [PubMed] [Google Scholar]
- 10.Velasquez MT, Lew SQ, von Albertini B, Mishkin GJ, Bosch JP: Control of hypertension is better during hemodialysis than during continuous ambulatory peritoneal dialysis in ESRD patients. Clin Nephrol 48: 341–345, 1997 [PubMed] [Google Scholar]
- 11.Rocco MV, Flanigan MJ, Beaver S, Frederick P, Gentile DE, McClellan WM, Polder J, Prowant BF, Taylor L, Helgerson SD, Report from the 1995 Core Indicators for Peritoneal Dialysis Study Group : Report from the 1995 Core Indicators for Peritoneal Dialysis Study Group. Am J Kidney Dis 30: 165–173, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Cocchi R, Degli Esposti E, Fabbri A, Lucatello A, Sturani A, Quarello F, Boero R, Bruno M, Dadone C, Favazza A, Scanziani R, Tommasi A, Giangrande A: Prevalence of hypertension in patients on peritoneal dialysis: Results of an Italian multicentre study. Nephrol Dial Transplant 14: 1536–1540, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Weiler EWJ, Saldanha LF, Khalil-Manesh F, Prins BA, Purdy RE, Gonick HC: Relationship of Na-K-ATPase inhibitors to blood-pressure regulation in continuous ambulatory peritoneal dialysis and hemodialysis. J Am Soc Nephrol 7: 454–463, 1996 [DOI] [PubMed] [Google Scholar]
- 14.Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S: Ambulatory blood pressure monitoring in haemodialysis and continuous ambulatory peritoneal dialysis (CAPD) patients. J Hum Hypertens 16: 585–589, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Udayaraj UP, Steenkamp R, Caskey FJ, Rogers C, Nitsch D, Ansell D, Tomson CR: Blood pressure and mortality risk on peritoneal dialysis. Am J Kidney Dis 53: 70–78, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Konings CJ, Kooman JP, Schonck M, Dammers R, Cheriex E, Palmans Meulemans AP, Hoeks AP, van Kreel B, Gladziwa U, van der Sande FM, Leunissen KM: Fluid status, blood pressure, and cardiovascular abnormalities in patients on peritoneal dialysis. Perit Dial Int 22: 477–487, 2002 [PubMed] [Google Scholar]
- 17.Tonbul Z, Altintepe L, Sözlü C, Yeksan M, Yildiz A, Türk S: The association of peritoneal transport properties with 24-hour blood pressure levels in CAPD patients. Perit Dial Int 23: 46–52, 2003 [PubMed] [Google Scholar]
- 18.Wang MC, Tseng CC, Tsai WC, Huang JJ: Blood pressure and left ventricular hypertrophy in patients on different peritoneal dialysis regimens. Perit Dial Int 21: 36–42, 2001 [PubMed] [Google Scholar]
- 19.Cannella G, Paoletti E, Ravera G, Cassottana P, Araghi P, Mulas D, Peloso G, Delfino R, Messa P: Inadequate diagnosis and therapy of arterial hypertension as causes of left ventricular hypertrophy in uremic dialysis patients. Kidney Int 58: 260–268, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Cheigh JS, Milite C, Sullivan JF, Rubin AL, Stenzel KH: Hypertension is not adequately controlled in hemodialysis patients. Am J Kidney Dis 19: 453–459, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Bishu K, Gricz KM, Chewaka S, Agarwal R: Appropriateness of antihypertensive drug therapy in hemodialysis patients. Clin J Am Soc Nephrol 1: 820–824, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Ertürk S, Ertuğ AE, Ateş K, Duman N, Aslan SM, Nergisoğlu G, Diker E, Erol C, Karatan O, Erbay B: Relationship of ambulatory blood pressure monitoring data to echocardiographic findings in haemodialysis patients. Nephrol Dial Transplant 11: 2050–2054, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Lazar AE, Smith MC, Rahman M: Blood pressure measurement in hemodialysis patients. Semin Dial 17: 250–254, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT, Jr, Smith MC: A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis 39: 1226–1230, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Inrig JK, Patel UD, Gillespie BS, Hasselblad V, Himmelfarb J, Reddan D, Lindsay RM, Winchester JF, Stivelman J, Toto R, Szczech LA: Relationship between interdialytic weight gain and blood pressure among prevalent hemodialysis patients. Am J Kidney Dis 50: 108–118, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal R: Volume-associated ambulatory blood pressure patterns in hemodialysis patients. Hypertension 54: 241–247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thompson AM, Pickering TG: The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int 70: 1000–1007, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Santos SF, Mendes RB, Santos CA, Dorigo D, Peixoto AJ: Profile of interdialytic blood pressure in hemodialysis patients. Am J Nephrol 23: 96–105, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Mendes RB, Santos SF, Dorigo D, Mansoor GA, Crowley ST, White WB, Peixoto AJ: The use of peridialysis blood pressure and intradialytic blood pressure changes in the prediction of interdialytic blood pressure in haemodialysis patients. Blood Press Monit 8: 243–248, 2003 [DOI] [PubMed] [Google Scholar]
- 30.Rohrscheib MR, Myers OB, Servilla KS, Adams CD, Miskulin D, Bedrick EJ, Hunt WC, Lindsey DE, Gabaldon D, Zager PG, DCI Medical Directors : Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol 3: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Argilés A, Mourad G, Mion C: Seasonal changes in blood pressure in patients with end-stage renal disease treated with hemodialysis. N Engl J Med 339: 1364–1370, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Conion PJ, Walshe JJ, Heinle SK, Minda S, Krucoff M, Schwab SJ: Predialysis systolic blood pressure correlates strongly with mean 24-hour systolic blood pressure and left ventricular mass in stable hemodialysis patients. J Am Soc Nephrol 7: 2658–2663, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R, Peixoto AJ, Santos SF, Zoccali C: Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 1: 389–398, 2006 [DOI] [PubMed] [Google Scholar]
- 34.K/DOQI Workgroup : K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45[Suppl 3]: S1–S153, 2005 [PubMed] [Google Scholar]
- 35.Davenport A, Cox C, Thuraisingham R: Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int 73: 759–764, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Ifudu O, Dawood M, Homel P, Friedman EA: Excess interdialytic weight gain provokes antihypertensive drug therapy in patients on maintenance hemodialysis. Dial Transplant 26: 541, 1997 [Google Scholar]
- 37.Iseki K, Nakai S, Shinzato T, Morita O, Shinoda T, Kikuchi K, Wada A, Kimata N, Akiba T: Prevalence and determinants of hypertension in chronic hemodialysis patients in Japan. Ther Apher Dial 11: 183–188, 2007 [DOI] [PubMed] [Google Scholar]
- 38.López-Gómez JM, Villaverde M, Jofre R, Rodriguez-Benítez P, Pérez-García R: Interdialytic weight gain as a marker of blood pressure, nutrition, and survival in hemodialysis patients. Kidney Int Suppl 93: S63–S68, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Rahman M, Dixit A, Donley V, Gupta S, Hanslik T, Lacson E, Ogundipe A, Weigel K, Smith MC: Factors associated with inadequate blood pressure control in hypertensive hemodialysis patients. Am J Kidney Dis 33: 498–506, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Luik AJ, Gladziwa U, Kooman JP, van Hooff JP, de Leeuw PW, van Bortel LMAB, Leunissen KML: Influence of interdialytic weight gain on blood pressure in hemodialysis patients. Blood Purif 12: 259–266, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Chazot C, Charra B, Laurent G, Didier C, Vo Van C, Terrat JC, Calemard E, Vanel T, Ruffet M: Interdialysis blood pressure control by long haemodialysis sessions. Nephrol Dial Transplant 10: 831–837, 1995 [PubMed] [Google Scholar]
- 42.Agarwal R, Metiku T, Tegegne GG, Light RP, Bunaye Z, Bekele DM, Kelley K: Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol 3: 1364–1372, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agarwal R: Role of home blood pressure monitoring in hemodialysis patients. Am J Kidney Dis 33: 682–687, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Agarwal R, Peixoto AJ, Santos SF, Zoccali C: Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit 14: 2–11, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D, American Heart Association. American Society of Hypertension. Preventive Cardiovascular Nurses Association : Call to action on use and reimbursement for home blood pressure monitoring: A joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension 52: 10–29, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O’Brien E, Ohkubo T, Padfield P, Palatini P, Pickering T, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G, ESH Working Group on Blood Pressure Monitoring : European Society of Hypertension guidelines for blood pressure monitoring at home: A summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens 26: 1505–1526, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Agarwal R: Managing hypertension using home blood pressure monitoring among haemodialysis patients—a call to action. Nephrol Dial Transplant 25: 1766–1771, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Agarwal R, Andersen MJ, Bishu K, Saha C: Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int 69: 900–906, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Agarwal R, Satyan S, Alborzi P, Light RP, Tegegne GG, Mazengia HS, Yigazu PM: Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol 30: 126–134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C: Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47: 62–68, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Moriya H, Ohtake T, Kobayashi S: Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant 22: 1198–1204, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Moriya H, Oka M, Maesato K, Mano T, Ikee R, Ohtake T, Kobayashi S: Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol 3: 416–422, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amar J, Vernier I, Rossignol E, Bongard V, Arnaud C, Conte JJ, Salvador M, Chamontin B: Nocturnal blood pressure and 24-hour pulse pressure are potent indicators of mortality in hemodialysis patients. Kidney Int 57: 2485–2491, 2000 [DOI] [PubMed] [Google Scholar]
- 54.Alborzi P, Patel N, Agarwal R: Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol 2: 1228–1234, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Agarwal R: Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 56: 512–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.da Silva GV, de Barros S, Abensur H, Ortega KC, Mion D, Jr, Cochrane Renal Group Prospective Trial Register: CRG060800146 : Home blood pressure monitoring in blood pressure control among haemodialysis patients: An open randomized clinical trial. Nephrol Dial Transplant 24: 3805–3811, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Kauric-Klein Z, Artinian N: Improving blood pressure control in hypertensive hemodialysis patients. CANNT J 17: 24–28, 31–36, quiz 29–30, 37–38, 2007 [PubMed] [Google Scholar]
- 58.Agarwal R, Light RP: Chronobiology of arterial hypertension in hemodialysis patients: Implications for home blood pressure monitoring. Am J Kidney Dis 54: 693–701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitra S, Chandna SM, Farrington K: What is hypertension in chronic haemodialysis? The role of interdialytic blood pressure monitoring. Nephrol Dial Transplant 14: 2915–2921, 1999 [DOI] [PubMed] [Google Scholar]
- 60.Agarwal R, Andersen MJ, Light RP: Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol 28: 210–217, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansoor GA, White WB: Ambulatory blood pressure monitoring is a useful clinical tool in nephrology. Am J Kidney Dis 30: 591–605, 1997 [DOI] [PubMed] [Google Scholar]
- 62.Peixoto AJ, Santos SF, Mendes RB, Crowley ST, Maldonado R, Orias M, Mansoor GA, White WB: Reproducibility of ambulatory blood pressure monitoring in hemodialysis patients. Am J Kidney Dis 36: 983–990, 2000 [DOI] [PubMed] [Google Scholar]
- 63.Townsend RR, Ford V: Ambulatory blood pressure monitoring: Coming of age in nephrology. J Am Soc Nephrol 7: 2279–2287, 1996 [DOI] [PubMed] [Google Scholar]
- 64.Agarwal R: Blood pressure and mortality among hemodialysis patients. Hypertension 55: 762–768, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peixoto AJ, Gray TA, Crowley ST: Validation of the SpaceLabs 90207 ambulatory blood pressure device for hemodialysis patients. Blood Press Monit 4: 217–221, 1999 [PubMed] [Google Scholar]
- 66.Agarwal R, Lewis RR: Prediction of hypertension in chronic hemodialysis patients. Kidney Int 60: 1982–1989, 2001 [DOI] [PubMed] [Google Scholar]
- 67.Kelley K, Light RP, Agarwal R: Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension 50: 143–150, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Agarwal R, Light RP: Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol 294: F303–F308, 2008 [DOI] [PubMed] [Google Scholar]
- 69.Ventura JE, Spósito M: Volume sensitivity of blood pressure in end-stage renal disease. Nephrol Dial Transplant 12: 485–491, 1997 [DOI] [PubMed] [Google Scholar]
- 70.Inrig JK, Oddone EZ, Hasselblad V, Gillespie B, Patel UD, Reddan D, Toto R, Himmelfarb J, Winchester JF, Stivelman J, Lindsay RM, Szczech LA: Association of intradialytic blood pressure changes with hospitalization and mortality rates in prevalent ESRD patients. Kidney Int 71: 454–461, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Inrig JK, Patel UD, Toto RD, Szczech LA: Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: A secondary analysis of the Dialysis Morbidity and Mortality Wave 2 Study. Am J Kidney Dis 54: 881–890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inrig JK: Intradialytic hypertension: A less-recognized cardiovascular complication of hemodialysis. Am J Kidney Dis 55: 580–589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chou KJ, Lee PT, Chen CL, Chiou CW, Hsu CY, Chung HM, Liu CP, Fang HC: Physiological changes during hemodialysis in patients with intradialysis hypertension. Kidney Int 69: 1833–1838, 2006 [DOI] [PubMed] [Google Scholar]
- 74.Agarwal R, Light RP: Intradialytic hypertension is a marker of volume excess. Nephrol Dial Transplant 25: 3355–3361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Buren PN, Kim C, Toto R, Inrig JK: Intradialytic hypertension and the association with interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 6: 1684–1691, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cirit M, Akçiçek F, Terzioğlu E, Soydaş C, Ok E, Ozbaşli CF, Başçi A, Mees EJ: ‘Paradoxical’ rise in blood pressure during ultrafiltration in dialysis patients. Nephrol Dial Transplant 10: 1417–1420, 1995 [PubMed] [Google Scholar]
- 77.Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Inrig JK, Van Buren P, Kim C, Vongpatanasin W, Povsic TJ, Toto R: Probing the mechanisms of intradialytic hypertension: A pilot study targeting endothelial cell dysfunction. Clin J Am Soc Nephrol 7: 1300–1309, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller BW, Cress CL, Johnson ME, Nichols DH, Schnitzler MA: Exercise during hemodialysis decreases the use of antihypertensive medications. Am J Kidney Dis 39: 828–833, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Kooman JP, van der Sande F, Leunissen K, Locatelli F: Sodium balance in hemodialysis therapy. Semin Dial 16: 351–355, 2003 [DOI] [PubMed] [Google Scholar]
- 81.Krautzig S, Janssen U, Koch KM, Granolleras C, Shaldon S: Dietary salt restriction and reduction of dialysate sodium to control hypertension in maintenance haemodialysis patients. Nephrol Dial Transplant 13: 552–553, 1998 [DOI] [PubMed] [Google Scholar]
- 82.Tomson CR: Advising dialysis patients to restrict fluid intake without restricting sodium intake is not based on evidence and is a waste of time. Nephrol Dial Transplant 16: 1538–1542, 2001 [DOI] [PubMed] [Google Scholar]
- 83.Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, Goldman L: Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 362: 590–599, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ozkahya M, Toz H, Qzerkan F, Duman S, Ok E, Basci A, Mees EJ: Impact of volume control on left ventricular hypertrophy in dialysis patients. J Nephrol 15: 655–660, 2002 [PubMed] [Google Scholar]
- 85.Ogden DA: A double blind crossover comparison of high and low sodium dialysis. Proc Clin Dial Transplant Forum 8: 157–165, 1978 [PubMed] [Google Scholar]
- 86.Cybulsky AV, Matni A, Hollomby DJ: Effects of high sodium dialysate during maintenance hemodialysis. Nephron 41: 57–61, 1985 [DOI] [PubMed] [Google Scholar]
- 87.Daugirdas JT, Al-Kudsi RR, Ing TS, Norusis MJ: A double-blind evaluation of sodium gradient hemodialysis. Am J Nephrol 5: 163–168, 1985 [DOI] [PubMed] [Google Scholar]
- 88.Barré PE, Brunelle G, Gascon-Barré M: A randomized double blind trial of dialysate sodiums of 145 mEq/L, 150 mEq/L, and 155 mEq/L. ASAIO Trans 34: 338–341, 1988 [PubMed] [Google Scholar]
- 89.Munoz Mendoza J, Sun S, Chertow GM, Moran J, Doss S, Schiller B: Dialysate sodium and sodium gradient in maintenance hemodialysis: A neglected sodium restriction approach? Nephrol Dial Transplant 26: 1281–1287, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Flanigan M: Dialysate composition and hemodialysis hypertension. Semin Dial 17: 279–283, 2004 [DOI] [PubMed] [Google Scholar]
- 91.Santos SF, Peixoto AJ: Revisiting the dialysate sodium prescription as a tool for better blood pressure and interdialytic weight gain management in hemodialysis patients. Clin J Am Soc Nephrol 3: 522–530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Paula FM, Peixoto AJ, Pinto LV, Dorigo D, Patricio PJ, Santos SF: Clinical consequences of an individualized dialysate sodium prescription in hemodialysis patients. Kidney Int 66: 1232–1238, 2004 [DOI] [PubMed] [Google Scholar]
- 93.Manlucu J, Gallo K, Heidenheim PA, Lindsay RM: Lowering postdialysis plasma sodium (conductivity) to increase sodium removal in volume-expanded hemodialysis patients: A pilot study using a biofeedback software system. Am J Kidney Dis 56: 69–76, 2010 [DOI] [PubMed] [Google Scholar]
- 94.Sang GL, Kovithavongs C, Ulan R, Kjellstrand CM: Sodium ramping in hemodialysis: A study of beneficial and adverse effects. Am J Kidney Dis 29: 669–677, 1997 [DOI] [PubMed] [Google Scholar]
- 95.Song JH, Lee SW, Suh CK, Kim MJ: Time-averaged concentration of dialysate sodium relates with sodium load and interdialytic weight gain during sodium-profiling hemodialysis. Am J Kidney Dis 40: 291–301, 2002 [DOI] [PubMed] [Google Scholar]
- 96.Davies S, Carlsson O, Simonsen O, Johansson AC, Venturoli D, Ledebo I, Wieslander A, Chan C, Rippe B: The effects of low-sodium peritoneal dialysis fluids on blood pressure, thirst and volume status. Nephrol Dial Transplant 24: 1609–1617, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stewart WK, Fleming LW: Blood pressure control during maintenance haemodialysis with isonatric (high sodium) dialysate. Postgrad Med J 50: 260–264, 1974 [Google Scholar]
- 98.Sinha AD, Agarwal R: Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial 22: 480–482, 2009 [DOI] [PubMed] [Google Scholar]
- 99.Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bridges N, Jarquin-Valdivia AA: Use of the Trendelenburg position as the resuscitation position: To T or not to T? Am J Crit Care 14: 364–368, 2005 [PubMed] [Google Scholar]
- 101.Monnet X, Teboul JL: Passive leg raising. Intensive Care Med 34: 659–663, 2008 [DOI] [PubMed] [Google Scholar]
- 102.Vertes V, Cangiano JL, Berman LB, Gould A: Hypertension in end-stage renal disease. N Engl J Med 280: 978–981, 1969 [DOI] [PubMed] [Google Scholar]
- 103.Kayikcioglu M, Tumuklu M, Ozkahya M, Ozdogan O, Asci G, Duman S, Toz H, Can LH, Basci A, Ok E: The benefit of salt restriction in the treatment of end-stage renal disease by haemodialysis. Nephrol Dial Transplant 24: 956–962, 2009 [DOI] [PubMed] [Google Scholar]
- 104.Goldstein SL, Smith CM, Currier H: Noninvasive interventions to decrease hospitalization and associated costs for pediatric patients receiving hemodialysis. J Am Soc Nephrol 14: 2127–2131, 2003 [DOI] [PubMed] [Google Scholar]
- 105.Rodriguez HJ, Domenici R, Diroll A, Goykhman I: Assessment of dry weight by monitoring changes in blood volume during hemodialysis using Crit-Line. Kidney Int 68: 854–861, 2005 [DOI] [PubMed] [Google Scholar]
- 106.Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, Flynn JT: A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol 2: 252–257, 2007 [DOI] [PubMed] [Google Scholar]
- 107.Reddan DN, Szczech LA, Hasselblad V, Lowrie EG, Lindsay RM, Himmelfarb J, Toto RD, Stivelman J, Winchester JF, Zillman LA, Califf RM, Owen WF, Jr: Intradialytic blood volume monitoring in ambulatory hemodialysis patients: A randomized trial. J Am Soc Nephrol 16: 2162–2169, 2005 [DOI] [PubMed] [Google Scholar]
- 108.Wizemann V, Wabel P, Chamney P, Zaluska W, Moissl U, Rode C, Malecka-Masalska T, Marcelli D: The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 24: 1574–1579, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Inrig JK, Patel UD, Toto RD, Reddan DN, Himmelfarb J, Lindsay RM, Stivelman J, Winchester JF, Szczech LA: Decreased pulse pressure during hemodialysis is associated with improved 6-month outcomes. Kidney Int 76: 1098–1107, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shoji T, Tsubakihara Y, Fujii M, Imai E: Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int 66: 1212–1220, 2004 [DOI] [PubMed] [Google Scholar]
- 111.Tattersall J, Martin-Malo A, Pedrini L, Basci A, Canaud B, Fouque D, Haage P, Konner K, Kooman J, Pizzarelli F, Tordoir J, Vennegoor M, Wanner C, ter Wee P, Vanholder R: EBPG guideline on dialysis strategies. Nephrol Dial Transplant 22[Suppl 2]: ii5–ii21, 2007 [DOI] [PubMed] [Google Scholar]
- 112.Foley RN, Gilbertson DT, Murray T, Collins AJ: Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 365: 1099–1107, 2011 [DOI] [PubMed] [Google Scholar]
- 113.Charra B, Calemard E, Ruffet M, Chazot C, Terrat J-C, Vanel T, Laurent G: Survival as an index of adequacy of dialysis. Kidney Int 41: 1286–1291, 1992 [DOI] [PubMed] [Google Scholar]
- 114.Brunet P, Saingra Y, Leonetti F, Vacher-Coponat H, Ramananarivo P, Berland Y: Tolerance of haemodialysis: A randomized cross-over trial of 5-h versus 4-h treatment time. Nephrol Dial Transplant 11[Suppl 8]: 46–51, 1996 [DOI] [PubMed] [Google Scholar]
- 115.Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A: Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 61: 2235–2239, 2002 [DOI] [PubMed] [Google Scholar]
- 116.Woods JD, Port FK, Orzol S, Buoncristiani U, Young E, Wolfe RA, Held PJ: Clinical and biochemical correlates of starting “daily” hemodialysis. Kidney Int 55: 2467–2476, 1999 [DOI] [PubMed] [Google Scholar]
- 117.Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane P, Hanly PJ: Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant 23: 673–679, 2008 [DOI] [PubMed] [Google Scholar]
- 118.Chan CT, Jain V, Picton P, Pierratos A, Floras JS: Nocturnal hemodialysis increases arterial baroreflex sensitivity and compliance and normalizes blood pressure of hypertensive patients with end-stage renal disease. Kidney Int 68: 338–344, 2005 [DOI] [PubMed] [Google Scholar]
- 119.Saad E, Charra B, Raj DS: Hypertension control with daily dialysis. Semin Dial 17: 295–298, 2004 [DOI] [PubMed] [Google Scholar]
- 120.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Agarwal R: Frequent versus standard hemodialysis. N Engl J Med 364: 975–976, author reply 976, 2011 [DOI] [PubMed] [Google Scholar]
- 123.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, Larive B, Chan CT, Chertow GM, Copland M, Hoy CD, Lindsay RM, Levin NW, Ornt DB, Pierratos A, Pipkin MF, Rajagopalan S, Stokes JB, Unruh ML, Star RA, Kliger AS, Kliger A, Eggers P, Briggs J, Hostetter T, Narva A, Star R, Augustine B, Mohr P, Beck G, Fu Z, Gassman J, Greene T, Daugirdas J, Hunsicker L, Larive B, Li M, Mackrell J, Wiggins K, Sherer S, Weiss B, Rajagopalan S, Sanz J, Dellagrottaglie S, Kariisa M, Tran T, West J, Unruh M, Keene R, Schlarb J, Chan C, McGrath-Chong M, Frome R, Higgins H, Ke S, Mandaci O, Owens C, Snell C, Eknoyan G, Appel L, Cheung A, Derse A, Kramer C, Geller N, Grimm R, Henderson L, Prichard S, Roecker E, Rocco M, Miller B, Riley J, Schuessler R, Lockridge R, Pipkin M, Peterson C, Hoy C, Fensterer A, Steigerwald D, Stokes J, Somers D, Hilkin A, Lilli K, Wallace W, Franzwa B, Waterman E, Chan C, McGrath-Chong M, Copland M, Levin A, Sioson L, Cabezon E, Kwan S, Roger D, Lindsay R, Suri R, Champagne J, Bullas R, Garg A, Mazzorato A, Spanner E, Rocco M, Burkart J, Moossavi S, Mauck V, Kaufman T, Pierratos A, Chan W, Regozo K, Kwok S, Frequent Hemodialysis Network (FHN) Trial Group : The effects of frequent nocturnal home hemodialysis: The Frequent Hemodialysis Network Nocturnal Trial. Kidney Int 80: 1080–1091, 2011. 21775973 [Google Scholar]
- 124.Twardowski ZJ: Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif 25: 90–98, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Katzarski KS, Charra B, Luik AJ, Nisell J, Divino Filho JC, Leypoldt JK, Leunissen KM, Laurent G, Bergström J: Fluid state and blood pressure control in patients treated with long and short haemodialysis. Nephrol Dial Transplant 14: 369–375, 1999 [DOI] [PubMed] [Google Scholar]
- 126.Agarwal R, Bouldin JM, Light RP, Garg A: Probing dry-weight improves left ventricular mass index. Am J Nephrol 33: 373–380, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hörl MP, Hörl WH: Drug therapy for hypertension in hemodialysis patients. Semin Dial 17: 288–294, 2004 [DOI] [PubMed] [Google Scholar]
- 128.Hayashi SY, Seeberger A, Lind B, Gunnes S, Alvestrand A, do Nascimento MM, Lindholm B, Brodin LA: Acute effects of low and high intravenous doses of furosemide on myocardial function in anuric haemodialysis patients: A tissue Doppler study. Nephrol Dial Transplant 23: 1355–1361, 2008 [DOI] [PubMed] [Google Scholar]
- 129.Hoyer J, Schulte KL, Lenz T: Clinical pharmacokinetics of angiotensin converting enzyme (ACE) inhibitors in renal failure. Clin Pharmacokinet 24: 230–254, 1993 [DOI] [PubMed] [Google Scholar]
- 130.Agarwal R, Weir MR: Dry-weight: A concept revisited in an effort to avoid medication-directed approaches for blood pressure control in hemodialysis patients. Clin J Am Soc Nephrol 5: 1255–1260, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zannad F, Kessler M, Lehert P, Grünfeld JP, Thuilliez C, Leizorovicz A, Lechat P: Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int 70: 1318–1324, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, Dohi Y: Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol Dial Transplant 21: 2507–2512, 2006 [DOI] [PubMed] [Google Scholar]
- 133.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: A prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 134.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V: Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet 373: 1009–1015, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Agarwal R, Sinha AD: Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertension 53: 860–866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Agarwal R, Sinha AD, Pappas MK, Abraham TN, Tegegne GG: Hypertension in hemodialysis treated with atenolol or lisinopril: A randomized controlled trial. Nephrol Dial Transplant 29: 672–681, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Buckner FS, Eschbach JW, Haley NR, Davidson RC, Adamson JW: Hypertension following erythropoietin therapy in anemic hemodialysis patients. Am J Hypertens 3: 947–955, 1990 [DOI] [PubMed] [Google Scholar]
- 138.Eschbach JW, Kelly MR, Haley NR, Abels RI, Adamson JW: Treatment of the anemia of progressive renal failure with recombinant human erythropoietin. N Engl J Med 321: 158–163, 1989 [DOI] [PubMed] [Google Scholar]
- 139.Eschbach JW, Abdulhadi MH, Browne JK, Delano BG, Downing MR, Egrie JC, Evans RW, Friedman EA, Graber SE, Haley NR, Korbet S, Krantz SB, Lundin AP, Nissenson AR, Ogden DA, Paganini EP, Rader B, Rutsky EA, Stivelman J, Stone WJ, Teschan P, Van Stone JC, Van Wyck DB, Zuckerman K, Adamson JW: Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med 111: 992–1000, 1989 [DOI] [PubMed] [Google Scholar]
- 140.Abraham PA, Macres MG: Blood pressure in hemodialysis patients during amelioration of anemia with erythropoietin. J Am Soc Nephrol 2: 927–936, 1991 [DOI] [PubMed] [Google Scholar]
- 141.Kaupke CJ, Kim S, Vaziri ND: Effect of erythrocyte mass on arterial blood pressure in dialysis patients receiving maintenance erythropoietin therapy. J Am Soc Nephrol 4: 1874–1878, 1994 [DOI] [PubMed] [Google Scholar]
- 142.Lebel M, Kingma I, Grose JH, Langlois S: Hemodynamic and hormonal changes during erythropoietin therapy in hemodialysis patients. J Am Soc Nephrol 9: 97–104, 1998 [DOI] [PubMed] [Google Scholar]
- 143.Wang XQ, Vaziri ND: Erythropoietin depresses nitric oxide synthase expression by human endothelial cells. Hypertension 33: 894–899, 1999 [DOI] [PubMed] [Google Scholar]
- 144.Bode-Böger SM, Böger RH, Kuhn M, Radermacher J, Frölich JC: Recombinant human erythropoietin enhances vasoconstrictor tone via endothelin-1 and constrictor prostanoids. Kidney Int 50: 1255–1261, 1996 [DOI] [PubMed] [Google Scholar]
- 145.Carlini R, Obialo CI, Rothstein M: Intravenous erythropoietin (rHuEPO) administration increases plasma endothelin and blood pressure in hemodialysis patients. Am J Hypertens 6: 103–107, 1993 [DOI] [PubMed] [Google Scholar]
- 146.Carlini RG, Dusso AS, Obialo CI, Alvarez UM, Rothstein M: Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int 43: 1010–1014, 1993 [DOI] [PubMed] [Google Scholar]
- 147.Eggena P, Willsey P, Jamgotchian N, Truckenbrod L, Hu MS, Barrett JD, Eggena MP, Clegg K, Nakhoul F, Lee DB: Influence of recombinant human erythropoietin on blood pressure and tissue renin-angiotensin systems. Am J Physiol 261: E642–E646, 1991 [DOI] [PubMed] [Google Scholar]
- 148.Hand MF, Haynes WG, Johnstone HA, Anderton JL, Webb DJ: Erythropoietin enhances vascular responsiveness to norepinephrine in renal failure. Kidney Int 48: 806–813, 1995 [DOI] [PubMed] [Google Scholar]
- 149.Amar J, Vernier I, Rossignol E, Lenfant V, Conte JJ, Chamontin B: Influence of nycthemeral blood pressure pattern in treated hypertensive patients on hemodialysis. Kidney Int 51: 1863–1866, 1997 [DOI] [PubMed] [Google Scholar]
- 150.Canadian Erythropoietin Study Group : Effect of recombinant human erythropoietin therapy on blood pressure in hemodialysis patients. Am J Nephrol 11: 23–26, 1991 [DOI] [PubMed] [Google Scholar]
- 151.Lebel M, Kingma I, Grose JH, Langlois S: Effect of recombinant human erythropoietin therapy on ambulatory blood pressure in normotensive and in untreated borderline hypertensive hemodialysis patients. Am J Hypertens 8: 545–551, 1995 [DOI] [PubMed] [Google Scholar]
- 152.Ishimitsu T, Tsukada H, Ogawa Y, Numabe A, Yagi S: Genetic predisposition to hypertension facilitates blood pressure elevation in hemodialysis patients treated with erythropoietin. Am J Med 94: 401–406, 1993 [DOI] [PubMed] [Google Scholar]
- 153.Zoccali C, Benedetto FA, Tripepi G, Cambareri F, Panuccio V, Candela V, Mallamaci F, Enia G, Labate C, Tassone F: Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int 53: 1078–1084, 1998 [DOI] [PubMed] [Google Scholar]
- 154.Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, Sharief I, Punjabi NM, Newman AB: Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol 17: 3503–3509, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Abdel-Kader K, Dohar S, Shah N, Jhamb M, Reis SE, Strollo P, Buysse D, Unruh ML: Resistant hypertension and obstructive sleep apnea in the setting of kidney disease. J Hypertens 30: 960–966, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Park J, Campese VM: Resistant hypertension and obstructive sleep apnea in end-stage renal disease. J Hypertens 30: 880–881, 2012 [DOI] [PubMed] [Google Scholar]
- 157.Tada T, Kusano KF, Ogawa A, Iwasaki J, Sakuragi S, Kusano I, Takatsu S, Miyazaki M, Ohe T: The predictors of central and obstructive sleep apnoea in haemodialysis patients. Nephrol Dial Transplant 22: 1190–1197, 2007 [DOI] [PubMed] [Google Scholar]
- 158.Dorhout Mees EJ: Hypertension in haemodialysis patients: Who cares? Nephrol Dial Transplant 14: 28–30, 1999 [DOI] [PubMed] [Google Scholar]
- 159.Duranti E, Imperiali P, Sasdelli M: Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl 55: S173–S174, 1996 [PubMed] [Google Scholar]
- 160.Fleischmann EH, Bower JD, Salahudeen AK: Are conventional cardiovascular risk factors predictive of two-year mortality in hemodialysis patients? Clin Nephrol 56: 221–230, 2001 [PubMed] [Google Scholar]
- 161.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49: 1379–1385, 1996 [DOI] [PubMed] [Google Scholar]
- 162.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD: Reverse epidemiology of hypertension and cardiovascular death in hemodialysis patients: The 58th annual fall conference and scientific sessions.. Hypertension 45: 811–817, 2005 [DOI] [PubMed] [Google Scholar]
- 163.Mazzuchi N, Carbonell E, Fernández-Cean J: Importance of blood pressure control in hemodialysis patient survival. Kidney Int 58: 2147–2154, 2000 [DOI] [PubMed] [Google Scholar]
- 164.Salem MM, Bower J: Hypertension in the hemodialysis population: Any relation to one-year survival? Am J Kidney Dis 28: 737–740, 1996 [DOI] [PubMed] [Google Scholar]
- 165.Kawamura M, Fijimoto S, Hisanaga S, Yamamoto Y, Eto T: Incidence, outcome, and risk factors of cerebrovascular events in patients undergoing maintenance hemodialysis. Am J Kidney Dis 31: 991–996, 1998 [DOI] [PubMed] [Google Scholar]
- 166.van der Sande FM, Hermans MM, Leunissen KM, Kooman JP: Noncardiac consequences of hypertension in hemodialysis patients. Semin Dial 17: 304–306, 2004 [DOI] [PubMed] [Google Scholar]
- 167.Savazzi GM, Cusmano F, Bergamaschi E, Vinci S, Allegri L, Garini G: Hypertension as an etiopathological factor in the development of cerebral atrophy in hemodialyzed patients. Nephron 81: 17–24, 1999 [DOI] [PubMed] [Google Scholar]
- 168.Takeda A, Toda T, Fujii T, Shinohara S, Sasaki S, Matsui N: Discordance of influence of hypertension on mortality and cardiovascular risk in hemodialysis patients. Am J Kidney Dis 45: 112–118, 2005 [DOI] [PubMed] [Google Scholar]
- 169.De Lima JJ, Lopes HF, Grupi CJ, Abensur H, Giorgi MC, Krieger EM, Pileggi F: Blood pressure influences the occurrence of complex ventricular arrhythmia in hemodialysis patients. Hypertension 26: 1200–1203, 1995 [DOI] [PubMed] [Google Scholar]
- 170.Tomita J, Kimura G, Inoue T, Inenaga T, Sanai T, Kawano Y, Nakamura S, Baba S, Matsuoka H, Omae T: Role of systolic blood pressure in determining prognosis of hemodialyzed patients. Am J Kidney Dis 25: 405–412, 1995 [DOI] [PubMed] [Google Scholar]
- 171.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33: 507–517, 1999 [DOI] [PubMed] [Google Scholar]
- 172.Salem MM: Hypertension in the haemodialysis population: Any relationship to 2-years survival? Nephrol Dial Transplant 14: 125–128, 1999 [DOI] [PubMed] [Google Scholar]
- 173.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54: 561–569, 1998 [DOI] [PubMed] [Google Scholar]
- 174.Lacson E, Jr, Lazarus JM: The association between blood pressure and mortality in ESRD-not different from the general population? Semin Dial 20: 510–517, 2007 [DOI] [PubMed] [Google Scholar]
- 175.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48: 606–615, 2006 [DOI] [PubMed] [Google Scholar]
- 176.Charra B: Control of blood pressure in long slow hemodialysis. Blood Purif 12: 252–258, 1994 [DOI] [PubMed] [Google Scholar]
- 177.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, Bedrick EJ, Meyer KB, Johnson HK, Zager PG, Medical Directors of Dialysis Clinic Inc : Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17: 513–520, 2006 [DOI] [PubMed] [Google Scholar]
- 178.Degoulet P, Legrain M, Réach I, Aimé F, Devriés C, Rojas P, Jacobs C: Mortality risk factors in patients treated by chronic hemodialysis. Report of the Diaphane collaborative study. Nephron 31: 103–110, 1982 [DOI] [PubMed] [Google Scholar]
- 179.Groothoff J, Gruppen M, de Groot E, Offringa M: Cardiovascular disease as a late complication of end-stage renal disease in children. Perit Dial Int 25[Suppl 3]: S123–S126, 2005 [PubMed] [Google Scholar]
- 180.Parekh RS, Carroll CE, Wolfe RA, Port FK: Cardiovascular mortality in children and young adults with end-stage kidney disease. J Pediatr 141: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 181.Mitsnefes M, Stablein D: Hypertension in pediatric patients on long-term dialysis: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Am J Kidney Dis 45: 309–315, 2005 [DOI] [PubMed] [Google Scholar]
- 182.Halbach SM, Martz K, Mattoo T, Flynn J: Predictors of blood pressure and its control in pediatric patients receiving dialysis. J Pediatr 160: 621–625, e1, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kramer AM, van Stralen KJ, Jager KJ, Schaefer F, Verrina E, Seeman T, Lewis MA, Boehm M, Simonetti GD, Novljan G, Groothoff JW: Demographics of blood pressure and hypertension in children on renal replacement therapy in Europe. Kidney Int 80: 1092–1098, 2011 [DOI] [PubMed] [Google Scholar]
- 184.Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF: Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol 16: 318–323, 2001 [DOI] [PubMed] [Google Scholar]
- 185.Bakkaloglu SA, Borzych D, Soo Ha I, Serdaroglu E, Büscher R, Salas P, Patel H, Drozdz D, Vondrak K, Watanabe A, Villagra J, Yavascan O, Valenzuela M, Gipson D, Ng KH, Warady BA, Schaefer F, International Pediatric Peritoneal Dialysis Network : Cardiac geometry in children receiving chronic peritoneal dialysis: Findings from the International Pediatric Peritoneal Dialysis Network (IPPN) registry. Clin J Am Soc Nephrol 6: 1926–1933, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, Strife CF: Severe left ventricular hypertrophy in pediatric dialysis: Prevalence and predictors. Pediatr Nephrol 14: 898–902, 2000 [DOI] [PubMed] [Google Scholar]
- 187.Hölttä T, Happonen JM, Rönnholm K, Fyhrquist F, Holmberg C: Hypertension, cardiac state, and the role of volume overload during peritoneal dialysis. Pediatr Nephrol 16: 324–331, 2001 [DOI] [PubMed] [Google Scholar]
- 188.Schärer K, Schmidt KG, Soergel M: Cardiac function and structure in patients with chronic renal failure. Pediatr Nephrol 13: 951–965, 1999 [DOI] [PubMed] [Google Scholar]
- 189.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents : The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114[Suppl 4th Report]: 555–576, 2004 [PubMed] [Google Scholar]
- 190.Kidney Disease Outcomes Quality Initiative (K/DOQI) : K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis 43[Suppl 1]: S1–S290, 2004 [PubMed] [Google Scholar]
- 191.Ulinski T, Genty J, Viau C, Tillous-Borde I, Deschênes G: Reduction of left ventricular hypertrophy in children undergoing hemodialysis. Pediatr Nephrol 21: 1171–1178, 2006 [DOI] [PubMed] [Google Scholar]
- 192.Thalange NK, Foster PJ, Gill MS, Price DA, Clayton PE: Model of normal prepubertal growth. Arch Dis Child 75: 427–431, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Paglialonga F, Ardissino G, Galli MA, Scarfia RV, Testa S, Edefonti A: Bioimpedance analysis and cardiovascular status in pediatric patients on chronic hemodialysis. Hemodial Int 16[Suppl 1]: S20–S25, 2012 [DOI] [PubMed] [Google Scholar]