Abstract
Sphingosine-1-phosphate (S1P), a bioactive sphingolipid metabolite, has been implicated in regulating vascular tone and participating in chronic and acute kidney injury. However, little is known about the role of S1P in the renal microcirculation. Here, we directly assessed the vasoresponsiveness of preglomerular and postglomerular microvascular segments to exogenous S1P using the in vitro blood-perfused juxtamedullary nephron preparation. Superfusion of S1P (0.001–10 μM) evoked concentration-dependent vasoconstriction in preglomerular microvessels, predominantly afferent arterioles. After administration of 10 μM S1P, the diameter of afferent arterioles decreased to 35%±5% of the control diameter, whereas the diameters of interlobular and arcuate arteries declined to 50%±12% and 68%±6% of the control diameter, respectively. Notably, efferent arterioles did not respond to S1P. The S1P receptor agonists FTY720 and FTY720-phosphate and the specific S1P1 receptor agonist SEW2871 each evoked modest afferent arteriolar vasoconstriction. Conversely, S1P2 receptor inhibition with JTE-013 significantly attenuated S1P-mediated afferent arteriolar vasoconstriction. Moreover, blockade of L-type voltage-dependent calcium channels with diltiazem or nifedipine attenuated S1P-mediated vasoconstriction. Intravenous injection of S1P in anesthetized rats reduced renal blood flow dose dependently. Western blotting and immunofluorescence revealed S1P1 and S1P2 receptor expression in isolated preglomerular microvessels and microvascular smooth muscle cells. These data demonstrate that S1P evokes segmentally distinct preglomerular vasoconstriction via activation of S1P1 and/or S1P2 receptors, partially via L-type voltage-dependent calcium channels. Accordingly, S1P may have a novel function in regulating afferent arteriolar resistance under physiologic conditions.
Sphingosine 1-phosphate (S1P) is recognized as an important signaling molecule in diverse biologic processes.1,2 Growing evidence indicates that S1P plays an important role in regulating vascular reactivity.3–5 S1P is a bioactive sphingolipid metabolite and is released from erythrocytes, platelets, and endothelial cells.6,7 The majority of S1P effects are mediated via five distinct receptors (S1P1–S1P5 receptors), which represent a family of small G protein–coupled receptors (GPCRs)5; however, S1P can also exist in the cytoplasm as a second messenger involved in Ca2+ mobilization or cell survival and proliferation.8,9 S1P1– S1P3 receptors are expressed by a wide variety of tissues, whereas S1P4 and S1P5 receptors are mainly expressed in cells of the immune and nervous systems.4,10 In the vasculature, endothelial cells mainly express S1P1 and S1P3 with variable expression of S1P2, whereas vascular smooth muscle cells express S1P2 and S1P3 with variable expression of S1P1.3–5 Studies in animals show that application of exogenous S1P causes either vasoconstriction or vasodilation of isolated arteries from several vascular beds.3,4
Although S1P receptor expression is detected in kidneys,11–13 little is known about the effects of S1P on renal microvascular function. Early studies showed that S1P evoked vasoconstriction in isolated intrarenal arteries.14 Intravenous infusion of S1P to rats in vivo decreased renal blood flow (RBF) without changing mean arterial pressure.15 Genetic studies found a significantly higher RBF in anesthetized S1P2 gene knockout mice compared with wild-type mice.16 These studies suggest that S1P may be important in regulating renal vascular function. More recent studies show that S1P signaling pathways are upregulated under several pathologic conditions including renal ischemia-reperfusion injury, diabetic nephropathy, and hypertensive renal injury.17 For example, administration of the S1P receptor agonist FTY720 significantly attenuated renal injury in 5/6 nephrectomy hypertensive rats by reducing lymphocyte infiltration in kidneys.18 Both mRNA and protein levels of S1P2 receptors are increased in diabetic rat kidney tissue and S1P-induced vasoconstriction is significantly enhanced in isolated-perfused diabetic rat kidneys.13 In addition, renal ischemia-reperfusion markedly increases mRNA expression of S1P1, S1P2, and S1P3 receptors in the renal cortex.19,20 Activation of S1P1 receptors19,21 or selective blockade of S1P2 receptors20 protects against renal ischemia-reperfusion injury. Overall, these studies indicate that alteration of S1P receptor signaling may contribute to renal injury under pathologic conditions. Therefore, it is important to determine the role of S1P in renal microvascular function. In this study, we focused on elucidating the influence of exogenous S1P on renal microvascular caliber and determining the distribution of S1P receptors in renal microvessels.
Results
Exogenous S1P Causes Segmentally Distinct Renal Microvascular Responses
Figure 1 illustrates the responses of different segments of the preglomerular and postglomerular microvasculature to increasing concentrations of S1P (0.001–10 μM) utilizing the in vitro blood-perfused juxtamedullary nephron (JMN) preparation. Baseline diameters averaged 14.7±0.4, 19.2±1.2, 27.4±2.0, and 56.8±7.6 μm for afferent and efferent arterioles and interlobular and arcuate arteries, respectively (Figure 1A). Superfusion of S1P evoked concentration-dependent vasoconstriction in all segments of preglomerular microvessels but predominantly in afferent arterioles. Afferent arterioles started to vasoconstrict upon administration of 0.001 µM S1P decreasing the diameter to 95%±1% of the control (Figure 1B), but significant vasoconstriction was observed from 0.01 µM, which reduced the diameter to 87%±3% of the control (P<0.05). When the concentration of S1P reached 10 µM, afferent arterioles showed potent vasoconstriction. The arteriolar diameter declined to 35%±5% of the control (P<0.05). S1P also vasoconstricted upstream arteries in a concentration-dependent fashion but was less efficacious. Diameters of interlobular and arcuate arteries declined to 50%±12% and 68%±6% of the control, respectively, in response to 10 µM S1P. Interestingly, efferent arterioles were completely unresponsive to S1P even at 10 µM, whereas superfusion of NE (0.1 µM) reduced the diameter of those efferent arterioles by 8.8%±2.4%.
Figure 1.
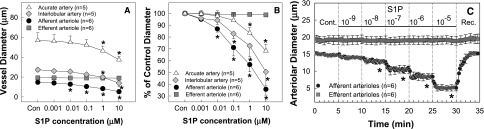
Effect of S1P on renal microvascular reactivity. (A) Superfusion of exogenous S1P is assessed on arcuate arteries (triangles), interlobular arteries (diamonds), afferent arterioles (circles), and efferent arterioles (squares) using the in vitro blood-perfused JMN preparation while perfusion pressure is held at 100 mmHg. Data represent the actual diameters of each renal microvascular segment. (B) The same data are normalized as a percentage of the control diameter for each group. (C) Time course of the S1P response on afferent and efferent arteriolar diameters. Each data point reflects the mean diameter response collected from afferent arterioles (circles) and efferent arterioles (squares) at 12-second intervals. Molar S1P concentrations are depicted in between the dashed vertical lines. Values are expressed as the mean±SEM. *P<0.05 versus the baseline diameter in the same group. n=5–6 in each group. Cont., control period; Rec., recovery period.
Figure 1C depicts the temporal behavior of S1P on afferent and efferent arteriolar reactivity. S1P-induced afferent arteriolar vasoconstriction occurred almost immediately after applying S1P on the kidney surface and achieved a steady state within 30–60 seconds, but arteriolar diameter quickly recovered to the control diameters after S1P was removed from superfusate. By contrast, efferent arterioles did not even produce a transient diameter response to S1P over the broad concentration range tested.
Effect of Intraluminal Administration of S1P on Renal Microvascular Reactivity
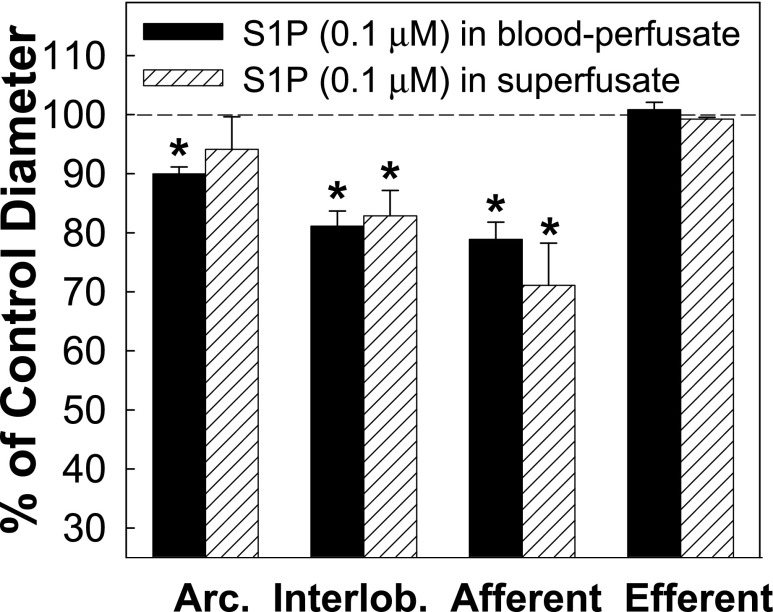
Because S1P is mainly produced from endothelial cells and circulating cells in vivo,6,7 perfusion of S1P in the blood might exert different effects on renal vascular reactivity. Figure 2 illustrates the renal microvascular responses to intraluminal administration of S1P (0.1 µM) compared with the effect of S1P delivered in superfusion solution (superfusion data were extracted from Figure 1B). Intraluminal administration of S1P reduced the diameters of arcuate and interlobular arteries and afferent arterioles to 90%±1%, 81%±3%, and 79%±3% of the control, respectively, similar to the vasoconstrictor responses induced by abluminal superfusion of S1P (P>0.05). The efferent arteriolar diameter still remained unchanged in response to intraluminal S1P delivery.
Figure 2.
Comparison of intraluminal and abluminal administration of exogenous S1P on renal microvascular reactivity. Renal microvascular reactivity is assessed using the in vitro blood-perfused JMN preparation while perfusion pressure is held at 100 mmHg. Baseline diameter is measured for arcuate arteries (Arc.; n=3), interlobular arteries (Interlob.; n=3), afferent arterioles (Afferent; n=9), and efferent arterioles (Efferent; n=3) before S1P exposure. S1P is administered luminally (black bars; n=4 rats) by the addition of S1P to the blood perfusate to achieve a plasma concentration of S1P approximating 0.1 µM and the diameters of the same microvascular locations are measured again. The second measurement depicts the effect of luminal S1P on the diameter of that renal microvascular segment. The results obtained with luminal S1P administration are compared with the responses of S1P (0.1 µM) delivered in the superfusion solution (white dash bars; data are extracted from Figure 1B), respectively. Data are normalized as the percentage of baseline diameter for each microvessel. Values are expressed as the mean±SEM. *P<0.05 versus the baseline diameter of the corresponding vessels.
Effects of S1P Receptor Agonists FTY720 and FTY720-Phosphate on Afferent Arteriolar Reactivity
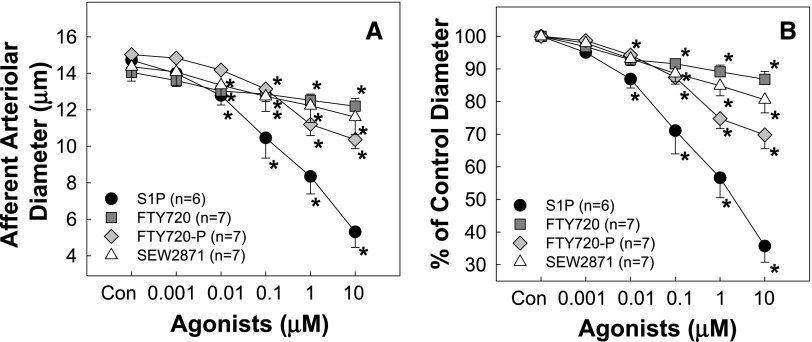
To determine which receptors mediate S1P-induced vasoconstriction of preglomerular microvessels, we assessed the afferent arteriolar response to FTY720, a S1P receptor agonist, for all S1P receptors except S1P2. FTY720 evoked concentration-dependent vasoconstriction of afferent arterioles (Figure 3). The baseline diameter averaged 14.1±0.3 µm (Figure 3A) and declined to 97%±1%, 93%±%2, 92%±2%, 89%±2%, and 87%±2% of the control in response to increasing concentrations of FTY720 (Figure 3B). Because FTY720 achieves its action by being converted to its biologically active form, FTY720-phosphate, in vivo,22 the modest vasoconstriction with FTY720 compared with S1P could reflect inefficient activation of the S1P receptor by FTY720. Accordingly, the response to FTY720-phosphate was assessed. As expected, FTY720-phosphate caused greater vasoconstriction than FTY720 over the same concentration range (Figure 3).
Figure 3.
Afferent arteriolar responses to S1P receptor activation. (A) Afferent arteriolar responses to increasing concentrations of FTY720 (a S1P receptor agonist except S1P2 receptors; squares), FTY720-phosphate (the active form of FTY720; diamonds), and SEW2871 (a selective S1P1 receptor agonist; triangles) compared with the vasoconstriction induced by S1P (circles; data from Figure 1). Data represent the actual diameters from each group. (B) The same data normalized as the percentage of control diameter for each group. Values are expressed as the mean±SEM. *P<0.05 versus the baseline diameter in the same group. n=6–7 in each group.
Effect of the S1P1 Receptor Agonist SEW2871 on Afferent Arteriolar Reactivity
We determined the effect of the selective S1P1 receptor agonist, SEW2871, on afferent arteriole diameter. SEW2871 evoked concentration-dependent vasoconstriction of afferent arterioles (Figure 3). Baseline diameter averaged 14.4±0.8 µm (Figure 3A) and declined to 98%±1%, 93%±2%, 88%±3%, 85%±3%, and 80%±4% of the control diameter (Figure 3B) in response to SEW2871. SEW2871 was a significantly less effective vasoconstrictor compared with S1P but was similar to FTY720 and FTY720-phosphate.
Effect of S1P2 Receptor Antagonism on S1P-Mediated Afferent Arteriolar Vasoconstriction
To assess the role of S1P2 receptors in S1P-induced vasoconstriction, we determined the effect of selective S1P2 receptor blockade with JTE-013 (1 or 10 µM) on the afferent arteriole response to S1P. During a 30-minute incubation period with JTE-013 (1 µM), the baseline arteriolar diameter averaged 15.9±0.4 µm and remained relatively constant (Figure 4A). The S1P concentration curve, however, was shifted significantly to the right by JTE-013 (Figure 4, B and C). S1P decreased arteriolar diameter to 101%±2%, 99%±3%, 90%±5%, 76%±7%, and 54%±8% of the control in the presence of JTE-013 compared with a 96%±2%, 89%±2%, 73%±2%, 53%±2%, and 35%±6% reduction in diameters in the absence of JTE-013, respectively. With 10 µM JTE-013, the arteriolar diameter increased significantly by 8%–9% during the first 10 minutes (P<0.05), but gradually declined to diameters similar to the control by 30 minutes (Figure 4A). The S1P concentration-response curve, however, was shifted further to the right with the diameter declining to 102%±1%, 99%±2%, 96%±2%, 85%±2%, and 65%±5% of the control (Figure 4, B and C). Accordingly, S1P-induced afferent arteriolar vasoconstriction was significantly attenuated by JTE-013 (P<0.05 versus S1P alone), implicating S1P2 receptors in the response. KCl-mediated vasoconstriction was unchanged by JTE-013 and averaged 46%±5% and 49%±4% of the control without and with JTE-013, respectively.
Figure 4.
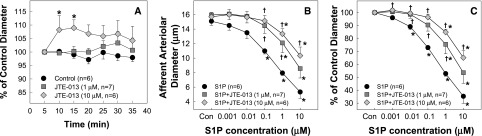
Effect of S1P2 receptor blockade on basal afferent arteriolar diameter and S1P-mediated afferent arteriolar vasoconstriction. (A) Effect of S1P2 receptor inhibition with a selective S1P2 receptor antagonist, JTE-013 (1 µM, squares; or 10 µM, diamonds, respectively), on basal afferent arteriole diameter during a 30-minute incubation period compared with control afferent arterioles superfused with a vehicle (circles). (B) Afferent arteriolar responses to increasing concentrations of S1P in the absence (circles) and presence of JTE-013 (1 μM, squares; or 10 μM, diamonds, respectively). Data represent the actual diameters from each group. (C) The same data are normalized as the percentage of control diameter for each group. S1P-induced afferent arteriolar vasoconstriction is significantly attenuated by JTE-013 concentration dependently. Values are expressed as the mean±SEM. *P<0.05 versus the control diameter in the same group; †P<0.05 versus vasoconstriction with superfusion of S1P alone. n=6–7 in each group. Con, control.
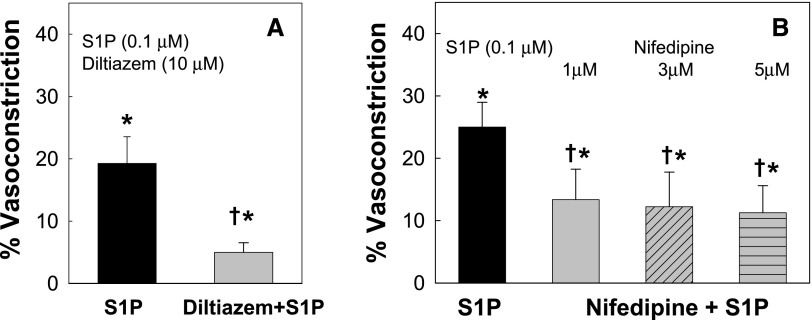
S1P-Mediated Afferent Arteriolar Vasoconstriction Was Blunted by Blocking Voltage-Dependent L-Type Calcium Channels
L-type calcium channels (L-VDCCs) are important regulators of preglomerular microvascular reactivity for many vasoconstrictors.23–26 To determine the role of L-VDCCs in S1P-mediated vasoconstriction, we examined the afferent arteriolar response to S1P in the presence of the L-VDCC blockers diltiazem or nifedipine. A single dose of S1P (0.1 µM), which caused a modest afferent arteriolar vasoconstriction (Figure 1), was chosen. As illustrated in Figure 5A, S1P alone reduced the arteriolar diameter by 19%±4%. Diltiazem (10 µM) significantly inhibited S1P-mediated vasoconstriction. The arteriolar diameter declined by only 5%±2% in response to S1P (P<0.05 versus S1P alone), suggesting that S1P-mediated vasoconstriction is achieved largely via activation of L-VDCCs. To confirm this finding, we used another L-VDCC inhibitor, nifedipine. S1P alone decreased the arteriolar diameter by 25%±4% (Figure 5B), and this response was reduced to just 13%±5% in the presence of nifedipine (1 µM, P<0.05 versus S1P alone). The magnitude of the inhibition by nifedipine was similar at nifedipine concentrations of 1, 3, or 5 µM.
Figure 5.
Effect of L-type calcium channel blockade on S1P-mediated afferent arteriolar vasoconstriction. (A) Effect of superfusion with diltiazem (10 μM) on S1P (0.1 μM) mediated afferent arteriolar vasoconstriction. (B) Effect of superfusion with nifedipine at 1, 3, or 5 μM, respectively, on S1P (0.1 μM) mediated afferent arteriolar vasoconstriction. Data are expressed as the percent reduction in diameter induced by S1P for each group. Values are expressed as the mean±SEM. *P<0.05 indicates a significant reduction in diameter by S1P versus control; †P<0.05 versus vasoconstriction with superfusion of S1P alone. n=6 in each group.
Effect of Intravenous Injections of Exogenous S1P on RBF in Anesthetized Rats In Vivo
To confirm the in vitro observation that S1P is a potent vasoconstrictor of preglomerular microvasculature, we performed an in vivo study by measuring RBF responses to bolus injections of S1P in anesthetized rats. Intravenous injection of exogenous S1P reduced RBF dose dependently (Figure 6A). RBF declined by 0.5±0.2 ml/min in response to S1P at 1.0 μg/kg and further decreased by 1.8±0.4, 4.2±0.4, 6.3±0.4, and 7.7±0.5 ml/min (P<0.05) after S1P was increased from 2.5 to 20 μg/kg, respectively, without significant changes in BP. In contrast, RBF remained stable during vehicle injections. Figure 6B shows original recordings of RBF and BP illustrating the rapid reduction and recovery in RBF in response to bolus injections of S1P.
Figure 6.
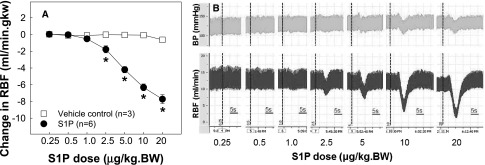
Effect of intravenous injections of exogenous S1P on RBF in anesthetized rats in vivo. RBF is assessed in anesthetized rats in vivo by bolus injections of S1P (circles; n=6) or methanol as vehicle control (squares; n=3) via the femoral vein at least at 5-minute intervals. Baseline RBF is similar between S1P and vehicle control groups (9.7±0.7 versus 9.5±1.3 ml/min g kidney weight). (A) Dose-dependent reduction in RBF in responses to bolus injections of S1P (circles, n=6) compared with vehicle control rats (squares, n=3). Data are expressed as the mean±SEM. *P<0.05 versus the baseline RBF before a bolus of S1P injection. (B) Original recording from a representative rat showing rapid and transient reductions in RBF (bottom traces) in response to increasing bolus injections of S1P without significant changes in BP (top traces). Vertical dash lines indicate S1P injection. Bar=5-second period. BW, body weight.
Protein Expression of S1P Receptors in Isolated Preglomerular Microvessels
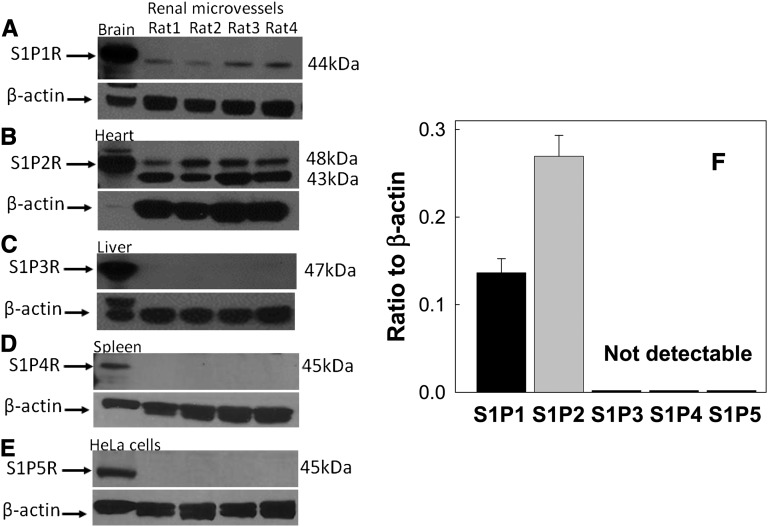
We examined S1P receptor expression in isolated preglomerular microvessels by Western blot analysis (Figure 7). S1P1 (44 kD) and S1P2 receptor protein (48 kD) were detected in isolated preglomerular microvessels (Figure 7, A and B). S1P3 (47 kD), S1P4 (45 kD), and S1P5 (45 kD) receptor protein were undetectable in preglomerular microvessels but detected in homogenates from liver, spleen, and HeLa cells, respectively, as positive controls (Figure 7, C–E). No bands were detected in renal microvessels or positive controls when normal rabbit serum IgG was incubated instead of primary S1P antibodies (data not shown). Figure 7F summarizes densitometry data revealing S1P1 and S1P2 receptor expression in preglomerular microvessels.
Figure 7.
Protein expression of S1P receptors in isolated rat preglomerular microvessels. Representative Western blot images for S1P1 (A), S1P2 (B), S1P3 (C), S1P4 (D), and S1P5 (E) receptor expression in isolated preglomerular microvessels. Homogenates from brain, heart, liver, spleen, and HeLa cells are used as positive control tissues for S1P1, S1P2, S1P3, S1P4, and S1P5 receptor expression, respectively. β-Actin serves as a loading control and is shown in the bottom of each panel. Densitometry analysis of S1P receptor protein expression in isolated preglomerular microvessels is summarized in F (n=4 rats for each receptor studied). Data are expressed as the mean±SEM.
S1P Receptor Expression in Isolated Preglomerular Microvascular Smooth Muscle Cells
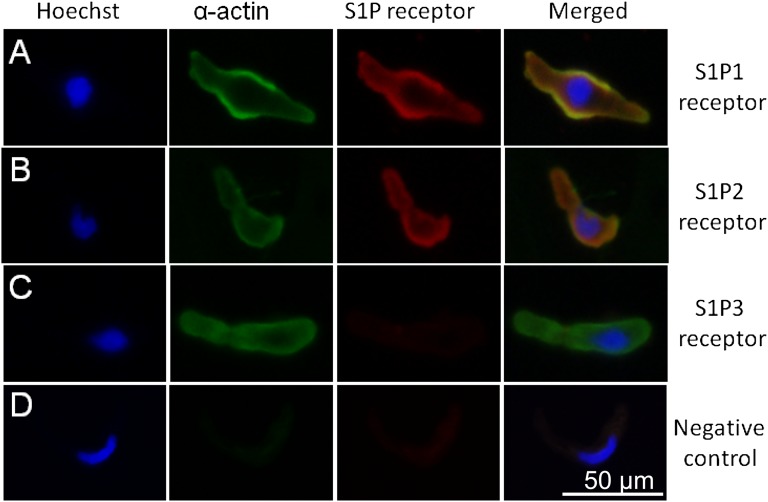
We also assessed S1P receptor expression using immunofluorescence staining in microvascular smooth muscle cells (MVSMCs) isolated from preglomerular microvessels. S1P1 and S1P2 receptors were detected in MVSMCs (Figure 8, A and B). No staining was observed in the cells stained with primary antibodies against S1P3 receptors (Figure 8C). Figure 8D shows negative control when the primary antibodies against S1P receptors and α-smooth muscle actin were omitted. Cells were confirmed to be MVSMCs by immunostaining with α-smooth muscle actin and the Hoechst nuclear stain.
Figure 8.
Immunofluorescence staining of S1P receptors in freshly isolated preglomerular MVSMCs. Representative images show immunofluorescence staining for the nucleus (blue), vascular smooth muscle α-actin (green), the corresponding S1P receptors (red), and the colocalization of S1P receptors and vascular smooth muscle cell (yellow) expression. (A) Positive fluorescence staining with S1P1 receptor antibodies. (B) Positive fluorescence staining with S1P2 receptor antibodies. (C) No significant S1P3 receptor staining is observed. (D) Negative control cells are stained by omitting primary S1P receptor antibody and α-smooth muscle actin antibody. Each cell is confirmed by positive nuclear staining with Hoechst and by positive vascular smooth muscle cell staining with α-smooth muscle actin antibody. Bar=50 µm.
Discussion
This study provides novel evidence that exogenous S1P evokes potent vasoconstriction of the preglomerular microvasculature but not efferent arterioles. Of the preglomerular vascular segments tested, afferent arterioles were the most reactive. The nonselective S1P receptor agonist FTY720 and its biologically active form, FTY720-phosphate, and the selective S1P1 receptor agonist SEW2871 also cause concentration-dependent vasoconstriction of afferent arterioles, but all were less potent than S1P. Furthermore, selective antagonism of S1P2 receptor activation with JTE-013 significantly attenuated S1P-induced vasoconstriction. S1P1 and S1P2, but not S1P3, S1P4, and S1P5, receptor proteins were detected in isolated preglomerular microvessels and MVSMC. In addition, S1P-induced vasoconstriction of afferent arterioles was largely inhibited by the L-VDCC blockers diltiazem and nifedipine. These studies demonstrate that S1P caused afferent arteriolar vasoconstriction by stimulating S1P1 and S1P2 receptors, mainly via calcium influx through L-VDCC. The potent vasoconstrictor effect of S1P on afferent arterioles but not on efferent arterioles suggests that S1P may function as a novel regulator of afferent arteriolar function under physiologic conditions.
Since the discovery of S1P as a ligand for a family of small GPCRs,5 S1P is recognized as an important signaling molecule involved in diverse biologic processes and function.1,2,5 Recent studies indicate that S1P plays an important role in regulating vascular tone in resistance vessels, including cerebral, coronary, and mesenteric arteries16,27–32; however, very little is known about the renal influence of S1P under either physiologic or pathophysiologic conditions. Bischoff et al. reported that S1P vasoconstricted isolated intrarenal arteries14 and reduced RBF without changing arterial pressure in anesthetized rats,15 indicating that S1P may be an important regulator of renal vascular function. The intrarenal arteries, however, contribute less to overall renal vascular resistance than microvessels and the S1P receptor signaling pathways involved in S1P-mediated renal vasoconstriction are also unknown. In this study, we utilized the blood-perfused JMN preparation to directly assess segmental renal microvascular reactivity to exogenous S1P. Our study demonstrates that S1P is a potent vasoconstrictor of afferent arterioles, whereas it is less effective on upstream renal arteries. Afferent arterioles responded to S1P at concentrations as low as 0.001 µM and reached a significant vasoconstriction at 0.01 µM, which is similar to the S1P concentration range found in interstitial fluid and plasma.3,6,33,34 Our results also show that afferent arterioles are highly sensitive to S1P compared with the resistance vessels from other vascular beds.27–29
Importantly, in contrast with the effect of S1P in preglomerular microvessels, we found efferent arterioles to be completely unresponsive to S1P. Despite the lack of an efferent arteriole response to S1P, NE vasoconstricted those same efferent arterioles as reported earlier,35 indicating that these arterioles were capable of responding to vasoconstrictor signals but just were not responsive to S1P. We also found that each segment of the renal microvasculature responded similarly to exogenous S1P regardless of whether S1P was administered intraluminally or abluminally. Efferent arterioles were still unresponsive to S1P, supporting the unique lack of reactivity of efferent arterioles to S1P. These segmentally distinct actions of S1P suggest intrarenal heterogeneity in S1P receptor expression and distribution.
The S1P receptors were initially named as endothelial differentiation gene 1 receptors.36 To date, five S1P receptors (S1P1–S1P5) have been identified and cloned. Generally, S1P1, S1P2, and S1P3 receptors are major receptors expressed in endothelial and vascular smooth muscle cells. Interestingly, S1P mainly acts at resistance vessels causing vasoconstriction or vasodilation,3,4,30,31,37,38 whereas it has little or no effect in conduit vessels such as the aorta, although S1P receptors were detected in aortic smooth muscle cells.37 S1P receptors (S1P1–SIP3) are detected in whole kidney tissue, renal medulla, and glomeruli11,12; however, the distribution of S1P receptor expression in the renal microvasculature has not been studied. We demonstrate here that both S1P1 and S1P2 proteins are abundantly present in isolated preglomerular microvessels, whereas S1P3, S1P4, and S1P5 receptor protein expression was not detected. Importantly, we did detect protein expression in positive controls for each S1P receptor, whereas no bands were evident in renal microvessels or positive control samples when normal rabbit serum IgG was substituted for primary S1P antibodies, confirming that the primary antibody detection is specific rather than nonspecific binding. In addition, the lack of detection of S1P4 receptors in renal microvessels agrees with the findings of Ota et al., who reported positive staining for S1P4 receptor protein in pulmonary arteries but not intrarenal arteries.39 Furthermore, to distinguish between endothelial or tubular cell expression,11,12 we isolated MVSMCs from preglomerular microvessels. Consistent with Western blot data, S1P1 and S1P2, but not S1P3, receptors were strongly detected in isolated MVSMCs. These observations are consistent with functional studies conducted using pharmacologic probes such as the selective S1P1 receptor agonist SEW2871 and the selective S1P2 receptor antagonist JTE-013.
FTY720 is a synthetic S1P analog whose bioactivity is enhanced by phosphorylation to FTY720-phosphate. FTY720-phosphate acts at S1P1, S1P3, S1P4, and S1P5, but not S1P2, receptors, whereas SEW2871 is a selective S1P1 receptor agonist. In this study, we found that the magnitude of afferent arteriolar vasoconstriction induced by SEW2871 is similar to responses produced by FTY720 and FTY720-phosphate but significantly less than responses evoked by S1P. We were unable to detect expression of S1P3, S1P4, and S1P5 receptors in preglomerular microvessels and/or MVSMCs, suggesting that S1P1 receptors are the major receptors contributing to FTY720 and FTY720-phosphate–mediated vasoconstriction of afferent arterioles.
Evidence indicates that stimulation of endothelial S1P1 receptors activates nitric oxide synthase, which in turn leads to nitric oxide release.40 Therefore, S1P1 receptor–mediated vasoconstriction may be modulated by S1P-induced endothelial nitric oxide production. This postulate needs further investigation. Overall, the SEW2871-evoked vasoconstriction of afferent arterioles combined with the evidence of S1P1 receptor protein expression indicates the existence of S1P1 receptors in preglomerular microvasculature.
Our results also demonstrate a role of S1P2 receptors contributing to S1P-mediated vasoconstriction of preglomerular microvessels. JTE-013 is a potent and selective S1P antagonist at S1P2 receptors.32,41 In this study, S1P-mediated afferent arteriolar vasoconstriction was significantly blunted by JTE-013 in a concentration-dependent manner, suggesting that S1P2 receptors participate in S1P-mediated vasoconstriction in rat kidneys. Because JTE-013 reportedly blocked endothelin or KCl-induced vasoconstriction of cerebral arteries, its specificity for S1P2 receptors was questioned.27 However, in our experimental setting, the vasoconstrictor response of afferent arterioles to KCl is similar with or without JTE-013, suggesting that the inhibitory effect of JTE-013 on S1P-induced vasoconstriction is unlikely to reflect a nonspecific inhibition of S1P2 receptors. Furthermore, JTE-013 completely inhibited the vasoconstriction induced by low S1P concentrations (0.001–0.1 µM) which are considered S1P2 receptor dependent.38 Interestingly, afferent arteriolar diameters tended to increase immediately after exposure to 10 µM JTE-013. Although the relaxation to JTE-013 was not statistically significant at the end of the incubation period, the tendency of JTE-013 to vasodilate implies that endogenous S1P may present. Our observation of an afferent arteriolar dilation during S1P2 receptor blockade is consistent with a study in anesthetized S1P2 gene knockout mice that exhibited significantly higher RBF compared with wild-type mice,16 suggesting that endogenous S1P increases renal vascular resistance through S1P2 receptor activation.
Intracellular S1P levels are tightly regulated by the balance between its synthesis by sphingosine kinase and degradation by S1P lyases and sphingosine phosphatase.1 S1P levels can be increased under inflammatory conditions, platelet activation, hypoxia, and atherosclerosis1–3,6,7; however, it is unclear what the normal stimuli are leading to increased production of S1P in blood vessels under physiologic conditions. Studies in hamster gracilis muscle resistance arteries and rabbit posterior cerebral arteries suggest that S1P can be stimulated in response to elevation of transmural pressure.31,38,42 Therefore, more studies are needed to explore the renal S1P signaling pathway to advance our current knowledge. Collectively, the functional data combined with the Western blot and immunofluorescence data suggest that S1P1 and S1P2 receptors are responsible for S1P-mediated vasoconstriction of the preglomerular microvasculature.
S1P receptor activation can trigger various intracellular signaling pathways, including intracellular Ca2+ release, RhoA/Rho kinase, generation of reactive oxygen species, and endothelial nitric oxide synthase activation.3,4,43 Because the S1P-induced afferent arteriolar vasoconstriction was significantly blocked by diltiazem or nifedipine, it is reasonable to conclude that L-VDCC activation plays an important role in S1P-mediated vasoconstriction of preglomerular microvessels. This is consistent with the observations by Bischoff et al. that Ca2+ influx plays a major role in regulating S1P-mediated renal vasoconstriction in anesthetized rats in vivo.44 L-VDCCs play a prominent role in controlling RBF and GFR by maintaining basal renal vascular tone and regulating preglomerular microvascular responses to various vasoconstrictor stimuli.23–26,45,46 Thus, the involvement of L-VDCCs in S1P-mediated vasoconstriction of preglomerular microvessels implies that S1P may play an important role in regulating renal vascular resistance and in controlling the renal microcirculation. Nevertheless, a small proportion of S1P-mediated afferent arteriolar vasoconstriction was retained in the presence of diltiazem or nifedipine, suggesting that other intracellular signaling pathways may also be involved.
Comparing S1P with many other common vasoconstrictors on afferent arteriolar reactivity shows that the S1P-mediated vasoconstriction of afferent arterioles is less potent than the vasoconstriction induced by endothelin 1,47 but similar to the vasoconstriction induced by other GPCR agonists, such as angiotensin II,35,48 arginine vasopressin,48 and NE,35 whereas S1P is more potent than ATP.49 It requires approximately 10 pM of endothelin-1, 6 nM of angiotensin II, 70 nM of S1P, 200 nM of arginine vasopressin, 300 nM of NE, and 100 µM of ATP to achieve a 25% reduction of afferent arteriolar diameters. The concentration of S1P (70 nM) required for this change is in the middle of those often vasoconstrictors, suggesting that biologic behavior of S1P is similar to a number of other important vasoconstrictors. Because the afferent arterioles are the primary renal resistance vessels and are vital in regulating RBF and glomerular filtration, the unique vasoconstrictor influence of S1P in afferent but not efferent arterioles suggests that endogenous S1P may be a novel regulator of afferent arteriolar function and thus glomerular hemodynamics. To further support our in vitro findings, we performed an in vivo study by measuring RBF responses to bolus injections of S1P in anesthetized rats. Consistent with the report by Bischoff et al.,15 intravenous injection of exogenous S1P decreased RBF without significant changes in BP. The rapid reduction and recovery in RBF in response to a bolus of S1P are consistent with the behavior of S1P on afferent arteriolar reactivity measured in the in vitro.
In summary, this study reveals that S1P is a potent vasoconstrictor of preglomerular microvessels. Furthermore, S1P1 and S1P2, but not S1P3, S1P4, and S1P5, receptor proteins are detected in the isolated preglomerular microvessels and MVSMCs. Our results demonstrate that S1P evokes afferent arteriolar vasoconstriction via activation of S1P1 and S1P2 receptors, mainly via L-VDCC activation. Because afferent arterioles are of major importance in regulating RBF and GFR, the exclusive vasoconstrictor influence of S1P in afferent but not efferent arterioles strongly implies a fundamental importance of S1P signaling in controlling glomerular capillary pressure and glomerular filtration. Altered S1P mediated reactivity in afferent arterioles might contribute to the development of renal injury under pathologic conditions, such as ischemia-reperfusion renal injury, hypertension, and diabetes. Therefore, a better understanding of the S1P signaling mechanisms in renal microvascular regulation is an essential step in predicting both the physiologic and potential therapeutic effects of S1P in patients with renal disease.
Concise Methods
Animals
Male Sprague-Dawley rats (n=178; Charles River Laboratories, Raleigh, NC) weighing 350–400 g were housed in a 12–12 light-controlled room and had free access to standard chow and drinking water. All experiments were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals using procedures approved by the Institutional Animal Care and Use Committee at Georgia Regents University (formerly Georgia Health Sciences University).
In Vitro Blood-Perfused JMN Preparation
JMN was prepared as previously described.50,51 Briefly, two rats (i.e., two rats per experiment) were anesthetized with pentobarbital (50 mg/kg intraperitoneally). The right kidney from the kidney donor was cannulated via the superior mesenteric artery and continuously perfused with Tyrode’s solution containing 5.2% BSA (Calbiochem, La Jolla, CA). Blood was collected from the kidney and blood donors into a heparinized syringe (500 IU) and processed by centrifuging, collecting the plasma fraction, and washing the erythrocyte fraction twice with saline. The plasma and washed erythrocytes were mixed to form a reconstituted blood perfusate with a hematocrit of approximately 33%.
The perfused kidney was harvested and prepared for videomicroscopy experiments.50,51 After completing the dissection procedures, the kidney was visualized under a light microscope (Nikon Eclipse E600FN; Nikon, Tokyo, Japan) fitted with a Nikon water-immersion objective (×40) and bathed with 37°C Tyrode’s buffer superfusate containing 1% BSA. The perfusate was switched to the reconstituted blood delivered from a sealed, pressurized reservoir continuously gassed with 95%O2/5%CO2 while perfusion pressure was held at 100 mmHg. Perfusion pressure was monitored using a polyethylene pressure cannula positioned within the perfusion cannula and connected to a pressure transducer (model TRN005; Kent Scientific Corporation, Torrington, CT). The focused image of the inner cortical surface of the kidney was displayed on a video monitor and recorded on DVD for later analysis. Afferent arteriolar inner diameters were measured at a single site, at 12-second intervals using a calibrated image-shearing monitor (model 908; Vista Electronics, Valencia, CA) and the mean diameter was calculated from the average of all diameter measurements obtained during the final 2 minutes of each treatment period.
Experimental Protocol
Each protocol began with a 5-minute control period to establish the steady state arteriolar diameter with perfusion pressure held at 100 mmHg after an initial equilibration period (at least 15 minutes). The following experiments were conducted.
Experiment 1: Effect of Exogenous S1P on Renal Microvascular Reactivity
After the control period, S1P concentrations over a log concentration scale from 0.001 to 10 μM were superfused directly onto the inner cortical surface for 5 minutes at each concentration while perfusion pressure was maintained at 100 mmHg. Only one vessel was studied from each kidney preparation for afferent or efferent arterioles and interlobular or arcuate arteries. Each group consisted of five to six kidneys. Because methanol was used for dissolving S1P, we conducted vehicle control experiments for methanol to establish its effect on afferent arteriolar reactivity (n=5). The effects of methanol on afferent arteriolar reactivity were assessed during exposure to increasing concentrations of methanol (0.000076%–0.76%) that mimic the methanol concentrations contained in the S1P solutions (0.001–10 μM). The results from the vehicle control experiments showed that the afferent arteriolar diameter remained unchanged by methanol up to 0.076% and declined slightly to 96%±3% of the control when methanol was increased to 0.76%, but did not reach statistical significance (P>0.05).
Experiment 2: Effect of Intraluminal Administration of S1P on Renal Microvascular Reactivity
Because S1P is mainly produced from endothelial cells and circulating cells in vivo,6,7 perfusion of S1P in the blood might exert different effects on renal vascular reactivity. To determine the effect of intraluminal administration of S1P on renal microvascular reactivity, we conducted a set of experiments (n=4 rats) by adding S1P to the blood perfusate. Briefly, during the control conditions, baseline diameters from different segments of renal microvasculature were recorded and measured. S1P was then directly added to the blood perfusate to achieve a plasma concentration of S1P approximating 0.1 µM. After a 15-minute stabilization period, the diameters of each microvascular segment were measured again at the same locations as in the control conditions. The results obtained with intraluminal S1P administration were compared with the responses of S1P (0.1 µM) delivered in superfusion solution (data were extracted from experiment 1).
Experiment 3: Effects of S1P Receptor Agonist FTY720 and Its Active Form FTY720-Phosphate on Afferent Arteriolar Reactivity
To define which subtypes of S1P receptors mediate the renal microvascular response to S1P, we assessed the effect of the S1P receptor agonist FTY702, which will activate S1P receptors except S1P2.52 Briefly, after the control period, FTY720 concentrations over a log concentration scale from 0.001 to 10 μM were superfused directly onto the inner cortical surface for 5 minutes at each concentration while perfusion pressure was maintained at 100 mmHg. Because FTY720 only caused modest afferent arteriolar vasoconstriction compared with S1P, we further assessed the effect of its biologically active form, FTY720-phosphate, on afferent arteriolar reactivity. Similar to the FTY720 experiments, FTY720-phosphate concentrations over a log concentration scale from 0.001 to 10 μM were superfused instead of FTY720. Each group consisted of six to seven kidneys. The degrees of afferent arteriolar vasoconstriction by FTY720 or FTY720-phosphate were compared with S1P-induced vasoconstriction performed in experiment 1.
Experiment 4: Effect of Selective S1P1 Receptor Agonist SEW2871 on Afferent Arteriolar Reactivity
The selective S1P1 receptor agonist SEW2871 was used to differentiate afferent arteriolar reactivity to S1P1 versus S1P3 receptor activation. Similar to the S1P experiments, SEW2871 concentrations over a log concentration scale from 0.001 to 10 μM were superfused (n=7). The degree of afferent arteriolar vasoconstriction by SEW2871 was compared with S1P-induced vasoconstriction performed in experiment 1. Because ethanol was used for dissolving SEW2871, the effects of ethanol on afferent arteriolar reactivity were assessed during exposure of increasing concentrations of ethanol (0.000009%–0.09%) that mimic the ethanol concentration used in the SEW2871 solutions (0.001–10 μM). Results from the vehicle control experiments showed that superfusion of ethanol up to 0.09% had no detectable effect on arteriolar diameter.
Experiment 5: Effect of Selective S1P2 Receptor Antagonist JTE-013 on S1P-Mediated Afferent Arteriolar Reactivity
To determine the contribution of S1P2 receptors in mediating S1P-induced vasoconstriction, afferent arteriolar reactivity to S1P was assessed in the presence of the selective S1P2 receptor inhibitor JTE-013. Briefly, after a 5-minute control period, the superfusate was continued (control) or switched to the superfusate containing JTE-013 at 1 or 10 μM for a 30-minute period and then S1P concentration-response (0.001–10 μM) was conducted during superfusion of JTE-013. At the end of the experiments, arterioles were exposed to KCl (55 mM) to determine the effect of depolarization on afferent arteriolar diameter in the presence of JTE-013. Each group consisted of six to seven kidneys.
Experiment 6: Effect of L-VDCC Blockers Diltiazem or Nifedipine on S1P-Induced Vasoconstriction
To elucidate the signaling pathway responsible for S1P-induced vasoconstriction of afferent arterioles, we examined a possible role of L-VDCCs in S1P-mediated vasoconstriction by application of L-VDCC blockers. A single dose of S1P (0.1 µM), which caused a modest afferent arteriolar vasoconstriction in experiment 1, was chosen. Briefly, after a 5-minute control period, the kidney was exposed to S1P (0.1 µM) for 5 minutes while perfusion pressure was held at 100 mmHg. After a 5-minute recovery period, the superfusate was switched to an identical solution containing diltiazem (10 μM) or nifedipine (1–5 μM) for 5 minutes before repeating S1P.
Effect of Intravenous Injections of S1P on RBF in Anesthetized Rats In Vivo
To support our in vitro findings, we conducted an in vivo study in pentobarbital-anesthetized rats by monitoring RBF and BP responses after bolus injections of S1P. The details of the surgical process were previously described.49 Briefly, rats were anesthetized with pentobarbital (50 mg/kg intraperitoneally) and the left carotid artery and femoral vein were cannulated to monitor arterial pressure and to deliver drugs, respectively. RBF from the left kidney was measured using an ultrasonic flow probe (Transonic Systems, Inc., Ithaca, NY). After a 60-minute stabilization period, rats were given intravenous bolus injections of S1P from 0.25 to 20 µg/kg body weight (n=6) or vehicle as the control (n=3) via the femoral vein at least at 5-minute intervals and RBF and BP were continuously monitored and recorded using CHART 5 (ADInstruments, Colorado Springs, CO).
Expression of S1P Receptors in Renal Microvessels by Western Blot
Preglomerular microvessels were collected as previously described.53 Briefly, rats were anesthetized with pentobarbital for retrograde perfusion through the abdominal aorta. The kidneys were flushed with 5.2% BSA solution followed by 5% Evan’s blue. The kidneys were removed and decapsulated in ice-cold physiologic salt solution (PSS). After removing the medulla and intrarenal and arcuate arteries, cortical tissue was gently pressed on a nylon membrane sieve (100-μm pore size; BioDesign, Inc., Carmel, NY) and rinsed with ice-cold PSS. The kidney tissue was transferred into a 20 ml-PSS–containing dithiothreitol, collagenase type II, trypsin inhibitor, and albumin (4 mg for each), and incubated for 20 minutes at 36.5°C. After digestion, the media was poured onto a 70-μm nylon sieve and rinsed with ice-cold PSS. Segments of interlobular arteries with attached afferent arterioles were identified and collected by microdissection under a stereoscope. The collected microvessels were quickly frozen with liquid nitrogen and stored at −80°C until analysis.
Protein expression of S1P receptors in preglomerular microvessels was determined by Western blot. Briefly, isolated renal microvessels were homogenized in radioimmunoprecipitation assay lysis buffer (EMD Millipore, Billerica, Temecula, CA) with protease inhibitor cocktail (One cOmplete tablet in 10 ml lysis buffer; Roche Diagnostics, Penzberg, Germany) using the Bullet blender (Next Advance, Inc., Averill Park, NY). Protein concentration was determined by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Protein aliquots (20 μg) were mixed with loading buffer (Invitrogen, Carlsbad, CA) and separated on a 4%–12% Bis-Tris electrophoresis gel (Invitrogen). Proteins were electrophoretically transferred from the gels onto nitrocellulose membranes (Bio-Rad Laboratories). After blocking nonspecific binding sites with 5% fat-free milk in Tris-buffered saline/Tween 20 buffer, the membranes were incubated overnight (4°C) with subtype-specific primary antibodies against S1P1 (1:500), S1P2 (1:500), S1P3 (1:500), S1P4 (1:200), or S1P5 (1:200) receptors, respectively. The washed membranes were incubated again with donkey anti-rabbit IgG horseradish peroxidase conjugate (GE HealthCare, Buckinghamshire, UK) for 1 hour at room temperature. Negative controls were prepared by incubation with normal rabbit serum IgG instead of primary S1P antibodies. Densitometry was performed using enhanced chemiluminescence detection (Konica Corporation, Tokyo, Japan) and was determined by β-actin expression using UN-SCAN-IT software (Silk Scientific, Inc., Orem, UT).
Purified polyclonal antibody for S1P1 (ab77076) and mAb for S1P3 (ab108370) receptors were purchased from Abcam (Cambridge, UK). Purified antibodies for S1P2 (sc-25491), S1P4 (sc-366771), and S1P5 (sc-25493) were from Santa Cruz Biotechnology (Santa Cruz, CA). The antibody specificity for S1P2 receptors was characterized by several studies including two studies in kidneys.11,12 The antibody specificity for S1P5 receptor was assessed in a previous study.54 Although the antibodies for S1P1 and S1P3 receptors are widely applied in mouse tissues rather than rat tissues, the epitope sequences for these antibodies are specific to the S1P1 and S1P3 receptors and do not cross-react with other family members. The polyclonal rabbit antibody for the S1P4 receptor is raised against the C terminus of the human S1P4 receptor protein. The S1P4 antibody does not cross-react with other family members as determined by BLAST searches.
Expression of S1P Receptors in Preglomerular MVSMCs by Immunofluorescence Staining
To eliminate the contamination of MVSMCs with tubular and endothelial cells, S1P receptor expression was examined in single MVSMCs. Briefly, preglomerular microvessels were isolated and digested as described above. The isolated renal microvessels were further digested in a 5 ml-PSS solution containing papain (7 mg), dithiothreitol (2.7 mg), and albumin (20 mg) at 36.5°C for 10–12 minutes. Cells were centrifuged and then fixed in 4% paraformaldehyde for 10 minutes and air-dried on glass slides at 37°C. The slides were rinsed in PSS and blocked in 10% normal goat serum for 1 hour. The cells were incubated with primary antibody for S1P1 (ab11424, 1:100), S1P2 (1:100), or S1P3 (1:100) receptors combined with primary antibody for α-smooth muscle actin (1:300; Biocare Medical, Concord, CA) overnight (4°C). The cells were rinsed in PSS and incubated with Alexa Fluor 594 goat anti-rabbit IgG (1:200; Invitrogen) and Alexa Fluor 488 donkey anti-mouse IgG (1:600; Invitrogen) for 1 hour and then with Hoechst nuclear stain, (1 mg/L; Invitrogen) for 5 minutes at room temperature. After a final rinse with PSS, samples were covered with Gel-Mount medium and coverslips. Negative controls were prepared by omitting the primary antibodies against S1P receptors and α-smooth muscle actin with the rest of process identical. Images of immunofluorescence stained cells were captured using an Olympus BX40F-3 fluorescence microscope equipped with an Olympus DP70 digital camera using DP Controller software (Olympus Optical Co. Ltd., Tokyo, Japan).
Drug Preparation
S1P (ENZO Life Sciences, Inc., Farmingdale, NY) was dissolved in methanol to make a stock solution of S1P at 1.3 mM according to the manufacturer’s instructions, and then aliquoted in Eppendorf tubes for storage at −20°C. S1P was diluted in Tyrode’s buffer containing 1% BSA on the day of experiments. JTE-013 (Tocris Bioscience, Ellisville, MO), FTY720 (Cayman Chemical, Ann Arbor, MI), and SEW2871 (Cayman Chemical) were dissolved in ethanol to yield final stock concentrations of 50, 60, and 10 mM, respectively, and were further diluted in Tyrode’s buffer containing 1% BSA before each experiment. FTY720-phosphate (Cayman Chemical) was dissolved in methanol to yield a final concentration of 2.6 mM and further diluted in Tyrode’s buffer containing 1% BSA before experiments.
Statistical Analyses
All values are expressed as means±SEM. Vascular responses were normalized as a percentage of the control diameter. Within-group analysis was performed using one-way ANOVA for repeated measures followed by post hoc analysis with Dunnett’s multiple range test. Significant differences between groups were determined using one-way ANOVA and Dunnett’s post hoc tests. A P value<0.05 was considered significant.
Disclosures
None.
Acknowledgments
This study is supported by the American Heart Association (National Scientist Development Grant 10SDG3770010 to Z.G.) and the National Institutes of Health (Grants DK44628, HL074167, and HL098135 to E.W.I., and HL095499 to J.S.P.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Maceyka M, Milstien S, Spiegel S: Sphingosine-1-phosphate: The Swiss army knife of sphingolipid signaling. J Lipid Res 50[Suppl]: S272–S276, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alvarez SE, Milstien S, Spiegel S: Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18: 300–307, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Hla T, Venkataraman K, Michaud J: The vascular S1P gradient-cellular sources and biological significance. Biochim Biophys Acta 1781: 477–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Igarashi J, Michel T: Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res 82: 212–220, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim RH, Takabe K, Milstien S, Spiegel S: Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta 1791: 692–696, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yatomi Y: Plasma sphingosine 1-phosphate metabolism and analysis. Biochim Biophys Acta 1780: 606–611, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Venkataraman K, Lee YM, Michaud J, Thangada S, Ai Y, Bonkovsky HL, Parikh NS, Habrukowich C, Hla T: Vascular endothelium as a contributor of plasma sphingosine 1-phosphate. Circ Res 102: 669–676, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goetzl EJ, Wang W, McGiffert C, Liao JJ, Huang MC: Sphingosine 1-phosphate as an intracellular messenger and extracellular mediator in immunity. Acta Paediatr Suppl 96: 49–52, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Meyer zu Heringdorf D, Liliom K, Schaefer M, Danneberg K, Jaggar JH, Tigyi G, Jakobs KH: Photolysis of intracellular caged sphingosine-1-phosphate causes Ca2+ mobilization independently of G-protein-coupled receptors. FEBS Lett 554: 443–449, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Sanchez T, Hla T: Structural and functional characteristics of S1P receptors. J Cell Biochem 92: 913–922, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Imasawa T, Kitamura H, Ohkawa R, Satoh Y, Miyashita A, Yatomi Y: Unbalanced expression of sphingosine 1-phosphate receptors in diabetic nephropathy. Exp Toxicol Pathol 62: 53–60, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Zhu Q, Xia M, Wang Z, Li PL, Li N: A novel lipid natriuretic factor in the renal medulla: Sphingosine-1-phosphate. Am J Physiol Renal Physiol 301: F35–F41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bautista-Pérez R, Arellano A, Franco M, Osorio H, Coronel I: Sphingosine-1-phosphate induced vasoconstriction is increased in the isolated perfused kidneys of diabetic rats. Diabetes Res Clin Pract 94: e8–e11, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Bischoff A, Czyborra P, Fetscher C, Meyer Zu Heringdorf D, Jakobs KH, Michel MC: Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br J Pharmacol 130: 1871–1877, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff A, Czyborra P, Meyer Zu Heringdorf D, Jakobs KH, Michel MC: Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br J Pharmacol 130: 1878–1883, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenz JN, Arend LJ, Robitz R, Paul RJ, MacLennan AJ: Vascular dysfunction in S1P2 sphingosine 1-phosphate receptor knockout mice. Am J Physiol Regul Integr Comp Physiol 292: R440–R446, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD: Sphingosine-1-phosphate receptors: Biology and therapeutic potential in kidney disease. Kidney Int 73: 1220–1230, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krämer S, Binder E, Loof T, Wang-Rosenke Y, Martini S, Khadzhynov D, Budde K, Neumayer HH, Peters H: The lymphocyte migration inhibitor FTY720 attenuates experimental hypertensive nephropathy. Am J Physiol Renal Physiol 297: F218–F227, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Awad AS, Ye H, Huang L, Li L, Foss FW, Jr, Macdonald TL, Lynch KR, Okusa MD: Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol 290: F1516–F1524, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Kim M, Brown KM, D’Agati VD, Lee HT: Inhibition of sphingosine 1-phosphate receptor 2 protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 23: 266–280, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajwa A, Jo SK, Ye H, Huang L, Dondeti KR, Rosin DL, Haase VH, Macdonald TL, Lynch KR, Okusa MD: Activation of sphingosine-1-phosphate 1 receptor in the proximal tubule protects against ischemia-reperfusion injury. J Am Soc Nephrol 21: 955–965, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Billich A, Bornancin F, Dévay P, Mechtcheriakova D, Urtz N, Baumruker T: Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem 278: 47408–47415, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Carmines PK, Fowler BC, Bell PD: Segmentally distinct effects of depolarization on intracellular [Ca2+] in renal arterioles. Am J Physiol 265: F677–F685, 1993 [DOI] [PubMed] [Google Scholar]
- 24.Conger JD, Falk SA: KCl and angiotensin responses in isolated rat renal arterioles: Effects of diltiazem and low-calcium medium. Am J Physiol 264: F134–F140, 1993 [DOI] [PubMed] [Google Scholar]
- 25.Inscho EW, Mason MJ, Schroeder AC, Deichmann PC, Stiegler KD, Imig JD: Agonist-induced calcium regulation in freshly isolated renal microvascular smooth muscle cells. J Am Soc Nephrol 8: 569–579, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Salomonsson M, Sorensen CM, Arendshorst WJ, Steendahl J, Holstein-Rathlou NH: Calcium handling in afferent arterioles. Acta Physiol Scand 181: 421–429, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, Waeber C: Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol 153: 140–147, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salomone S, Yoshimura S, Reuter U, Foley M, Thomas SS, Moskowitz MA, Waeber C: S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur J Pharmacol 469: 125–134, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Choi SK, Ahn DS, Lee YH: Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc Res 82: 324–332, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Bolz SS, Vogel L, Sollinger D, Derwand R, Boer C, Pitson SM, Spiegel S, Pohl U: Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation 108: 342–347, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Keller M, Lidington D, Vogel L, Peter BF, Sohn HY, Pagano PJ, Pitson S, Spiegel S, Pohl U, Bolz SS: Sphingosine kinase functionally links elevated transmural pressure and increased reactive oxygen species formation in resistance arteries. FASEB J 20: 702–704, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, Ozaki Y: Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res 58: 170–177, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Cyster JG, Schwab SR: Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu Rev Immunol 30: 69–94, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T: Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J 397: 461–471, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inscho EW, Imig JD, Cook AK: Afferent and efferent arteriolar vasoconstriction to angiotensin II and norepinephrine involves release of Ca2+ from intracellular stores. Hypertension 29: 222–227, 1997 [DOI] [PubMed] [Google Scholar]
- 36.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T: Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science 279: 1552–1555, 1998 [DOI] [PubMed] [Google Scholar]
- 37.Coussin F, Scott RH, Wise A, Nixon GF: Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: Differential role in vasoconstriction. Circ Res 91: 151–157, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Peter BF, Lidington D, Harada A, Bolz HJ, Vogel L, Heximer S, Spiegel S, Pohl U, Bolz SS: Role of sphingosine-1-phosphate phosphohydrolase 1 in the regulation of resistance artery tone. Circ Res 103: 315–324, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota H, Beutz MA, Ito M, Abe K, Oka M, McMurtry IF: S1P(4) receptor mediates S1P-induced vasoconstriction in normotensive and hypertensive rat lungs. Pulm Circ 1: 399–404, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Igarashi J, Erwin PA, Dantas AP, Chen H, Michel T: VEGF induces S1P1 receptors in endothelial cells: Implications for cross-talk between sphingolipid and growth factor receptors. Proc Natl Acad Sci U S A 100: 10664–10669, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osada M, Yatomi Y, Ohmori T, Ikeda H, Ozaki Y: Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299: 483–487, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Lim M, Choi SK, Cho YE, Yeon SI, Kim EC, Ahn DS, Lee YH: The role of sphingosine kinase 1/sphingosine-1-phosphate pathway in the myogenic tone of posterior cerebral arteries. PLoS ONE 7: e35177, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanimoto T, Lungu AO, Berk BC: Sphingosine 1-phosphate transactivates the platelet-derived growth factor beta receptor and epidermal growth factor receptor in vascular smooth muscle cells. Circ Res 94: 1050–1058, 2004 [DOI] [PubMed] [Google Scholar]
- 44.Bischoff A, Finger J, Michel MC: Nifedipine inhibits sphinogosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn Schmiedebergs Arch Pharmacol 364: 179–182, 2001 [DOI] [PubMed] [Google Scholar]
- 45.Inscho EW, Cook AK, Webb RC, Jin LM: Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol 296: F590–F597, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arendshorst WJ, Thai TL: Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Curr Opin Nephrol Hypertens 18: 40–49, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Inscho EW, Imig JD, Cook AK, Pollock DMET: ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol 146: 1019–1026, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ikenaga H, Fallet RW, Carmines PK: Basal nitric oxide production curtails arteriolar vasoconstrictor responses to ANG II in rat kidney. Am J Physiol 271: F365–F373, 1996 [DOI] [PubMed] [Google Scholar]
- 49.Guan Z, Giddens MI, Osmond DA, Cook AK, Hobbs JL, Zhang S, Yamamoto T, Pollock JS, Pollock DM, Inscho EW: Immunosuppression preserves renal autoregulatory function and microvascular P2X(1) receptor reactivity in ANG II-hypertensive rats. Am J Physiol Renal Physiol 304: F801–F807, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inscho EW, Cook AK, Clarke A, Zhang S, Guan Z: P2X1 receptor-mediated vasoconstriction of afferent arterioles in angiotensin II-infused hypertensive rats fed a high-salt diet. Hypertension 57: 780–787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guan Z, Pollock JS, Cook AK, Hobbs JL, Inscho EW: Effect of epithelial sodium channel blockade on the myogenic response of rat juxtamedullary afferent arterioles. Hypertension 54: 1062–1069, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Albert R, Hinterding K, Brinkmann V, Guerini D, Müller-Hartwieg C, Knecht H, Simeon C, Streiff M, Wagner T, Welzenbach K, Zécri F, Zollinger M, Cooke N, Francotte E: Novel immunomodulator FTY720 is phosphorylated in rats and humans to form a single stereoisomer. Identification, chemical proof, and biological characterization of the biologically active species and its enantiomer. J Med Chem 48: 5373–5377, 2005 [DOI] [PubMed] [Google Scholar]
- 53.Zhao X, Cook AK, Field M, Edwards B, Zhang S, Zhang Z, Pollock JS, Imig JD, Inscho EW: Impaired Ca2+ signaling attenuates P2X receptor-mediated vasoconstriction of afferent arterioles in angiotensin II hypertension. Hypertension 46: 562–568, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Rodgers A, Mormeneo D, Long JS, Delgado A, Pyne NJ, Pyne S: Sphingosine 1-phosphate regulation of extracellular signal-regulated kinase-1/2 in embryonic stem cells. Stem Cells Dev 18: 1319–1330, 2009 [DOI] [PubMed] [Google Scholar]